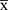Back to Journals » Drug Design, Development and Therapy » Volume 16
Ropivacaine with Dexmedetomidine or Dexamethasone in a Thoracic Paravertebral Nerve Block Combined with an Erector Spinae Plane Block for Thoracoscopic Lobectomy Analgesia: A Randomized Controlled Trial
Authors Yang J, Zhao M, Zhang XR, Wang XR, Wang ZH, Feng XY, Lei YJ, Zhang JW
Received 14 March 2022
Accepted for publication 13 May 2022
Published 26 May 2022 Volume 2022:16 Pages 1561—1571
DOI https://doi.org/10.2147/DDDT.S366428
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Jing Yang,1 Min Zhao,1 Xiao-Rui Zhang,1 Xiao-Rui Wang,1 Zhi-Hao Wang,1 Xiao-Yue Feng,2 Ya-Juan Lei,1 Jian-Wen Zhang1,3
1Department of Anesthesiology, Third Hospital of Shanxi Medical University, Shanxi Bethune Hospital, Shanxi Academy of Medical University, Tongji Shanxi Hospital, Taiyuan, 030032, People’s Republic of China; 2Department of Pain Management, Third Hospital of Shanxi Medical University, Shanxi Bethune Hospital, Shanxi Academy of Medical University, Tongji Shanxi Hospital, Taiyuan, 030032, People’s Republic of China; 3Department of Day Surgery, Third Hospital of Shanxi Medical University, Shanxi Bethune Hospital, Shanxi Academy of Medical University, Tongji Shanxi Hospital, Taiyuan, 030032, People’s Republic of China
Correspondence: Jian-Wen Zhang, Department of Anesthesiology and Department of Day Surgery, Third Hospital of Shanxi Medical University, Shanxi Bethune Hospital, Shanxi Academy of Medical University, Tongji Shanxi Hospital, No. 99 of Longcheng Street, Xiaodian District, Taiyuan, 030032, People’s Republic of China, Tel +86 13994299284, Email [email protected]
Objective: This study aimed to investigate the effect of ropivacaine with dexmedetomidine or dexamethasone in a thoracic paravertebral nerve block (TPVB) combined with an erector spinae plane block (ESPB) for thoracoscopic lobectomy analgesia.
Methods: A total of 97 patients undergoing thoracoscopic lobectomy under general anesthesia were enrolled in this study and randomly divided into three groups, ie, a ropivacaine group (Group R), a ropivacaine + dexmedetomidine group (Group R1), and a ropivacaine + dexamethasone group (Group R2). Ultrasound-guided TPVB combined with an erector spinae plane block was given after anesthesia induction. The following were applied to each group: Group R received 30 mL of 0.5% ropivacaine + 5 mL of a normal saline mixture; Group R1 received 30 mL of 0.5% ropivacaine + 5 mL of a 1 μg/kg dexmedetomidine mixture; Group R2 received 30 mL of 0.5% ropivacaine + 5 mL of an 8 mg dexamethasone mixture. The primary observation index was the time to the first postoperative remedial analgesia. The secondary observation indexes were the intraoperative consumption of propofol and sufentanil, time to waking from anesthesia, time to extubation, postoperative numerical rating scaltpe (NRS) score, postoperative sufentanil consumption, remedial analgesic dosage, and adverse reactions.
Results: When compared with Group R, the time to first postoperative remedial analgesia was longer, the intraoperative and postoperative sufentanil consumption and flurbiprofen axetil remedial analgesic dose were lower, and the time to waking from anesthesia and time to extubation were shorter in groups R1 and R2 (P < 0.05). The NRS scores at 1, 6, 12, and 24 h postoperatively in groups R1 and R2 were lower than in Group R at the same time points (P < 0.05).
Conclusion: Ropivacaine with dexmedetomidine or dexamethasone in TPVB combined with ESPB could prolong the time to first postoperative remedial analgesia, reduce perioperative sufentanil and postoperative remedial analgesic drug consumption, and decrease the postoperative NRS score in patients undergoing thoracoscopic lobectomy.
Keywords: ropivacaine, local anesthetic adjuvant, dexmedetomidine, dexamethasone, thoracic paravertebral nerve block, erector spinae plane block, thoracoscopic surgery, perioperative analgesia
Corrigendum for this paper has been published.
Background
The importance of early rehabilitation after surgery has accelerated the application of thoracoscopic techniques in the field of thoracic surgery. Stimulation of the organs caused by internal trauma, injury of the pleura and lung parenchyma, traction of the intercostal nerve, and stimulation of the thoracic drainage tube during thoracoscopic surgery may cause a notable intraoperative stress response and postoperative pain, which, in turn, may lead to complications, such as atelectasis, pulmonary infection, and myocardial ischemia.1
Ultrasound-guided thoracic paravertebral nerve block (TPVB)2 and an erector spinae plane block (ESPB)3–5 can be used alone or as a supplement to general anesthesia for perioperative analgesia in thoracoscopic surgery. However, the limited diffusion range of TPVB6 and the wide diffusion range and poor accuracy of ESPB7,8 limit their widespread application in the perioperative period.
