Back to Journals » Patient Related Outcome Measures » Volume 14
The Burden of Sickle Cell Disease on Children and Their Caregivers: Caregiver Reports of Children’s Health-Related Quality of Life and School Experiences, Caregiver Burden, and Their Association with Frequency of Vaso-Occlusive Crises
Authors Campbell A, Rizio AA , McCausland KL, Iorga S, Yen GP, Paulose J, Lee S
Received 3 May 2023
Accepted for publication 28 October 2023
Published 28 November 2023 Volume 2023:14 Pages 369—381
DOI https://doi.org/10.2147/PROM.S419607
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lynne Nemeth
Andrew Campbell,1 Avery A Rizio,2 Kristen L McCausland,2 Serban Iorga,3 Glorian P Yen,3 Jincy Paulose,3 Soyon Lee3
1Children’s National Hospital, Washington, DC, USA; 2QualityMetric Incorporated LLC, Johnston, RI, USA; 3Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA
Correspondence: Avery A Rizio, QualityMetric Incorporated, LLC, 1301 Atwood Avenue, Suite 216E, Johnston, RI, 02919, USA, Tel +1 401-903-4631, Email [email protected]
Background: Children with sickle cell disease (SCD) experience a multiplex of disease-related symptoms and complications, including vaso-occlusive crises (VOCs), episodes characterized by extreme pain.
Methods: A cross-sectional observational survey examined the health-related quality of life (HRQoL) and school experiences of children with SCD 2 months– 11 years, burden experienced by their caregivers, and associations between these outcomes and VOC frequency. Caregivers (N=167) of children with SCD in the US completed the Infant-Toddler Quality of Life-Short Form 47 (ITQoL-SF47) for children 2 months– 4 years, the Child Health Questionnaire–Parent Form 50 (CHQ-PF50) and PROMIS Pain Interference and Sleep Disturbance Parent Proxy short forms for children 5– 11 years, and a study-specific survey of school experiences.
Results: Children with SCD 2 months– 4 years had lower ITQoL-SF47 scores (ie, worse HRQoL, p< 0.001) than a normative sample of children; across domains, differences ranged from 18.73– 45.03 points and exceeded minimal important difference (MID) thresholds. Except for the behavior domain, children with SCD 5– 11 years had lower scores on all CHQ-PF50 domains than the normative sample (p< 0.001); differences ranged from 6.78– 36.37 points and exceeded MID thresholds. Children with more frequent VOCs had lower HRQoL and worse school experiences than children with less frequent VOCs (p< 0.05, except for behavior domains). The largest differences based on VOC frequency were observed for overall health and bodily pain/discomfort among children 2 months– 4 years (differences=40.88 and 32.50 points, respectively), and bodily pain and role/social limitations due to physical health among children 5– 11 years (differences=38.99 and 37.80, respectively). Caregivers of children with more frequent VOCs experienced greater burden than caregivers of children with less frequent VOCs, though specific areas of impact (eg, caregiver emotions, time) differed across child age groups.
Conclusion: VOC frequency is negatively associated with HRQoL, highlighting the burden experienced by children with SCD and their caregivers.
Keywords: pediatric, child, toddler, well-being, pain crisis, vaso-occlusion
Introduction
Sickle cell disease (SCD) is a group of genetic blood disorders that cause a mutant form of hemoglobin that is less soluble than normal hemoglobin and is prone to polymerization upon deoxygenation.1,2 The polymerization of hemoglobin causes deformation of red blood cells, giving them a sickle shape and causing chronic hemolysis, anemia, and vaso-occlusion.2,3
Early symptoms of SCD in infants include swelling of the hands and feet, anemia, jaundice, and enlarged spleen.4 Other symptoms and complications observed among pediatric patients include fever, stroke, asthma, impaired immunity, avascular necrosis, chronic ulcers, chronic pain, and vaso-occlusive crises (VOCs).4 VOCs are a hallmark of SCD, are characterized by episodes of severe pain, and can begin within the first few months of life, with frequency and severity increasing through toddlerhood and childhood.5,6 VOCs are associated with complications such as acute chest syndrome and long term damage to muscles, bones, and organs.7,8
A review reported that children with SCD experience widespread impairments in health-related quality of life (HRQoL).9 While some studies have reported that infants and toddlers with SCD also experience deficits in HRQoL, particularly in areas related to physical well-being,10 others have failed to find evidence of such deficits among these youngest patients.11 Frequency of VOCs, as measured through healthcare visits, is related to physical and psychosocial HRQoL among children ages 2–19.12 Current research exploring patterns of HRQoL among infants/toddlers and children with SCD is limited, especially in children under age 2. In addition to HRQoL burden, school experiences may also be negatively affected by SCD. A survey of children with SCD revealed that they miss an average of 16 days of school each year, and are not always given the support they need to catch up.13 Reports of a broad range of school experiences, and their association with VOC frequency, are limited.
