Back to Journals » Clinical Interventions in Aging » Volume 18
Effect of Dexmedetomidine on Postoperative Plasma Neurofilament Light Chain in Elderly Patients Undergoing Thoracoscopic Surgery: A Prospective, Randomized Controlled Trial
Authors Hou YR, Xu CY, An MZ , Li ZP, Ni H, Chen T , Zhou QH
Received 9 June 2023
Accepted for publication 7 September 2023
Published 14 September 2023 Volume 2023:18 Pages 1565—1576
DOI https://doi.org/10.2147/CIA.S422560
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Maddalena Illario
Yue-ru Hou,1,2 Cheng-yun Xu,1,2 Ming-zi An,1,2 Zhen-ping Li,2 Hua-dong Ni,2 Tao Chen,3 Qing-he Zhou2
1Anesthesia Medicine, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, People’s Republic of China; 2Department of Anesthesiology and Pain Medicine, the Affiliated Hospital of Jiaxing University, Jiaxing, Zhejiang, People’s Republic of China; 3Department of Anesthesiology, Tongxiang First People’s Hospital, Tongxiang, Zhejiang, People’s Republic of China
Correspondence: Tao Chen, Department of Anesthesiology, Tongxiang First People’s Hospital, No. 1918, Xiaochang Road, Tongxiang, Zhejiang, 314599, People’s Republic of China, Tel +8613967327686, Fax +86 0573 8802 5098, Email [email protected] Qing-he Zhou, Department of Anesthesiology and Pain Medicine, the Affiliated Hospital of Jiaxing University, No. 1882, Ring 2nd South Road, Jiaxing, Zhejiang, 314000, People’s Republic of China, Tel +8613732573379, Fax +86 0573 8999 7760, Email [email protected]
Purpose: Dexmedetomidine exerts a neuroprotective effect, however, the mechanism underlying this effect remains unclear. This study aimed to explore whether dexmedetomidine can reduce the increase in neurofilament light chain (NfL) protein concentration to play a neuroprotective role during thoracoscopic surgery.
Patients and Methods: Patients aged ≥ 60 years undergoing general anesthesia for thoracoscopic surgery were randomly assigned to receive dexmedetomidine (group D) or not receive dexmedetomidine (group C). Patients in group D received a loading dose of dexmedetomidine 0.5 μg/kg before anesthesia induction and a continuous infusion at 0.5 μg·kg− 1·h− 1 until the end of the surgery. Dexmedetomidine was not administered in group C. The primary outcome was the NfL concentration on postoperative day 1. The concentrations of procalcitonin (PCT), serum amyloid A (SAA), and high-sensitivity C-reactive protein (hs-CRP) were detected preoperatively and on postoperative day 1. In addition, the numerical rating scale (NRS) and quality of recovery-40 (QoR-40) scores were evaluated.
Results: A total of 38 patients in group D and 37 in group C were included in the analysis. No differences were observed between the groups in terms of the plasma concentration of NfL preoperatively and on postoperative day 1 (11.17 [8.86, 13.93] vs 13.15 [10.76, 15.56] pg/mL, P > 0.05; 16.70 [12.23, 21.15] vs 19.48 [15.25, 22.85] pg/mL, P > 0.05, respectively). However, the postoperative plasma NfL concentration was significantly higher than the preoperative value in both groups (both P < 0.001). The groups exhibited no differences in PCT, SAA, hs-CRP, NRS, and QoR-40 (all P > 0.05).
Conclusion: Intraoperative administration of dexmedetomidine at a conventional dose does not appear to significantly reduce the increase in postoperative plasma NfL concentration in elderly patients undergoing thoracoscopic surgery. This finding suggests that the neuroprotective effect of dexmedetomidine at a conventional dose was not obvious during general anesthesia.