Zengin et al9 revealed that combining TPVB and ESPB enabled effective postoperative pain management along with the use of morphine in acceptable quantities in patients undergoing video-assisted thoracic surgery. Another advantage of this technique is that it provides a safer and more comfortable block for both patients and practitioners due to the simultaneous use of two different blocks with the insertion of a single needle.
Local anesthetic adjuvants can prolong the effective duration and enhance the quality of a peripheral nerve block. Gao et al10 revealed that dexmedetomidine, as an adjuvant to ropivacaine in ESPB, could prolong the sensory nerve block time, effectively control acute postoperative pain, reduce the need for postoperative remedial analgesia, and shorten the postoperative hospital stay of patients undergoing thoracoscopic surgery. Zhang et al11 showed that dexamethasone, combined with local anesthetics for ultrasound-guided horizontal transverse abdominal plane block, could reduce the pain score after abdominal surgery, prolong the time to first postoperative remedial analgesia, and reduce the consumption of morphine and the incidence of nausea and vomiting.
The purpose of this study was to investigate the effect of ropivacaine with dexmedetomidine or dexamethasone in TPVB combined with ESPB for thoracoscopic lobectomy analgesia. The researchers hypothesized that ropivacaine with dexmedetomidine or dexamethasone in TPVB combined with ESPB would be able to prolong the time to first postoperative remedial analgesia, reduce perioperative sufentanil and postoperative remedial analgesic drug consumption, and decrease the postoperative numerical rating scale (NRS) score in patients undergoing thoracoscopic lobectomy.
Methods
General Information
The present study was approved by the Ethics Committee of the Shanxi Bethune Hospital (YXLL-2020-071) and registered at the China Clinical Trial Registration Center (ChiCTR2100043516). All patients signed an informed consent form to confirm their participation in the research. A total of 90 patients undergoing thoracoscopic lobectomy under selective general anesthesia in the Shanxi Bethune Hospital from March 2021 to October 2021 were enrolled.
Inclusion Criteria
The inclusion criteria were as follows: patients aged 30–70 years with a body mass index (BMI) of 18–30 kg/m2 and an American Society of Anesthesiologists (ASA) grade of I or II.
Exclusion Criteria
The exclusion criteria were as follows: a history of any severe central nervous system or respiratory diseases, severe abnormal liver and kidney function, spinal deformity, infection at or near the intended puncture site, abnormal coagulation function, glucocorticoid contraindications, treatment for chronic pain or the long-term use of steroids, allergy to any of the drugs used in the study, or a refusal to sign the informed consent form.
Elimination Criteria
The elimination criteria were as follows: the decision to perform a thoracotomy during the perioperative period, perioperative bleeding ≥500 mL, or if the patient was transferred to the intensive care unit (ICU) postoperatively.
For this randomized controlled study, the SAS v9.4 statistical software program was used to generate a random number table, and the 90 patients participating in the study were randomly divided into three equal groups, ie, the ropivacaine group (Group R), the ropivacaine + dexmedetomidine group (Group R1), and the ropivacaine + dexamethasone group (Group R2). The attending anesthesiologist was informed about the grouping of the patients, but the patients and data acquisition recorders were blinded to the trial grouping. The same team of thoracic physicians performed all of the surgeries using single-port thoracoscopic techniques. The chest drainage tube was removed 3 days after surgery.
Anesthesia Protocol
All subjects fasted from food for 8 h and water for 4 h preoperatively and did not receive medication before being anesthetized. The electrocardiogram, noninvasive blood pressure, heart rate (HR), pulse oxygen saturation (SpO2), respiratory rate, and bispectral index (BIS) were monitored once the patient entered the operating room. Following on, the peripheral vein of the upper limb was opened to inject Ringer’s lactate solution. The patient then inhaled oxygen through a mask (5 L/min). For routine intravenous rapid anesthesia induction, midazolam (0.05 mg/kg), sufentanil citrate (0.5 μg/kg), rocuronium bromide (0.6 mg/kg), and etomidate fat emulsion (0.3 mg/kg) were successively injected intravenously; oxygen was supplied through mask pressurization for 3 min, and double-lumen endotracheal tube intubation was performed under a visible laryngoscope. With the help of a fiberoptic bronchoscope, the endotracheal tube was positioned and confirmed, and the anesthesia ventilator was connected for mechanical ventilation. The parameters for dual lung ventilation were as follows: tidal volume, 8 mL/kg; respiratory rate, 12 times/min; inspiratory-to-expiratory ratio, 1:2; oxygen flow, 2 L/min. The parameters for single-lung ventilation were as follows: tidal volume, 5 mL/kg; respiratory rate, 14 times/min; inspiratory-to-expiratory ratio, 1:2; oxygen flow, 2 L/min. During the surgery, the end-expiratory carbon dioxide partial pressure was maintained at 35–45 mmHg by changing the respiratory parameters, such as the tidal volume and inspiratory-to-expiratory ratio. At the end of single lung ventilation and following full suction of the sputum, the lung was inflated to promote lung recruitment.