This cross-sectional observational study sought to address 3 main research goals: 1) to quantify the HRQoL burden on children with SCD under the age of 12; 2) to examine the association between VOC frequency and child HRQoL and school experiences; and 3) to describe the burden of caregivers of children with SCD.
Methods
Study Sample and Procedures
A cross-sectional survey of caregivers of children with SCD was fielded online in the United States (US) between February and April 2021. Data were collected from caregivers of children with SCD ages 2 months–17 years. This manuscript reports on data collected from a subset of caregivers whose children were between the ages of 2 months and 11 years, to focus on the experiences of young children who may not be able to reliably self-report on their experiences. Self-reported data from adolescents with SCD (ages 12–17) will be reported in a separate manuscript.
The study was approved by an independent review board; all participants provided informed consent prior to completing the online survey.
A convenience sample of caregivers was recruited by a vendor, Global Perspectives, who used existing panels of relevant individuals and social media engagement. Caregivers were screened over the phone and deemed eligible if they were ≥18 years old, currently living in the US, and the primary caregiver of a child with SCD between the ages of 2 months and 17 years (inclusive). Caregivers of children with sickle cell trait were excluded. To ensure exclusion of caregivers of children with sickle cell trait, those who did not know their child’s sickle cell type were also excluded. Only 1 caregiver per household was eligible to participate, and each caregiver reported on a single child. A set of sampling quotas for children’s VOC frequency and age was instituted to ensure representation across the full range of these variables.
Upon completion of an informed consent form, caregivers were provided a link to the online survey. Caregivers who completed the survey were offered a $60 honorarium.
Study Measures
Health-Related Quality of Life
The Infant-Toddler Quality of Life-Short Form 47 (ITQoL-SF47) measured HRQoL for children 2 months–4 years. The ITQoL-SF47 is a 47-item, generic caregiver-reported survey that assesses domains of HRQoL relevant to infants/toddlers.14 The ITQoL-SF47 includes 9 scales to assess child well-being and 3 scales to assess caregiver impact. Scale scores range from 0–100, with 100 representing best HRQoL.14 Developer-provided US-based normative data, obtained from caregivers of infants/toddlers ages 2–71 months, provide information on the HRQoL of the average US-based infant/toddler and their caregiver.15 Normative scores are available for 6 child HRQoL scales and all 3 caregiver impact scales. The means and standard deviations (SDs) of the normative values were adjusted to reflect the average scores among a general population sample of caregivers of infants/toddlers ages 2–59 months to better align with the age range of the study sample (see Supplemental Table 1 for adjusted normative scores).
The Child Health Questionnaire – Parent Form 50 (CHQ-PF50) measured HRQoL for children 5–11 years. The CHQ-PF50 is a generic 50-item caregiver-reported survey.16 Eleven scales measure child HRQoL and 4 scales measure caregiver/family impacts. CHQ-PF50 produces individual scale scores and 2 summary scores: Psychosocial Summary and Physical Summary. All scale scores range from 0–100, with higher scores indicating better HRQoL. Developer-provided US-based normative data, obtained from caregivers of children ages 5–18, provide information on the HRQoL of the average US-based child and their caregiver.16 Normative scores are available for 8 scales and both summary scores relating to child HRQoL, and all 4 caregiver/family impact scales. The means and SDs of these normative values were adjusted to reflect the average scores among a general population sample of children ages 5–12 to better align with the age range of the study sample (see Supplemental Table 1 for adjusted normative scores).