Keywords: dexmedetomidine, thoracoscopic surgery, neurofilament light chain, general anesthesia
Introduction
At present, over 300 million surgical procedures are performed worldwide annually, and a large proportion of these cases involve the use of general anesthesia. General anesthesia and surgery have been demonstrated to be associated with central nervous system damage,1 particularly in the form of postoperative cognitive impairment.2,3 As previously reported, the incidence of postoperative delirium (POD) among surgical patients is approximately 36.8%,4 and it is associated with increased rates of dementia and mortality, especially in elderly surgical patients.4,5 The literature includes studies reporting the direct neurotoxic effects of general anesthetics and surgery.1,6 In addition, a study by Laszlo Vutskits et al reported that anesthetics can trigger long-term morphological and functional alterations in the brain.7
Dexmedetomidine, a potent and highly selective agonist of α2-adrenergic receptors, is routinely used as an adjunct to anesthesia. Previous studies have demonstrated that dexmedetomidine exerts a neuroprotective effect;8–10 it has also been reported to inhibit neuroendocrine and inflammatory responses11,12 and blunt stress response by reducing the levels of serum catecholamine, cortisol, and cytokine.13 Recent experimental studies have also reported that dexmedetomidine exerts long-term effects on the brain, including neuroprotective activity against excitotoxic damage through α2-adrenergic receptors.14 Although previous studies have shown the neuroprotective effects of dexmedetomidine, the mechanisms underlying these effects remain unclear.
Neurofilament light chain (NfL), a specific molecular biomarker of axonal damage, has been shown to cause neuronal damage in several central nervous system-related disorders,15–17 such as trauma, cerebrovascular diseases, and POD.18–21 NfL is a subunit of neurofilaments (NFs), located in the axons of neurons. When axonal damage, axonal loss, and neuronal death occur, NfL is released from the axon to the cerebrospinal fluid and blood.22 The NfL concentration ratio in cerebrospinal fluid to plasma is reportedly 40:1.21 The current advancements in immunoassay technology have enabled the measurement of the plasma NfL concentration at the pg/mL level using a single-molecule enzyme-linked immunosorbent assay (Simoa).23–25 Isolation and detection of single protein molecules present a promising approach to the measurement of the precise concentrations of NfL, enabling the detection of even small changes. A recent study by Evered et al reported that an elevated plasma NfL concentration over 48 h after anesthesia and surgery may be associated with neuronal damage.1
During thoracoscopic surgery, the high intensity of surgical stimulation leads to significant hemodynamic fluctuations, and one-lung ventilation leads to hypoxemia, both of which may result in a high incidence of central nervous system damage,1,4,18 particularly in elderly patients. The present study aimed to investigate whether intraoperative administration of dexmedetomidine can alleviate central nervous system damage caused by general anesthesia and surgery by decreasing the increase in plasma NfL concentration in elderly patients undergoing thoracoscopic pulmonary surgery.
Materials and Methods
Trial Design
This randomized, double-blind, placebo-controlled trial with two parallel arms was conducted at the affiliated hospital of Jiaxing University. It was approved by ethics committee of the same institution (LS2021-KY-388) before the initiation of the experiment and conducted in accordance with the principles of the Helsinki Declaration. In addition, the study was registered in the Chinese Clinical Trial Registry (ChiCTR2200055869; http://www.chictr.org.cn/showproj.aspx?proj=136049) on January 22, 2022. Written informed consent was obtained from all patients or their legal representatives before participating in the study.
Participants and Baseline Data Collection
Potential participants were screened by the investigators the day before surgery. We included patients aged ≥ 60 years, with American Society of Anesthesiologists (ASA) grades I to III, and scheduled to undergo elective video-assisted thoracoscopic surgery for pulmonary wedging, lung segmental resection, or lung lobectomy under general anesthesia with an expected duration of 60 min or more were included. They stayed at the hospital for more than 48 h postoperatively. The exclusion criteria were as follows: lacking written informed consent; past history of schizophrenia, epilepsy, Parkinson’s disease, use of preoperative antipsychotic medication, or alcohol withdrawal delirium; preoperative plasma NfL concentration of > 50 pg/mL;26 visual, hearing, language, or other barriers that impede communication and assessment of delirium; acute myocardial infarction or stroke within at last 3 months; severe hepatic dysfunction (Child–Pugh grade C) or kidney failure (serum creatinine level of > 442 μmol/L or requirement for renal replacement therapy); allergy to any medication associated with the trial; severe bradycardia (heart rate of < 40 beats/min); sick sinus syndrome, or atrioventricular block of grade 2 or higher, but with no pacemaker.