Following the induction of anesthesia, the patient was placed in the lying position with the affected side up and ultrasound-guided TPVB, combined with ESPB, was performed. The procedure was performed as follows. Following routine disinfection, a surgical drape and towel were placed. A SonoSite® S-Nerve portable ultrasound machine (Fujifilm, WA, USA) was adopted, and the probe was placed at the T5–6 spinous process space of the patient on the affected side to conduct median sagittal scanning. The moving probe displayed the T5–6 transverse process and pleura, ie, the “landscape sign,” and the paravertebral space was presented as a wedge-shaped hypoechoic region. The puncture needle was inserted between the transverse costal process fascia and pleura in the paravertebral space of the thoracic spine using the out-of-plane puncturing technique. After confirming that no blood or cerebrospinal fluid could be observed upon withdrawing the needle, 15 mL of preconfigured local anesthetic was injected according to the patient grouping. The diffusion of liquid medicine and the downward movement of the pleura were clearly observed under ultrasound, ie, the “ebb-tide sign.” The ultrasonic probe was then moved to the T5 and T6 transverse processes, and the trapezius, rhomboid, and erector spinae muscles were penetrated in turn using the out-of-plane puncturing technique until the needle tip was between the deep surface of the erector spinae muscle and the T5 and T6 transverse processes. After confirming that no blood or cerebrospinal fluid could be observed when withdrawing the needle, 20 mL of preconfigured local anesthetic was injected according to the patient grouping. The prescription for the nerve block in each group was as follows.
Group R: 30 mL of 0.5% ropivacaine + 5 mL of a normal saline mixture (total = 35 mL);
Group R1: 30 mL of 0.5% ropivacaine + 1 μg/kg of a dexmedetomidine mixture (total = 35 mL);
Group R2: 30 mL of 0.5% ropivacaine + 8 mg of a dexamethasone mixture (total = 35mL).
In the perioperative period, total intravenous anesthesia was maintained. The infusion volume of propofol was adjusted to maintain a BIS value of 40–60. The infusion volume of sufentanil was adjusted to maintain the mean arterial pressure (MAP) between the preoperative base value ± 20%. Rocuronium bromide was given as required. A restrictive intravenous infusion was given during the surgery. During the perioperative period, when the MAP was <80% of the base value, 10 mg of ephedrine hydrochloride was injected intravenously; when the MAP was >120% of the base value, 0.3 mg of nicardipine was injected intravenously; when the HR was <80% of the base value, 0.5 mg of atropine sulfate was injected intravenously; and when the HR was >120% of the base value, 10 mg of esmolol was injected intravenously.
For all of the participants, the infusion of propofol and sufentanil was stopped during skin suturing, and 12.5 mg of dolasetron mesylate was injected intravenously. Once the patient regained consciousness and spontaneous breathing was recovered, the tracheal tube was removed, and the patient was escorted to the post-anesthesia recovery room. The indications for extubation were as follows: the patient was able to open their eyes, raise their head, raise their hands, and breathe autonomously according to instructions; tidal volume in spontaneous breathing >6 mL/kg; a respiratory rate of 10–18 times/min and SpO2 >95% after 5 min of deoxygenated breathing. Postoperatively, patient-controlled intravenous analgesia was started for 48 h. The analgesic pump formula was as follows: 100 μg of sufentanil citrate and 25 mg of dolasetron mesylate diluted to 100 mL with 0.9% normal saline. The analgesic pump setting was as follows: a loading dose of 2 mL, a background dose of 1 mL/h, a single patient-controlled additional dose of 2 mL, and a locking time of 15 min. The pain state of patients was evaluated using the NRS, and 50 mg of flurbiprofen axetil was used for remedial analgesia when the NRS score was ≥4. Dexmedetomidine and dexamethasone were not used within 48 h postoperatively.
Observation Indexes
Primary Observation Index
The primary observation index was the time to first postoperative remedial analgesia.
Secondary Observation Indexes
The following were used as secondary observation indexes.
- The perioperative consumption of propofol and sufentanil, time to waking from anesthesia (from the time the patient stopped anesthesia to the time they opened their eyes after being called), and time to extubation (from the time the patient stopped anesthesia to the time the tracheal catheter was removed).
- The NRS scores at 1, 6, 12, 24, and 48 h postoperatively (0 points indicated no pain, a score <4 indicated mild pain; a 4–7 score indicated moderate pain, a score >7 points indicated severe pain, and a score of 10 indicated unbearably severe pain.
- The total consumption of sufentanil and the remedial analgesic dose of flurbiprofen axetil in the analgesic pump within 48 h postoperatively.
- The incidence of hypotension, bradycardia, respiratory depression, agitation, pulmonary infection, nausea and vomiting, and neurotoxicity (abnormal sensation or a loss of sensation in the block area) within 48 h postoperatively.