Two short form measures from the PROMIS family of instruments – Pain Interference and Sleep Disturbance – served as supplemental measures of HRQoL among children ages 5–11. The Parent Proxy Pain Interference – Short Form 8a is a generic measure of the degree to which pain interferes with everyday activities. The Parent Proxy Sleep Disturbance – Short Form 4a is a generic measure of sleep difficulty. Each short form produces a single score standardized to a US general population mean of 50 and an SD of 10.17–19 Higher scores represent more pain interference or sleep disturbance.
School Experiences
School experiences were evaluated using survey items written for this study. Caregivers were asked to indicate whether their child currently attends school in any capacity (eg, in-person, remote), and if so, whether they participated in school classes or activities over the past 4 weeks. Eleven questions evaluated experiences including days of missed school, ability to complete schoolwork, perceptions of having to work harder than other kids, and difficulty enjoying school. All items assessed experiences related specifically to their child’s SCD. Skip logic was used so that caregivers of children who were not enrolled in school were not asked any additional questions about their school experiences; caregivers of children who were enrolled but did not participate in school classes or activities over the past 4 weeks only received a subset of relevant items.
The factor structure of these items was previously explored using data from adolescents ages 12–17, with a series of factor analyses indicating that a 3-factor model provided the best fit.20 Based on these results, a 3-factor confirmatory factor analysis was conducted using the caregiver data. Good model fit was observed (root mean square error of approximation=0.065, comparative fit index=0.995, Tucker Lewis index=0.993). Internal consistency reliability of each scale was good; Cronbach’s alpha ranged from 0.86–0.93, indicating that each scale measures a single underlying construct. The 3 individual scale scores, recent school experiences, general school experiences, and teacher support, were used for analyses.
VOC Frequency
VOC frequency was evaluated through caregiver-report at screening. The caregiver was asked to report how many VOCs their child had experienced in the past 12 months. Caregivers provided the exact number of VOCs (ie, rather than selecting a categorical response such as 0–2). VOCs were referred to as “sickle cell pain attacks (crises)” without any further definition or restriction.
Statistical Analyses
Analyses were conducted using SAS software, Version 9.4 of the SAS System for Microsoft Windows. Copyright © 2016. SAS Institute Inc. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA.
Sample Characteristics
Descriptive statistics summarized caregiver and child demographic characteristics. Summaries were reported for each domain from the ITQoL-SF47, CHQ-PF50, PROMIS short forms, and school experiences survey.
HRQoL of Children with SCD Compared to Normative Samples
ITQoL-SF47 and CHQ-PF50 domains pertaining to child HRQoL were compared to the adjusted normative scores from the US general population. Scores from the PROMIS Pain Interference and Sleep Disturbance Parent Proxy short forms were compared to the US general population score of 50. Welch’s t-tests were used to test for differences between the SCD sample means and normative sample means at an alpha level of 0.05.
A minimal important difference (MID) is the smallest difference in a scale score that is perceived by the respondent as important. While various methods can be used to estimate an MID, estimates based on the distribution of scores are common.21,22 For this study, the MID was defined as one-half SD of the normative score (see Supplemental Table 1 for thresholds). If the average ITQoL-SF47 or CHQ-PF50 score of the study sample was at least one-half SD lower than the normative sample, it was determined that children with SCD (or their caregivers) experienced meaningful burden. Because higher scores are worse for the PROMIS Pain Interference and Sleep Disturbance short forms (ie, scored in the opposite direction as the ITQoL-SF47 and CHQ-PF50), study sample scores that were at least one-half SD higher than the normative sample indicated meaningful burden for these outcomes.
Association Between VOC Frequency and Child HRQoL/School Experiences
A series of general linear models were conducted to examine the association between VOC frequency and child HRQoL/school experiences. Each model included VOC frequency (0–2 VOCs in the past 12 months; ≥3 VOCs in the past 12 months) as the independent variable and an HRQoL or school experience scale score as the dependent variable. Child’s age and household income were included as covariates. Benjamini-Hochberg (BH) adjusted p-values were used to correct for multiple comparisons.23
Burden on Caregivers of Children with SCD
Caregiver burden scores were obtained from the ITQoL-SF47 (caregivers of children ages 2 months–4 years) and CHQ-PF50 (caregivers of children 5–11 years). Average scores from the caregiver/family impact domains were compared to scores from the adjusted US general population, following the same analytic strategy used to examine child burden.