After obtaining written informed consent, patients’ baseline data (including demographic data, surgical diagnosis, comorbidities, and main physical and laboratory findings) were collected. Preoperatively and on postoperative day 1, two questionnaires were administered to patients in the ward, and they completed the questionnaires in their own time. One questionnaire was an 11-point numerical rating scale (NRS) that measures pain intensity. The other was the quality of recovery-40 (QoR-40) questionnaire, with scores ranging from 0 to 200; higher scores indicate better health status. The QoR-40 is a validated measure of a patient’s quality of recovery postoperatively.27,28
Randomization and Blinding
The patients were assigned to either of the trial groups using computer-generated random numbers (https://www.random.org). Even integers were assigned to group C and odd integers to group D. Patients in group D received dexmedetomidine treatment, whereas those in group C received 0.9% NaCl. The trial participants, anesthetists, staff collecting follow-up data, and laboratory staff were blinded to the group assignments.
Intervention, Anesthesia, and Perioperative Care
After entering the operating room, intravenous access was established, and a multifunctional monitor was connected for monitoring of hemodynamics, electrocardiography (ECG), noninvasive blood pressure (NIBP), blood oxygen saturation, and heart rate. Arterial-partial pressures of carbon dioxide (PaCO2) and oxygen (PaO2) were assessed via arterial blood gas analysis by puncture of the radial artery before preoxygenation. After a period of preoxygenation, general anesthesia was induced using intravenous sufentanil 0.3–0.5 μg/kg, followed by propofol 2.0–2.5 mg/kg and rocuronium 0.6–0.8 mg/kg. After 3 min, the patients were intubated with a double-lumen tube to achieve lung isolation, and fiberoptic bronchoscopy was adopted to confirm correct positioning. After tracheal intubation, mechanical ventilation was initiated with the following parameters: tidal volume, 8–10 mL/kg; respiratory rate, 12–16 times/min; expiration-to-inspiration ratio, 1:2; and oxygen flow rate, 2 L/min to maintain the end-tidal partial pressure of carbon dioxide (PetCO2) within the normal range of 35–45 mmHg. During surgery, oxygen was continuously administered at 2 L/min; sevoflurane 1.0%–1.5%, remifentanil 0.1–0.3 μg·kg−1·min−1, and propofol 0.4–0.8 mg·kg−1·h−1 were also administered to maintain the bispectral index (BIS) within the range of 40–60. On the basis of the muscle relaxation monitoring results, 0.15 mg/kg rocuronium was added every 30 min. Unless contraindicated, all patients received sufentanil 100 μg plus granisetron 6 mg diluted in 92 mL 0.9% NaCl for patient-controlled intravenous analgesia (PCIA). Furthermore, a dexmedetomidine loading dose of 0.5 µg/kg or the same volume of normal saline was pumped within 10 min before anesthesia induction and maintained at 0.5 µg·kg−1·h−1 until the end of the surgery.
During surgery, anesthetics were adjusted according to blood pressure, heart rate, and depth of anesthesia. Anesthetics, except trial drugs, were adjusted first, and the trial drugs were adjusted or stopped if the patient experienced continuous sinus bradycardia and hypotension. The patients’ vital signs were recorded before anesthesia induction (T1), after anesthesia induction (T2), during intubation (T3), at the moment of cutting the skin (T4), 30 min after the start of surgery (T5), at the end of surgery (T6), and 30 min after extubation (T7). The anesthetics used and adverse events during surgery were also recorded.