Statistical Analysis
Referring to Wang12 and Kumar,13 the sample size was 90 patients, with 30 patients in each of the three groups. The data were statistically analyzed using the SPSS Statistics 21.0 (IBM®, NY, USA) software program. The measurement data were expressed as mean ± standard deviation, and the count data were expressed as frequencies or percentages. A multigroup comparison of the normally distributed measurement data with homogeneity of variance was conducted using one-way analysis of variance, and intergroup pairwise comparison was conducted using the least significant difference method. The Kruskal–Wallis H-test was used to compare measurement data that were not normally distributed or that had heterogenous variance among multiple groups, and the Friedman test was used to compare repeated measurement data that were not normally distributed; for this data, the Bonferroni method was used to correct the P-value in the pairwise comparison. Categorical and count data were compared using the chi-squared (x2) test, and P < 0.05 was considered statistically significant.
Results
General Data
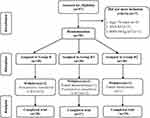
A total of 97 patients undergoing thoracoscopic lobectomy under general anesthesia and endotracheal intubation were selected for this study, and 7 were excluded because they did not meet the inclusion criteria (n = 3, age >70; n = 3, ASA = III; n = 1, BMI >30 kg/m2). Therefore, 90 patients were enrolled in the trial. During the trial, another 6 patients were excluded from the final data analysis because 1 patient received a thoracotomy and 5 were transferred to the ICU postoperatively (see Figure 1). The differences in general data among the three groups were not statistically significant (P > 0.05; see Table 1).
 |
Table 1 Comparison of General Data Among Three Groups |
Comparison of the Observation Indexes Between the Three Groups
Intraoperative Propofol Consumption
There was no significant difference in intraoperative propofol consumption between the three groups (H = 0.048 and P = 0.976; see Table 2).
 |
Table 2 Comparison of Intraoperative Consumption of Propofol and Sufentanil, Awakening Time from Anesthesia and Extubation Time Among the Three Groups ( |
Intraoperative Sufentanil Consumption
Compared with Group R, the intraoperative consumption of sufentanil was significantly lower in groups R1 and R2 (29.96 ± 8.94, 30.90 ± 4.59, and 47. 71 ± 16.57 μg, respectively; H = 26.208 and P < 0.001). There was no significant difference between groups R1 and R2 (P > 0.05; see Table 2).
Time Until Waking Up from Anesthesia
When compared with Group R, the time to waking from anesthesia was significantly shorter in groups R1 and R2 (14.81 ± 3.40, 14.48 ± 4.50, and 23.93 ± 3.45 min, respectively; F = 54.869 and P < 0.001). However, there was no significant difference between groups R1 and R2 (P > 0.05; see Table 2).
Time to Extubation After Anesthesia
When compared with Group R, the time to extubation after anesthesia was significantly shorter in groups R1 and R2 (18.96 ± 4.04, 18.17 ± 4.63, and 30.39 ± 4.67 min, respectively; F = 65.883 and P < 0.001). However, there was no significant difference between groups R1 and R2 (P > 0.05; see Table 2).
Time to the First Postoperative Remedial Analgesia
When compared with Group R, the time to first postoperative remedial analgesia was longer in groups R1 and R2 (24.07 ± 3.73, 23.83 ± 3.27, and 13.21 ± 2.77 h, respectively; H = 54.847 and P < 0.001). However, there was no significant difference between groups R1 and R2 (P > 0.05; see Table 3).
 |
Table 3 The Time to the First Postoperative Remedial Analgesia (T0), Consumption of Sufentanil and Remedial Dose of Flurbiprofen Axetil in Three Groups |
Postoperative Sufentanil Consumption
When compared with Group R, the postoperative sufentanil consumption was significantly lower in groups R1 and R2 (54.44 ± 5.70, 56.76 ± 5.85, and 74.00 ± 12.68 μg, respectively; H = 42.089 and P < 0.001). There was no significant difference between groups R1 and R2 (P > 0.05; see Table 3).
Postoperative Remedial Flurbiprofen Axetil Dose
Compared with Group R, the postoperative remedial dose of flurbiprofen axetil was significantly lower in groups R1 and R2 (68.52 ± 34.38, 72.41 ± 34.29, and 162.50 ± 46.40 mg, respectively; H = 43.849 and P < 0.001). However, there was no significant difference between groups R1 and R2 (P > 0.05; see Table 3).