Associations between VOC frequency and caregiver burden were examined following the same procedure used for child HRQoL analyses (including use of covariates).
Results
Sample Demographics
The analytic sample included 167 caregivers of children with SCD between the ages of 2 months and 11 years (Figure 1). Characteristics of the caregivers and of the children about whom caregivers reported are presented in Table 1. Caregivers were on average 34 years old; the majority were female (77.84%) and Black (92.81%). The children about whom the caregivers reported were on average 5.4 years old; approximately half (49%) were female, and the majority were Black (94.61%). The most frequently reported SCD type among the children was Hb-SS (75.45%), followed by Hb-SC (23.35%). On average, children experienced 5.11 VOCs in the past 12 months; 71.26% of the children experienced at least 3 VOCs. The most frequently reported current treatments for SCD included folic acid (72.46%), antibiotics (68.26%), blood transfusions (50.90%), hydroxyurea (49.70%), and nonsteroidal anti-inflammatory drugs (44.31%).
 |
Table 1 Characteristics of Caregivers and Their Children with SCD, Ages 2 Months – 11 Years |
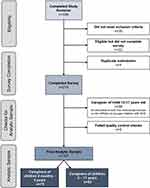 |
Figure 1 Flowchart of participant disposition. Abbreviations: HRQoL, health-related quality of life; SCD, sickle cell disease. |
HRQoL of Children with SCD Compared to Normative Samples
Average HRQoL scores for the study sample are presented in Supplemental Table 2. Compared to the normative sample, children with SCD ages 2 months–4 years experienced deficits (ie, lower scores) across all domains of HRQoL (p<0.001 for all domains; Figure 2A). Differences in all domains were meaningful, as they exceeded the MID threshold. The largest numerical deficit was observed for general health perceptions, where the average score for children with SCD was 45 points lower than that of the average US-based child (M=33.33 vs 78.36, p<0.001).
Children with SCD ages 5–11 years also exhibited deficits in HRQoL relative to a normative sample of children. For all domains of the CHQ-PF50 except behavior, children with SCD had lower HRQoL scores (p=0.936 for behavior, p<0.001 for all other domains, Figure 3A). Differences in all domains except behavior were also meaningful, as they exceeded the MID threshold. The largest numerical difference was observed for general health perceptions, where the average score for children with SCD was 36 points lower than that of the average US-based child (M=36.82 vs 73.19, p<0.001). Children with SCD also experienced more pain interference and sleep disturbance than the average US-based child (pain interference: 55.40 vs 50.00, p<0.001; sleep disturbance: 57.13 vs 50.00, p<0.001); average scores among children with SCD were meaningfully different from the normative score of 50 (Figure 3C).
Association Between VOC Frequency and Child HRQoL
Among children ages 2 months–4 years, associations were observed between VOC frequency and HRQoL for all domains of HRQoL, except for combined behavior/getting along and change in health, such that children who experienced ≥3 VOCs in the past 12 months had worse HRQoL (ie, lower scores) than children who experienced 0–2 VOCs (p=0.091 for combined behavior/getting along, p=0.199 for change in health; p<0.05 for all other domains; Figure 2B). The largest numerical differences were observed for overall health and bodily pain/discomfort, where both differed by >30 points (overall health: adjusted M=14.87 vs 55.75, p<0.001; bodily pain/discomfort: adjusted M=47.80 vs 80.30, p<0.001).
Among children ages 5–11 years, associations were observed between VOC frequency and HRQoL for all domains of the CHQ-PF50, except for behavior and global behavior, such that children with more frequent VOCs experienced worse HRQoL (ie, lower scores) than children with less frequent VOCs (p=0.547 for behavior, p=0.339 for global behavior, p<0.01 for all other domains; Figure 3B). The largest numerical differences were observed for global health (adjusted M=21.74 vs 56.42, p<0.001), physical functioning (adjusted M=56.04 vs 87.40, p<0.001), role/social limitations due to physical health (adjusted M=51.21 vs 89.01, p<0.001), and bodily pain (adjusted M=49.80 vs 88.79, p<0.001), all of which differed by >30 points. This pattern of results was also observed for pain interference and sleep disturbance, as measured by PROMIS short forms, where children with more frequent VOCs had higher scores, indicating more pain interference and sleep disturbance, than children with less frequent VOCs (p<0.001 for both domains, Figure 3D).