Blood Sample Processing
Blood samples (5 mL) were collected preoperatively and on postoperative day 1 and stored in vacutainer tubes (Kang Wei Shi Medical Device Co, LTD) containing the anticoagulant ethylenediaminetetraacetic acid (EDTA). Within 1 h, the tubes were centrifuged for 10 min at 3000 rpm, and then 500-μL aliquots were pipetted into Eppendorf tubes (AXYGEN). The samples were stored at −80°C in the laboratory of the affiliated hospital of Jiaxing University before being transported by a medical courier on dry ice at −80°C for analysis. A total of 156 blood samples were collected from 78 patients. EDTA plasma NfL concentration was quantified using ultrasensitive Simoa technology (Quanterix, MA, US) on the automated Simoa HD-X platform (GBIO, Hangzhou, Zhejiang Province, China) according to the manufacturer’s protocols. The NF-Light (Cat No: 103186) assay kit was purchased from Quanterix and used accordingly. The plasma samples were diluted at a 1:4 ratio. The calibrators and quality controls were measured in duplicate. All sample measurements were performed by board-certified laboratory technicians in one round of experiment using one batch of reagents. The operators were unaware of the disease status of the participants. The intra-assay coefficients of variation were 3.73% for a quality control sample with an NfL concentration of 4.045 pg/mL and 5.55% for a quality control sample with an NfL concentration of 272.817 pg/mL. The lower limit of quantification was 0.2380 pg/mL.
Procalcitonin (PCT), serum amyloid A (SAA), and high-sensitivity C-reactive protein (hs-CRP) were also detected preoperatively and on postoperative day 1.
Outcome Assessment
The NfL and inflammatory mediator concentration were measured preoperatively and on postoperative day 1. The secondary outcomes were the QoR-40 and NRS scores and postoperative hospital stay of both groups. The incidence of adverse events was evaluated from the start of the study drug administration to 2 h postoperatively. Sinus bradycardia was defined as a heart rate of <60 beats/min and sinus tachycardia as a heart rate of >100 beats/min. Hypotension was defined as systolic blood pressure level of <90 mmHg, diastolic blood pressure level of <60 mmHg, or a reduction of >30% over baseline. Hypertension was defined as systolic blood pressure level of >140 mmHg, diastolic blood pressure level of >90 mmHg, or an increase of >30% over baseline. Hypoxemia was defined as oxygen saturation of <90%.29
Sample Size Calculation
The primary outcome was the NfL concentration on postoperative day 1. The sample size was calculated using PASS version 15.0. According to our preliminary experiments, the plasma NfL concentrations in groups D and C on postoperative day 1 were 14.79 ± 5.88 and 19.21 ± 6.68 pg/mL, respectively. With the power set at 80% and a two-sided significance level of 0.05, 66 patients were required to detect a difference. Considering a loss-to-follow-up rate of approximately 15%, 78 patients were included in the study.
Statistical Analysis
Statistical analyses were conducted using SPSS version 25.0 (IBM Corp, Armonk, NY, USA). Data were examined and tested for distribution using the Shapiro–Wilk test. All data were expressed as mean (Standard deviation; SD) or median (25–75% range) as appropriate. An unpaired Student’s t-test and the Mann–Whitney U-test were used to compare normally and nonnormally distributed data, respectively, between the groups. Repeated-measurement data were analyzed using the generalized estimating equation (GEE). Categorical variables were analyzed using the χ²-test, continuity correction χ²-test, or likelihood ratio χ²-test, as appropriate. Statistical significance was set at P < 0.05.
Results
Patient Characteristics
From January to June 2022, 131 patients were screened for eligibility. Of whom 80 were enrolled and randomized. In total, 75 individuals and 150 samples were included in the final analysis. A flow diagram of the study is presented in Figure 1. Patient characteristics are shown in Table 1; as can be seen from the table, and the groups showed no differences in the baseline data.
 |
Table 1 Comparison of Patient Characteristics |
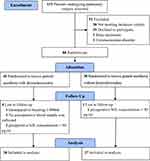 |
Figure 1 Patient flow though the study. |
Neurofilament Light
In group D, the plasma NfL concentration significantly increased from 11.17 [8.86, 13.93] pg/mL preoperatively to 16.70 [12.23, 21.15] pg/mL on postoperative day 1 (P < 0.001); in group C, the plasma NfL concentration significantly increased from 13.15 [10.76, 15.56] pg/mL preoperatively to 19.48 [15.25, 22.85] pg/mL on postoperative day 1(P < 0.001) (Figure 2). However, the plasma NfL concentration at baseline and on postoperative day 1 did not differ between the groups (both P > 0.05).