Numerical Rating Scores
The intragroup comparison demonstrated significant differences in the NRS scores in each of the three groups at different time points postoperatively (Group R: χ2 = 86.335 and P < 0.001; Group R1: χ2 = 97.374 and P < 0.001; Group R2: χ2 = 108.733 and P < 0.001). The results of the pairwise comparison revealed the NRS scores in the three groups decreased gradually over time, and there were significant differences in the NRS scores at different time points (P < 0.05; see Table 4).
 |
Table 4 NRS Scores at Different Time Points After Operation in Three Groups |
The intergroup comparison revealed that, compared with the same time points in Group R, the NRS score was lower 1, 6, 12, and 24 h postoperatively in groups R1 and R2 (NRS at 1 h: H = 32.469 and P < 0.001; NRS at 6 h: H = 18.363 and P < 0.001; NRS at 12 h: H = 21.301 and P < 0.001; NRS at 24 h: H = 20.363 and P < 0.001). There was no significant difference in the NRS score 48 h postoperatively between the three groups (H = 5.737 and P = 0.057; see Table 4).
Incidence of Adverse Reactions
No respiratory depression, pulmonary infection, or neurotoxicity occurred in any of the groups. There were no significant differences in the incidence of hypotension, bradycardia, agitation, or nausea and vomiting between the three groups within 48 h postoperatively (P > 0.05; see Table 5).
 |
Table 5 Comparison of Postoperative Adverse Reactions Among the Three Groups [Case Number (%)] |
Discussion
Dexmedetomidine is a highly selective alpha (α)-2 adrenergic receptor agonist with sedative, analgesic, anti-inflammatory, and antisympathetic effects. With dexmedetomidine as a ropivacaine adjuvant, ESPB can prolong postoperative analgesic time and reduce perioperative opioid consumption in patients undergoing a thoracotomy. Ding et al14 revealed that TPVB with dexmedetomidine as a ropivacaine adjuvant could satisfactorily control the postoperative pain of patients undergoing thoracoscopic lobectomy.
The present study revealed that, compared with a ropivacaine nerve block without a local anesthetic adjuvant, ropivacaine with dexmedetomidine in TPVB combined with ESPB could prolong the time to first postoperative remedial analgesia, reduce perioperative sufentanil and postoperative remedial analgesic drug consumption, and decrease the postoperative NRS score in patients undergoing thoracoscopic lobectomy. The mechanism is posited to be as follows.
- The peripheral analgesic effect of dexmedetomidine. Dexmedetomidine inhibits pain transmission by binding with the peripheral nerve α2 adrenergic receptor to reduce the release of norepinephrine from nerve endings and influences the direct action on the action potential of aδ/C-fibers.15–17
- The anti-inflammatory effect of dexmedetomidine. Animal experiments have explored the mechanism of perineural dexmedetomidine administration. Huang et al18 found that injecting 20 μg/kg of dexmedetomidine around the sciatic nerve in rats reduced inflammatory cytokines interleukin-6, tumor necrosis factor alpha, and the local inflammatory reaction by inhibiting the nuclear factor kappa-light-chain-enhancer of the activated B-cell signaling pathway. Li et al19 revealed that a femoral nerve block with 1 μg/kg of dexmedetomidine combined with ropivacaine had a better peripheral anti-inflammatory effect and pain control after total knee arthroplasty than ropivacaine only.
- The vasoconstrictive effect of dexmedetomidine. Dexmedetomidine induces peripheral vasoconstriction, resulting in the delayed absorption and prolonged action of local anesthetics by binding to the α2 adrenergic receptors in the peripheral nerves or peripheral blood vessels after local absorption into the blood.20
Dexamethasone is a long-acting steroid drug with anti-inflammatory, anti-allergic, antirheumatic, and immunosuppressive effects. In the past decade, it has been widely used in peripheral nerve blocks as a local anesthetic adjuvant. Gupta et al21 revealed that ropivacaine plus dexamethasone could significantly prolong the postoperative analgesic time during an abdominal transverse plane block. Pande et al22 also revealed that the analgesic time of a supraclavicular brachial plexus block could be significantly prolonged by adding a low dose of dexamethasone to the local anesthetic solution. Mao et al23 revealed that, as an adjuvant of ropivacaine for TPVB, dexamethasone could significantly reduce the dosage of perioperative anesthetic drugs, effectively control postoperative acute pain, reduce postoperative complications, shorten rehabilitation time, and reduce the incidence of chronic pain.
The present study revealed that compared with a ropivacaine nerve block without a local anesthetic adjuvant, ropivacaine with dexamethasone in TPVB combined with ESPB could prolong the time to first postoperative remedial analgesia, reduce perioperative sufentanil and postoperative remedial analgesic drug consumption, and decrease the postoperative NRS score in patients undergoing thoracoscopic lobectomy. The mechanism involved in this process may be as follows.
- The peripheral analgesic effect of dexamethasone. Dexamethasone inhibits the upregulation of nociceptive C-fiber K+ channel messenger ribonucleic acid and affects the activity of nerve fibers by binding to peripheral glucocorticoid receptors.24
- The peripheral anti-inflammatory effect of dexamethasone. Stan et al25 revealed that the addition of glucocorticoids to local anesthetics inhibited the synthesis and secretion of a variety of inflammatory mediators, resulting in the extension of the analgesic time of an axillary brachial plexus nerve block to 48 h.