Association Between VOC Frequency and School Experiences
Average school experience scores are presented in Supplemental Table 2 and item-level responses are presented in Supplemental Table 3. Among children ages 11 and under who were enrolled in school at the time of the survey, those with more frequent VOCs experienced worse (ie, lower scores) recent and general school experiences than children with less frequent VOCs (Recent experiences: adjusted M=69.29 vs 90.80, p<0.001; general experiences: adjusted M=41.20 vs 62.99, p<0.01; Figure 4). Teacher support did not differ according to VOC frequency (adjusted M=70.94 vs 71.44, adjusted p=0.938).
Burden on Caregivers of Children with SCD
Average caregiver impact domain scores are presented in Supplemental Table 4. Compared to the normative sample, caregivers of children with SCD ages 2 months–4 years reported lower mean scores (ie, greater burden) on the parent impact – emotion domain (ie, experiences worry due to their child’s health; M=53.78 vs 89.97, p<0.001), parent impact – time domain (ie, experiences limitations in time for personal needs due to their child’s health; M=40.92 vs 92.59, p<0.001), and family cohesion domain (ie, experiences difficulty in getting along; M=72.67 vs 80.44, p<0.01). The differences observed on emotion and time (but not family cohesion) also exceeded the MID threshold (Figure 5A). Only the parent impact – time domain was associated with VOC frequency, such that caregivers of children with more frequent VOCs experienced greater limitations in time for personal needs than caregivers of children with less frequent VOCs (adjusted M=36.37 vs 56.42, p<0.01; Figure 5B).
Compared to the normative sample, caregivers of children with SCD 5–11 years reported lower scores (ie, greater burden) on the parent impact – emotion domain (M=54.35 vs 81.42, p<0.001), parent impact – time domain (M=63.77 vs 88.67, p<0.001), and family activities domain (ie, their child’s health limits family activities and/or causes family tension; M=69.38 vs 89.36, p<0.001); these differences were also meaningful, as they exceeded the MID threshold (Figure 5C). Differences between caregivers of children with SCD and the normative sample were not observed for the family cohesion domain (M=69.46 vs 71.96, p=0.391). Three domains – parent impacts on emotions, parent impacts on time, and family activities – were also associated with VOC frequency (Figure 5D); caregivers of children with more frequent VOCs experienced more worry due to their child’s health (emotions: adjusted M=45.85 vs 71.07, p<0.001), more limitations in time for personal needs (time: adjusted M=57.10 vs 76.88, p<0.01), and more family tension (family activities: adjusted M=62.09 vs 83.74, p<0.001) than caregivers of children with less frequent VOCs.
Discussion
Results of this caregiver-reported, cross-sectional, observational study show that children with SCD have worse HRQoL than normative samples of children; these deficits exist both for infants/toddlers 2 months–4 years and children 5–11 years. Across both age groups, the largest deficits were related to general health (eg, beliefs that the caregiver’s child is less healthy than other children, expectations for living a healthy life), while in the older children equally large deficits were also observed for physical functioning and pain. Older children did not exhibit impairment in the behavior domain, relative to the normative sample. Across both age groups, children who experienced ≥3 VOCs had worse HRQoL than children with 0–2 VOCs. VOC frequency was not associated with combined behavior/getting along in the youngest children, nor was it associated with behavior in the older children or teacher support among children enrolled in school.
This study complements and extends previous findings on the HRQoL of children with SCD, providing needed insight into the experiences of younger children and the association between HRQoL and VOC frequency. One recent meta-analysis reported that children with SCD have worse overall HRQoL than healthy peers,24 while a second review reported more specifically on observed impairments in physical functioning, including both performance-based assessments and physical functioning domains of HRQoL.25 Results of the current study indicate that many other specific domains of HRQoL are impacted by SCD, including growth and development, general health, and pain for young children, and role/social limitations, general health, mental health, pain, and sleep for older children. These findings are of particular importance, as they highlight impairments experienced by children across a relatively wide range, including very young children. While some previous research has suggested that infants experience deficits in HRQoL relative to the general population,26 others have indicated that HRQoL of young children (ages 0–5) does not differ from the general population.11 As such, the current study provides valuable insight into the type and extent of HRQoL burden experienced by the youngest children with SCD.