NRS and QoR-40
The two groups showed exhibited no significant difference in the area under the curve values for pain NRS scores at 6, 12 and 24 h (all P > 0.05), as presented in Table 2. The two groups also showed no differences in the QoR-40 scores obtained preoperatively (group D vs group C, 194.4 ± 3.4 vs 196.0 ± 4.2; P > 0.05) and on postoperative day 1 (group D vs group C, 180.4 ± 8.2 vs 182.9 ± 7.8, P > 0.05). The QoR-40 score obtained on postoperative day 1 was significantly lower than the preoperative score in both groups (both P < 0.001; Figure 3).
 |
Table 2 AUC of Pain NRS Scores Over Time in the Two Groups |
Intraoperative Characteristics and Postoperative Outcomes
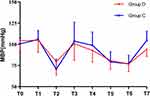
The intraoperative characteristics and postoperative outcomes are presented in Table 3 and Table 4. Propofol consumption in group D was significantly lower than that in group C (P < 0.001). The incidence of hypotension in group D was higher than in group C, and the number of patients requiring vasoactive drugs in group D was higher than that in group C (P < 0.05). The PACU duration in group D was longer than that in group C (P < 0.05). Other results did not differ between the two groups. Intraoperative mean arterial blood pressure (MABP) and heart rate (HR) were analyzed using the GEE (Figures 4 and 5). The GEE analysis revealed no difference in MABP between the two groups after the intervention (P = 0.477), but HR significantly differed between them (P = 0.024).
 |
Table 3 Intraoperative Data |
 |
Table 4 Postoperative Complications |
Inflammatory Factors
The PCT, SAA, and hs-CRP levels were measured pre- and postoperatively and did not show significant intergroup differences. The PCT and SAA levels on postoperative day 1 were higher than the preoperative levels in both groups (both P < 0.05) (Table 5).
 |
Table 5 Inflammatory Related Factors |
Discussion
This study demonstrated that intraoperative infusion of dexmedetomidine at a conventional dose could not reduce the increase in plasma NfL concentration in elderly patients undergoing thoracoscopic pulmonary surgery. This suggests that conventional doses of dexmedetomidine could not exert a definite neuroprotective effect during general anesthesia.
NfL is a cylindrical protein specifically located in the cytoplasm of neurons.21 Neuronal damage caused by certain diseases may lead to an increase in NfL concentration in the cerebrospinal fluid or blood.30,31 Our study showed that the plasma NfL concentration on postoperative day 1 significantly increased from baseline, indicating that neuronal damage had occurred after surgery and anesthesia induction. These results were consistent with the findings of a previous study showing that anesthesia and surgery were factors causing neuronal damage, and that the plasma NfL concentration was significantly increased postoperatively.1 Simultaneously, in the present study, a loading dose of 0.5 µg/kg was administered and a dose of 0.5 μg·kg−1·h−1 was maintained, and the routine intraoperative infusion of dexmedetomidine did not exert a significant effect on reducing the increase in postoperative plasma NfL concentration. To demonstrate its neuroprotective effect, a suitable dosage of dexmedetomidine needs to be explored.
In the present study, patients who received dexmedetomidine had a higher incidence of intraoperative hypotension and required more vasoactive drugs. Furthermore, patients administered dexmedetomidine had a longer stay in PACU than those given propofol. These results were correlated with the characteristics of dexmedetomidine.32 Intraoperative hypotension contributes to brain hypoperfusion, which can cause neuronal damage.33 In this study, patients who received dexmedetomidine had a higher incidence of intraoperative hypotension and required more vasoactive drugs. Although the intraoperative hypotension had a short duration and was corrected by the rational use of vasoactive drugs and replenishment of the circulating blood volume, transient intraoperative hypotension may also cause neuronal damage to some extent. Thus, the neuronal damage caused by intraoperative hypotension may partly be the reason why dexmedetomidine failed to exert a neuroprotective effect by preventing the increase in postoperative plasma NfL concentration.