- The vasoconstriction of dexamethasone. Dexamethasone induces a degree of peripheral vasoconstriction resulting in the delayed absorption and prolonged action time of local anesthetics by binding to glucocorticoid receptors in peripheral nerves or local vessels after peripheral local absorption into the blood.26
The study results showed that there were no significant differences in the time to first postoperative remedial analgesia, perioperative sufentanil consumption, postoperative flurbiprofen axetil remedial analgesia dose, or postoperative NRS scores between ropivacaine combined with dexmedetomidine or dexamethasone. This was consistent with the results obtained by Song et al27 and Yaghobi et al,28 which revealed that dexamethasone and dexmedetomidine as local anesthetic adjuvants had equivalent analgesic effects in TPVB combined with ESPB.
The present study revealed that there was no significant difference in intraoperative propofol consumption between the three groups. However, when compared with Group R, the perioperative consumption of sufentanil was reduced and the time to waking and extubation was shorter in groups R1 and R2, suggesting that dexmedetomidine or dexamethasone as a ropivacaine adjuvant could enhance the analgesic effect of ropivacaine in TPVB combined with ESPB.
The side effects of dexmedetomidine, such as bradycardia, hypertension, and hypotension, increased with an increase in dose. In animal experiments, Wang et al29 revealed that a low dose of dexmedetomidine (1 or 2 μg/mL) combined with 0.25% ropivacaine continuously blocked the femoral nerve of rabbits, and no obvious neurotoxic injury was found. However, a high dose (3 µg/mL) caused neurotoxic injury, thus indicating a dose-dependent neurotoxic reaction. As the incidence of adverse events may increase with an increase in the dexmedetomidine dose, according to the safety of the perineural medication, in this study, after referring to the literature, a dose of 1 µg/mL dexmedetomidine was used as the test dose.10,14,30,31
Kumar et al13 revealed that adding 8 mg of dexamethasone in 0.375% ropivacaine for an ultrasound-guided serratus anterior plane block could prolong T0 for patients undergoing a modified radical mastectomy. In a randomized controlled trial, Ibrahim et al32 revealed that 0.5% bupivacaine plus 8 mg of dexamethasone for an ultrasound-guided adductor canal block could prolong the postoperative analgesic time and reduce the consumption of postoperative analgesic drugs in arthroscopic anterior cruciate ligament reconstruction. Baloda et al33 revealed that adding 8 mg of dexamethasone to 0.5% levobupivacaine for a supraclavicular brachial plexus nerve block could shorten the onset time of sensory and motor nerve block and prolong the duration of analgesia. After referring to the abovementioned literature, a dose of 8 mg of dexamethasone was used as the test dose in this study.
The limitations of this study and related subsequent studies include the following. (1) The current study was exploratory, single-center research with a small sample size, and the universality of the research results is uncertain. Therefore, subsequent studies should verify the research conclusions by expanding the sample size and conducting multicenter trials. (2) This study only discussed observations of clinical efficacy after a fixed dose of local anesthetic and adjuvants; further studies are needed to explore the best dose compatibility scheme of local anesthetics and adjuvants to improve the quality of anesthesia, increase patient safety, and realize precise anesthesia doses and rapid postoperative rehabilitation. (3) This study only investigated the peripheral nerve block effects of dexmedetomidine or dexamethasone in combination with ropivacaine; the potential synergistic effects of these require additional research in the future.
Conclusion
In summary, ropivacaine with dexmedetomidine or dexamethasone in TPVB combined with ESPB could prolong the time to first postoperative remedial analgesia, reduce perioperative sufentanil and postoperative remedial analgesic drug consumption, and decrease the postoperative NRS score in patients undergoing thoracoscopic lobectomy.
Data Sharing Statement
We declared that materials described in the manuscript, including all relevant raw data, will be freely available to any scientist wishing to use them for non-commercial purposes, without breaching participant confidentiality. The data can be obtained from the corresponding author upon reasonable request from the publication date.
Ethics Approval and Consent to Participate
This study was conducted with approval from the Ethics Committee of Shanxi Bethune Hospital (No: YXLL-2020-071), and registered in the Chinese Clinical Trial Register website (www.chictr.org.cn, ChiCTR-2100043516). This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants.
Consent for Publication
All participants signed a document of informed consent.
Disclosure
The authors declare that they have no competing interests.
References
1. Kashiwagi Y, Iida T, Kunisawa T, et al. Efficacy of ultrasound-guided thoracic paravertebral block compared with the epidural analgesia in patients undergoing video-assisted thoracoscopic surgery. Masui. 2015;64(10):1010–1014.
2. Abd-Elshafy SK, Abdallal F, Kamel EZ, et al. Paravertebral dexmedetomidine in video-assisted thoracic surgeries for acute and chronic pain prevention. Pain Physician. 2019;22(3):271–280.