Associations between HRQoL and clinical indicators have been reported among children with SCD, but few have explored the relationship between HRQoL and VOC experiences. For example, healthcare resource utilization (emergency room visits and hospitalizations) was associated with overall HRQoL among children with SCD ages 8–17, such that those with higher utilization had lower HRQoL.27 Among children and adolescents with SCD ages 2–19 years, frequency of VOCs resulting in healthcare resource utilization was also negatively associated with 2 broad domains of HRQoL (physical and psychosocial).12 The current study considered the frequency of all VOCs experienced, including those that may be treated at home, and evaluated multiple domains of HRQoL as well as school experiences, rather than focusing only on component scores that aggregate across unique domains. As such, the results provide new information on the relationship between VOCs and specific domains of HRQoL and school experiences, helping to identify areas of burden more clearly. These results also emphasize the potential that even infants/toddlers could benefit from disease modifying therapy aimed at decreasing VOCs or preventing other complications, which could improve domains most impacted by VOCs, such as physical health and pain.
This study also provides insight into the experiences of caregivers of children with SCD. Relative to caregivers from the general population, caregivers of children with SCD reported worse scores (ie, greater burden) on domains related to family activities (only evaluated for caregivers of children ages 5–11), emotion, and time to care for their own needs. One example includes a recent study in which caregivers of children with SCD in Nigeria reported lost income, tension, or hostility in their home/with their spouse, and feeling they have neglected other family members.28 While this burden may be experienced by any caregiver as they face difficulties caused by caring for a young child regardless of the presence of a chronic disease, the current study quantifies the degree of burden relative to the US general population, clarifying the relative impact of caring for a child with SCD. Nevertheless, caregivers of children with SCD did not report lower perceptions of family cohesion than the general population, nor was VOC frequency associated with this domain. Through qualitative interviews, caregivers of children with SCD have emphasized the importance of family resilience, including flexibility, family and community connections, and social support.29 Though not directly evaluated in the current study, it is possible that resilience can help support a maintained sense of family cohesion, despite experiencing worry due to their child’s health and limitations in caring for their own needs.
Select limitations of this study should be noted. The study design was observational and cross-sectional; no causal or longitudinal relationships can be inferred. The purpose of the study was to describe HRQoL rather than to develop the best predictive model; other uncontrolled or unmeasured variables (eg, treatment that improves HRQoL by mechanisms beyond VOC reduction),30,31 could contribute to the relationships reported here. Moreover, inclusion of such variables in a model (eg, receipt of disease modifying therapy, SCD genotype) could also change the observed associations between HRQoL and VOC frequency. Data collection occurred during the COVID-19 pandemic, and before US-based children and infants were eligible for vaccinations. Hybrid or distance learning may have reduced school absences due to SCD, while potentially making other experiences more difficult. As such, generalizing these findings outside this context may be difficult. Finally, the study relied on caregiver report; HRQoL in infants may be difficult to evaluate (though infant-specific measures, such as the one used in this study may alleviate that), and for older children, caregiver reports may not always correspond exactly with reports that the child would provide.32
Despite these limitations, the study also had several strengths, particularly in the context of previously published work. Analyses presented here included a wide age range of children (2 months–11 years) and evaluated HRQoL with a set of surveys that produce scores for many specific domains, resulting in a more detailed assessment of burden. The study-specific measure of school experiences also evaluated a broad array of experiences that extend beyond absences or grades. Not restricting evaluation of VOC frequency to those resulting in healthcare resource utilization likely resulted in a more accurate depiction of children’s VOC experiences. A recent review suggests that while most studies consider only VOCs that are managed in an inpatient or outpatient setting, imposing such definitions on these experiences risks undercounting their true frequency, as many VOCs are treated at home.33 Thus, the associations between HRQoL and VOC frequency reported in the current study provide a more complete description of the patient experience.
Conclusion
Together, the findings of this study call for early attention to the burden of SCD experienced by children and their caregivers. Future research should focus on examining how treatment, and possibly non-pharmacologic interventions, may reduce this burden, focusing on the child and on the entire family unit.