NfL has been shown to be associated with brain trauma, inflammation of the central nervous system, multiple sclerosis, and axonal damage caused by traumatic or vascular injury.30 In the present study, the two groups exhibited no significant differences in the PCT, SAA, and hs-CRP levels; however, these levels of all significantly increased postoperatively. These findings were consistent with the change trend in plasma NfL concentration in both groups but differed from the findings of previous reports showing that dexmedetomidine can exert neuroprotective effects and reduce inflammation.8–10 These results also further indicate that intraoperative dexmedetomidine infusion at a regular dose may not exert neuroprotective effects in elderly patients undergoing thoracoscopic pulmonary surgery.
Postoperative pain is associated with inflammation and, in some cases, neuronal damage.34,35 In this study, no significant difference was observed in the NRS scores between the two groups. The results were consistent with the change trend in the plasma NfL and inflammatory mediator concentrations in both groups. This could be because the analgesia methods were the same in the two groups, both including intercostal nerve block and PCIA. Except for the intraoperative infusion of dexmedetomidine in group D, other treatments were the same in both groups. The aforementioned factors may be related to the lack of difference in the QoR-40 scores between the two groups, which was used to assess the quality of patient recovery.27,28 These results indicate that the use of dexmedetomidine does not improve patients’ postoperative pain and quality of recovery.
This study had some limitations. First, the sample size was relatively small, and the results require further exploration and validation in studies with larger sample sizes. Second, a previous study reported that POD is associated with an increase in the plasma NfL concentration on postoperative day 1.19 The present study did not involve POD observation; thus, it was impossible to further determine the correlation between POD and the plasma NfL concentration. Third, in this study, only a conventional dose of dexmedetomidine was administered; other doses may lead to different results.
Conclusion
Our study demonstrated that general anesthesia and surgery induced an increase in plasma NfL concentration following thoracic surgery in elderly patients. However, the plasma NfL concentration on postoperative day 1 did not differ between patients who received a conventional dose of dexmedetomidine and those who did not, indicating that intraoperative dexmedetomidine administration at a conventional dose during thoracic surgery in elderly patients did not exert a neuroprotective effect. To demonstrate its neuroprotective effect, a suitable dosage of dexmedetomidine needs to be explored.
Abbreviations
ASA, American Society of Anesthesiologists; BIS, the bispectral index; ECG, electrocardiography; EDTA, ethylenediaminetetraace-tic acid; GEE, generalized estimating equation; hs-CRP, high-sensitivity C-reactive protein; NfL, Neurofilament light chain; NFs, neurofilaments; NRS, numerical rating scale; PaO2, Arterial-partial pressures of oxygen; PaCO2, Arterial-partial pressures of carbon dioxide; PCIA, patient-controlled intravenous analgesia; PCT, procalcitonin; PetCO2, the end-tidal partial pressure of carbon dioxide; POD, postoperative delirium; QoR-40, the Quality of Recovery-40 Questionnaire; SAA, serum amyloid A; Simoa, Single-molecule enzyme-linked immunosorbent array.
Data Sharing Statement
All the data and material generated during the current study are available from the corresponding author upon reasonable request ([email protected]).
Ethics Approval and Informed Consent
This trial was approved by the ethics committee of the Affiliated Hospital of Jiaxing University, China, on November 25th, 2021 (LS2021-KY-388) before experiment was started and that has been conducted in accordance with the principles set forth in the Helsinki Declaration. Written informed consent was obtained from all participants or their guardians.
Consent for Publication
The details of any images, videos, recordings, etc can be published.
Acknowledgments
The authors thank Meng-jie Chen, Qun-fang Gu and their colleagues for helping to collect blood samples.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas and took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published. Yue-ru Hou contributed to the conception, design, data acquisition, data analysis, and draft preparation of this study. Qing-He Zhou contributed substantially to the conception, design, data analysis, and substantial revision of the paper. Tao Chen contributed substantially to the conception, design, and revision of the manuscript, as well as providing a critical review. Cheng-yun Xu, Ming-zi An, Zhen-Ping Li, and Hua-dong Ni contributed to the execution of the study, the acquisition of data, and substantially revising the manuscript. All authors agreed on submitting to the journal of Clinical Interventions in Aging. All authors reviewed and agreed on all versions of the manuscript before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage. All authors agree to take responsibility and be accountable for all aspects of the article.