3. Shim JG, Ryu KH, Kim PO, et al. Evaluation of ultrasound-guided erector spinae plane block for postoperative management of video-assisted thoracoscopic surgery: a prospective, randomized, controlled clinical trial. J Thorac Dis. 2020;12(8):4174–4182. doi:10.21037/jtd-20-689
4. Ciftci B, Ekinci M, Celik EC, et al. Efficacy of an ultrasound-guided erector spinae plane block for postoperative analgesia management after video-assisted thoracic surgery: a prospective randomized study. J Cardiothorac Vasc Anesth. 2020;34(2):444–449. doi:10.1053/j.jvca.2019.04.026
5. Fang B, Wang ZM, Huang XJ. Ultrasound-guided preoperative single-dose erector spinae plane block provides comparable analgesia to thoracic paravertebral block following thoracotomy: a single center randomized controlled double-blind study. Ann Transl Med. 2019;7(8):174. doi:10.21037/atm.2019.03.53
6. Santonastaso DP, Chiara AD, Rispoli M, et al. Real-time view of anesthetic solution spread during an ultrasound-guided thoracic paravertebral block. Tumori. 2018;104(6):NP50–NP52. doi:10.1177/0300891618763212
7. Cassai AD, Tonetti T. Local anesthetic spread during erector spinae plane block. J Clin Anesth. 2018;48:60–61. doi:10.1016/j.jclinane.2018.05.003
8. Adhikary SD, Bernard S, Hector Lopez H, et al. Erector spinae plane block versus retrolaminar block: a magnetic resonance imaging and anatomical Study. Reg Anesth Pain Med. 2018;43(7):756–762.
9. Zengin M, Baldemir R, Ulger G, et al. Postoperative analgesic efficacy of thoracic paravertebral block and erector spinae plane block combination in video-assisted thoracic surgery. Cureus. 2021;13(6):e15614. doi:10.7759/cureus.15614
10. Gao ZX, Xiao YM, Wang Q, et al. Comparison of dexmedetomidine and dexamethasone as adjuvant for ropivacaine in ultrasound-guided erector spinae plane block for video-assisted thoracoscopic lobectomy surgery: a randomized, double-blind, placebo-controlled trial. Ann Transl Med. 2019;7(22):668.
11. Zhang DH, Zhou C, Wei D, et al. Dexamethasone added to Local anesthetics in ultrasound-guided transversus abdominis plain (TAP) block for analgesia after abdominal surgery: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2019;14(1):e0209646. doi:10.1371/journal.pone.0209646
12. Wang Q, Li HX, Wei SJ, et al. Dexmedetomidine added to ropivacaine for ultrasound-guided erector spinae plane block prolongs analgesia duration and reduces perioperative opioid consumption after thoracotomy: a randomized, controlled clinical study. Clin J Pain. 2021;38(1):8–14. doi:10.1097/AJP.0000000000000992
13. Kumar V, Sirohiya P, Gupta N, et al. Effect of adding dexamethasone to ropivacaine for ultrasound-guided serratus anterior plane block in patients undergoing modified radical mastectomy: a preliminary trial. Indian J Anaesth. 2020;64(12):1032–1037. doi:10.4103/ija.IJA_261_20
14. Ding WG, Chen YN, Li DM, et al. Investigation of single-dose thoracic paravertebral analgesia for postoperative pain control after thoracoscopic lobectomy-A randomized controlled trial. Int J Surg. 2018;57:8–14. doi:10.1016/j.ijsu.2018.07.006
15. Nguyen V, Tiemann D, Park E, et al. Alpha-2 Agonists. Anesthesiol Clin. 2017;35(2):233–245. doi:10.1016/j.anclin.2017.01.009
16. Tang C, Xia Z. Dexmedetomidine in perioperative acute pain management: a non-opioid adjuvant analgesic. J Pain Res. 2017;10:1899–1904. doi:10.2147/JPR.S139387
17. Wang K, Wang LJ, Yang TJ, et al. Dexmedetomidine combined with local anesthetics in thoracic paravertebral block: a systematic review and meta-analysis of randomized controlled trials. Medicine. 2018;97(46):e13164. doi:10.1097/MD.0000000000013164
18. Huang Y, Lu Y, Zhang L, et al. Perineural dexmedetomidine attenuates inflammation in rat sciatic nerve via the NF-κB pathway. Int J Mol Sci. 2014;15(3):4049–4059. doi:10.3390/ijms15034049
19. Li J, Wang HH, Dong BH, et al. Adding dexmedetomidine to ropivacaine for femoral nerve block inhibits local inflammatory response. Minerva Anestesiol. 2017;83(6):590–597. doi:10.23736/S0375-9393.17.11430-6
20. Talke P, Stapelfeldt C, Lobo E, et al. Effect of alpha2B-adrenoceptor polymorphism on peripheral vasoconstriction in healthy volunteers. Anesthesiology. 2005;102(3):536–542. doi:10.1097/00000542-200503000-00010
21. Gupta A, Gupta A, Yadav N. Effect of dexamethasone as an adjuvant to ropivacaine on duration and quality of analgesia in ultrasound-guided transversus abdominis plane block in patients undergoing lower segment cesarean section-A prospective, randomised, single-blinded study. Indian J Anaesth. 2019;63(6):469–474. doi:10.4103/ija.IJA_773_18
22. Pande A, Mohini Sen I, Gupta A, et al. Perineural low dexamethasone dose as adjuvant in supraclavicular brachial plexus block for arteriovenous fistula creation in end stage renal disease: a randomized controlled trial. Braz J Anesthesiol. 2021. doi:10.1016/j.bjane.2021.10.012
23. Mao Y, Zuo YM, Mei B, et al. Efficacy of perineural dexamethasone with ropivacaine in thoracic paravertebral block for postoperative analgesia in elective thoracotomy: a randomized, double-blind, placebo-controlled trial. J Pain Res. 2018;11:1811–1819. doi:10.2147/JPR.S164225
24. Attardi B, Takimoto K, Gealy R, et al. Glucocorticoid induced up-regulation of a pituitary K+ channel m RNA in vitro and in vivo. Receptors Channels. 1993;1(4):287–293.