Abbreviations
CHQ-PF50, Child Health Questionnaire – Parent Form 50; HRQoL, Health-related quality of life; ITQoL-SF47, Infant-Toddler Quality of Life-Short Form 47; MID, Minimal important difference; SCD, Sickle cell disease; SD, Standard deviation; VOC, Vaso-occlusive crisis.
Ethics and Informed Consent
All procedures involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The informed consent form, protocol, and recruitment materials were approved by the Western Copernicus Group Independent Review Board (WCG IRB # 20203536). WCG is an independent review board, as opposed to a board that is affiliated with a specific hospital, university, or other institution. As this was not site-based research, approval from individual institutions’ boards was not necessary. All participants consented to participating in the study.
Acknowledgments
We would like to thank the caregivers who participated in this study; their time, interest, and insight is greatly appreciated.
Disclosure
GPY, JP, SL, and SI are employees of Novartis Pharmaceuticals Corporation. GPY and JP also own stock in the company. AC has received consultancy and research funding from Novartis Pharmaceuticals Corporation. He also reports grants for clinical trial and/or consultancy from Global Blood Therapeutics, Agios, Vertex, and Bluebird Bio, outside the submitted work. AAR and KLM are employees of QualityMetric Incorporated, LLC, and received funding from Novartis Pharmaceuticals Corporation to conduct this research. After the study was conducted, QualityMetric acquired ownership of the HealthActCHQ family of surveys, which includes the CHQ-PF50 and the ITQoL-SF47. The authors report no other conflicts of interest in this work.
References
1. Ware RE, de Montalembert M, Tshilolo L, Abboud MR. Sickle cell disease. Lancet. 2017;390(10091):311–323. doi:10.1016/S0140-6736(17)30193-9
2. Kato GJ, Piel FB, Reid CD, et al. Sickle cell disease. Nat Rev Dis Primers. 2018;4:18010. doi:10.1038/nrdp.2018.10
3. Piel FB, Steinberg MH, Rees DC, Longo DL. Sickle cell disease. N Engl J Med. 2017;376(16):1561–1573. doi:10.1056/NEJMra1510865
4. Kanter J, Kruse-Jarres R. Management of sickle cell disease from childhood through adulthood. Blood Rev. 2013;27(6):279–287. doi:10.1016/j.blre.2013.09.001
5. Stinson J, Naser B. Pain management in children with sickle cell disease. Pediatr Drugs. 2003;5(4):229–241. doi:10.2165/00128072-200305040-00003
6. Brandow AM, DeBaun MR. Key Components of Pain Management for Children and Adults with Sickle Cell Disease. Hematol Oncol Clin North Am. 2018;32(3):535–550. doi:10.1016/j.hoc.2018.01.014
7. Osunkwo I, Manwani D, Kanter J. Current and novel therapies for the prevention of vaso-occlusive crisis in sickle cell disease. Ther Adv Hematol. 2020;11:2040620720955000. doi:10.1177/2040620720955000
8. Jain S, Bakshi N, Krishnamurti L. Acute Chest Syndrome in Children with Sickle Cell Disease. Pediatr Allergy Immunol Pulmonol. 2017;30(4):191–201. doi:10.1089/ped.2017.0814
9. Ojelabi A, Graham Y, Ling J. Health-related quality of life predictors in children and adolescents with sickle cell disease: a systematic review. Int J Trop Dis Health. 2017;22(2):1–14. doi:10.9734/IJTDH/2017/31954
10. Beverung LM, Bemrich-Stolz C, Torres S, Panepinto JA. Health-related quality of life in infants with sickle cell disease. J Pediatr Hematol Oncol. 2015;37(8):590–594. doi:10.1097/MPH.0000000000000434
11. Houwing ME, Muntendam MJ, van Muilekom MM, et al. Health-related quality of life in infants, toddlers and young children with sickle cell disease. Pediatr Blood Cancer. 2022;69(1):e29358. doi:10.1002/pbc.29358
12. Schlenz AM, Schatz J, McClellan CB, Roberts CW. Responsiveness of the PedsQL to pain-related changes in health-related quality of life in pediatric sickle cell disease. J Pediatr Psychol. 2012;37(7):798–807. doi:10.1093/jpepsy/jss051
13. Dyson SM, Abuateya H, Atkin K, et al. Reported school experiences of young people living with sickle cell disorder in England. Br Educ Res J. 2010;36(1):125–142. doi:10.1080/01411920902878941