Funding
This study was supported by Zhejiang medical and health science and technology plan project (2023KY341), the Key Discipline Established with Zhejiang Provincial Traditional Chinese Medical Innovation Team (No. 2022-19), Zhejiang Provincial Key Clinical Specialty-Anesthesiology (2023-ZJZK-001) and Jiaxing Key Discipline of Medicine-Anesthesiology (2023-ZC-001). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors declare that they have no competing interests.
References
1. Evered L, Silbert B, Scott DA, Zetterberg H, Blennow K. Association of changes in plasma neurofilament light and tau levels with anesthesia and surgery results from the CAPACITY and ARCADIAN studies. JAMA Neurol. 2018;75(5):542–547. doi:10.1001/jamaneurol.2017.4913
2. van Harten AE, Scheeren TW, Absalom AR. A review of postoperative cognitive dysfunction and neuroinflammation associated with cardiac surgery and anaesthesia. Anaesthesia. 2012;67(3):280–293. doi:10.1111/j.1365-2044.2011.07008.x
3. Monk TG, Weldon BC, Garvan CW, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108(1):18–30. doi:10.1097/01.anes.0000296071.19434.1e
4. McDaniel M, Brudney C. Postoperative delirium: etiology and management. Curr Opin Crit Care. 2012;18(4):372–376. doi:10.1097/MCC.0b013e3283557211
5. Crocker E, Beggs T, Hassan A, et al. Long-term effects of postoperative delirium in patients undergoing cardiac operation: a systematic review. Ann Thorac Surg. 2016;102(4):1391–1399. doi:10.1016/j.athoracsur.2016.04.071
6. Cascella M, Muzio MR, Bimonte S, Cuomo A, Jakobsson JG. Postoperative delirium and postoperative cognitive dysfunction: updates in pathophysiology, potential translational approaches to clinical practice and further research perspectives. Minerva Anestesiol. 2018;84(2):246–260. doi:10.23736/S0375-9393.17.12146-2
7. Vutskits L, Xie Z. Lasting impact of general anaesthesia on the brain: mechanisms and relevance. Nat Rev Neurosci. 2016;17(11):705–717. doi:10.1038/nrn.2016.128
8. Ramsay MA, Luterman DL. Dexmedetomidine as a total intravenous anesthetic agent. Anesthesiology. 2004;101(3):787–790. doi:10.1097/00000542-200409000-00028
9. Paris A, Mantz J, Tonner PH, Hein L, Brede M, Gressens P. The effects of dexmedetomidine on perinatal excitotoxic brain injury are mediated by the alpha2A-adrenoceptor subtype. Anesth Analg. 2006;102(2):456–461. doi:10.1213/01.ane.0000194301.79118.e9
10. Degos V, Charpentier TL, Chhor V, et al. Neuroprotective effects of dexmedetomidine against glutamate agonist-induced neuronal cell death are related to increased astrocyte brain-derived neurotrophic factor expression. Anesthesiology. 2013;118(5):1123–1132. doi:10.1097/ALN.0b013e318286cf36
11. Tasdogan M, Memis D, Sut N, Yuksel M. Results of a pilot study on the effects of propofol and dexmedetomidine on inflammatory responses and intraabdominal pressure in severe sepsis. J Clin Anesth. 2009;21(6):394–400. doi:10.1016/j.jclinane.2008.10.010
12. Li B, Li Y, Tian S, et al. Anti-inflammatory effects of perioperative dexmedetomidine administered as an adjunct to general anesthesia: a meta-analysis. Sci Rep. 2015;5(1):12342. doi:10.1038/srep12342
13. Li Y, Wang B, Zhang LL, et al. Dexmedetomidine combined with general anesthesia provides similar intraoperative stress response reduction when compared with a combined general and epidural anesthetic technique. Anesth Analg. 2016;122(4):1202–1210. doi:10.1213/ANE.0000000000001165
14. Ma D, Hossain M, Rajakumaraswamy N, et al. Dexmedetomidine produces its neuroprotective effect via the alpha 2A-adrenoceptor subtype. Eur J Pharmacol. 2004;502(1–2):87–97. doi:10.1016/j.ejphar.2004.08.044
15. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi:10.1016/0022-3956(75)90026-6
16. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948. doi:10.7326/0003-4819-113-12-941
17. Ashton NJ, Janelidze S, Al Khleifat A, et al. A multicentre validation study of the diagnostic value of plasma neurofilament light. Nat Commun. 2021;12(1):3400. doi:10.1038/s41467-021-23620-z
18. Fong TG, Vasunilashorn SM, Ngo L, et al. Association of plasma neurofilament light with postoperative delirium. Ann Neurol. 2020;88(5):984–994. doi:10.1002/ana.25889
19. Casey CP, Lindroth H, Mohanty R, et al. Postoperative delirium is associated with increased plasma neurofilament light. Brain. 2020;143(1):47–54. doi:10.1093/brain/awz354
20. Gaetani L, Blennow K, Calabresi P, Di Filippo M, Parnetti L, Zetterberg H. Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry. 2019;90(8):870–881. doi:10.1136/jnnp-2018-320106
21. Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14(10):577–589. doi:10.1038/s41582-018-0058-z
22. Yuan A, Rao MV, Nixon RA. Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb Perspect Biol. 2017;9(4):a018309. doi:10.1101/cshperspect.a018309
23. Todd J, Freese B, Lu A, et al. Ultrasensitive flow-based immunoassays using single-molecule counting. Clin Chem. 2007;53(11):1990–1995. doi:10.1373/clinchem.2007.091181
24. Tessler LA, Reifenberger JG, Mitra RD. Protein quantification in complex mixtures by solid phase single-molecule counting. Anal Chem. 2009;81(17):7141–7148. doi:10.1021/ac901068x
25. Rissin DM, Kan CW, Campbell TG, et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat Biotechnol. 2010;28(6):595–599. doi:10.1038/nbt.1641
26. Palmqvist S, Janelidze S, Stomrud E, et al. Performance of fully automated plasma assays as screening tests for Alzheimer disease-related β-amyloid status. JAMA Neurol. 2019;76(9):1060–1069. doi:10.1001/jamaneurol.2019.1632
27. Myles PS, Weitkamp B, Jones K, Melick J, Hensen S. Validity and reliability of a postoperative quality of recovery score: the QoR-40. Br J Anaesth. 2000;84(1):11–15. doi:10.1093/oxfordjournals.bja.a013366
28. Chen Y, Wang J, Liu S, et al. Development and validation of the Chinese version of the quality of recovery-40 questionnaire. Ther Clin Risk Manag. 2020;16:1165–1173. doi:10.2147/TCRM.S281572
29. Casey JD, Janz DR, Russell DW, et al. Bag-mask ventilation during tracheal intubation of critically ill adults. N Engl J Med. 2019;380(9):811–821. doi:10.1056/NEJMoa1812405
30. Gisslén M, Price RW, Andreasson U, et al. Plasma concentration of the Neurofilament Light Protein (NFL) is a biomarker of CNS injury in HIV infection: a cross-sectional study. EBioMedicine. 2016;3:135–140. doi:10.1016/j.ebiom.2015.11.036
31. Disanto G, Barro C, Benkert P, et al. Serum Neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol. 2017;81(6):857–870. doi:10.1002/ana.24954
32. Nguyen V, Tiemann D, Park E, et al. Alpha-2 Agonists. Anesthesiol Clin. 2017;35(2):233–245. doi:10.1016/j.anclin.2017.01.009
33. Stahel PF, Smith WR, Moore EE. Hypoxia and hypotension, the “lethal duo” in traumatic brain injury: implications for prehospital care. Intensive Care Med. 2008;34(3):402–404. doi:10.1007/s00134-007-0889-3
34. Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618–1625. doi:10.1016/S0140-6736(06)68700-X
35. Pak DJ, Yong RJ, Kaye AD, et al. Chronification of pain: mechanisms, current understanding, and clinical implications. Curr Pain Headache Rep. 2018;22(2):9. doi:10.1007/s11916-018-0666-8
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.