25. Stan T, Goodman EJ, Bravo-Fernandez C, et al. Adding methylprednisolone to local anesthetic increases the duration of axillary block. Reg Anesth Pain Med. 2004;29(4):380–381. doi:10.1097/00115550-200407000-00021
26. Sakae TM, Marchioro P, Schuelter-Trevisol F, et al. Dexamethasone as a ropivacaine adjuvant for ultrasound-guided interscalene brachial plexus block: a randomized, double-blinded clinical trial. J Clin Anesth. 2017;38:133–136. doi:10.1016/j.jclinane.2017.02.004
27. Song ZG, Pang SY, Wang GY, et al. Comparison of postoperative analgesic effects in response to either dexamethasone or dexmedetomidine as local anesthetic adjuvants: a systematic review and meta-analysis of randomized controlled trials. J Anesth. 2021;35(2):270–287. doi:10.1007/s00540-021-02895-y
28. Yaghoobi S, Shahamat H, Alizadeh A, et al. Comparing postoperative analgesic effect of dexmedetomidine or dexamethasone added to lidocaine through infraclavicular block in forearm surgery. Clin J Pain. 2019;35(9):766–771. doi:10.1097/AJP.0000000000000736
29. Wang HL, Zhang GY, Dai WX, et al. Dose-dependent neurotoxicity caused by the addition of perineural dexmedetomidine to ropivacaine for continuous femoral nerve block in rabbits. J Int Med Res. 2019;47(6):2562–2570. doi:10.1177/0300060519847368
30. Li X, Liu YC, Zhao J, et al. The safety and efficacy of ultrasound-guided Serratus Anterior Plane Block (SAPB) combined with dexmedetomidine for patients undergoing Video-Assisted Thoracic Surgery (VATS): a randomized controlled trial. J Pain Res. 2020;13:1785–1795. doi:10.2147/JPR.S258443
31. Gao XJ, Zhao TH, Xu GJ, et al. The efficacy and safety of ultrasound-guided, bi-level, erector spinae plane block with different doses of dexmedetomidine for patients undergoing video-assisted thoracic surgery: a randomized controlled trial. Front Med. 2021;8:577885. doi:10.3389/fmed.2021.577885
32. Ibrahim AS, Aly MG, Farrag WS, et al. Ultrasound-guided adductor canal block after arthroscopic anterior cruciate ligament reconstruction: effect of adding dexamethasone to bupivacaine, a randomized controlled trial. Eur J Pain. 2019;23(1):135–141. doi:10.1002/ejp.1292
33. Baloda R, Bhupal JPS, Kumar P, et al. Supraclavicular brachial plexus block with or without dexamethasone as an adjuvant to 0.5% Levobupivacaine: a comparative study. J Clin Diagn Res. 2016;10(6):UC09–12. doi:10.7860/JCDR/2016/18325.8048
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
A Randomized Controlled Trial to Compare the Efficacy of Single versus Triple Injection Technique for Ultrasound-Guided Infraclavicular Block in Upper Limb Surgeries
Vedavyas R, Saravanan R, Mirunalini G, Gayathri B
Local and Regional Anesthesia 2023, 16:51-58
Published Date: 18 May 2023
Comparison of the Effects of Dexmedetomidine and Lidocaine on Stress Response and Postoperative Delirium of Older Patients Undergoing Thoracoscopic Surgery: A Randomized Controlled Trial
Lai Y, Chen Q, Xiang C, Li G, Wei K
Clinical Interventions in Aging 2023, 18:1275-1283
Published Date: 3 August 2023
Effect of Dexmedetomidine on Postoperative Plasma Neurofilament Light Chain in Elderly Patients Undergoing Thoracoscopic Surgery: A Prospective, Randomized Controlled Trial
Hou YR, Xu CY, An MZ, Li ZP, Ni H, Chen T, Zhou QH
Clinical Interventions in Aging 2023, 18:1565-1576
Published Date: 14 September 2023