14. Health ActCHQ. Itqol-SF47 Scoring Manual. Boston, MA: Health ActCHQ; 2015.
15. Health ActCHQ. Itqol-SF47 US Norms. Boston, MA: HealthActCHQ; 2017.
16. Health ActCHQ. The CHQ Scoring and Interpretation Manual. Boston, MA: Health ActCHQ; 2013.
17. Varni JW, Thissen D, Stucky BD, et al. PROMIS® Parent Proxy Report Scales: an item response theory analysis of the parent proxy report item banks. Qual Life Res. 2012;21(7):1223–1240. doi:10.1007/s11136-011-0025-2
18. Irwin DE, Gross HE, Stucky BD, et al. Development of six PROMIS pediatrics proxy-report item banks. Health Qual Life Outcomes. 2012;10(1):22. doi:10.1186/1477-7525-10-22
19. Forrest CB, Meltzer LJ, Marcus CL, et al. Development and validation of the PROMIS Pediatric Sleep Disturbance and Sleep-Related Impairment item banks. Sleep. 2018;41(6). doi:10.1093/sleep/zsy054
20. Campbell A, Rizio AA, McCausland KL, et al. The burden of sickle cell disease on adolescents: results of an observational study of vaso-occlusive crises, health-related quality of life, and school experiences; 2023.
21. Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–592. doi:10.1097/01.MLR.0000062554.74615.4C
22. Guyatt GH, Osoba D, Wu AW, Wyrwich KW, Norman GR. Methods to explain the clinical significance of health status measures. Mayo Clin Proc. 2002;77(4):371–383. doi:10.4065/77.4.371
23. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Statist Soc Ser B. 1995;57(1):289–300.
24. Stokoe M, Zwicker HM, Forbes C, et al. Health related quality of life in children with sickle cell disease: a systematic review and meta-analysis. Blood Rev. 2022;56:100982. doi:10.1016/j.blre.2022.100982
25. Marchese V, Rock K, Harpold A, Salazar A, Williams M, Shipper AG. Physical impairment and function in children and adolescents with sickle cell disease: a systematic review. Arch Phys Med Rehabil. 2022;103(6):1144–1167.e2. doi:10.1016/j.apmr.2021.08.022
26. Beverung LM, Strouse JJ, Hulbert ML, et al. Health-related quality of life in children with sickle cell anemia: impact of blood transfusion therapy. Am J Hematol. 2015;90(2):139–143. doi:10.1002/ajh.23877
27. Moody KL. Healthcare utilization and the quality of life of children and adolescents with sickle cell disease. Pediatr Blood Cancer. 2022;69(8):e29685. doi:10.1002/pbc.29685
28. Adegoke SA, Kuteyi EA. Psychosocial burden of sickle cell disease on the family, Nigeria. Afr J Prim Health Care Fam Med. 2012;4(1):289. doi:10.4102/phcfm.v4i1.380
29. Reader SK, Pantaleao A, Keeler CN, et al. Family resilience from the perspective of caregivers of youth with sickle cell disease. J Pediatr Hematol Oncol. 2020;42(2):100–106. doi:10.1097/MPH.0000000000001682
30. Thornburg CD, Calatroni A, Panepinto JA. Differences in health-related quality of life in children with sickle cell disease receiving hydroxyurea. J Pediatr Hematol Oncol. 2011;33(4):251–254. doi:10.1097/MPH.0b013e3182114c54
31. Bhatia M, Kolva E, Cimini L, et al. Health-related quality of life after allogeneic hematopoietic stem cell transplantation for sickle cell disease. Biol Blood Marrow Transplant. 2015;21(4):666–672. doi:10.1016/j.bbmt.2014.12.007
32. Upton P, Lawford J, Eiser C. Parent-child agreement across child health-related quality of life instruments: a review of the literature. Qual Life Res. 2008;17(6):895–913. doi:10.1007/s11136-008-9350-5
33. Zaidi AU, Glaros AK, Lee S, et al. A systematic literature review of frequency of vaso-occlusive crises in sickle cell disease. Orphanet J Rare Dis. 2021;16(1):460. doi:10.1186/s13023-021-02096-6
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




