Back to Journals » Clinical Interventions in Aging » Volume 18
Comparison of the Effects of Dexmedetomidine and Lidocaine on Stress Response and Postoperative Delirium of Older Patients Undergoing Thoracoscopic Surgery: A Randomized Controlled Trial
Authors Lai Y, Chen Q , Xiang C, Li G, Wei K
Received 4 May 2023
Accepted for publication 28 July 2023
Published 3 August 2023 Volume 2023:18 Pages 1275—1283
DOI https://doi.org/10.2147/CIA.S419835
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Maddalena Illario
Yuan Lai,1,2,* Qi Chen,1,3,* Chunfang Xiang,4 Guanzhu Li,5 Ke Wei1
1Department of Anesthesiology, First Clinical College of Chongqing Medical University, Chongqing, People’s Republic of China; 2Department of Anesthesiology, Affiliated People’s Hospital of Chongqing Three Gorges Medical College, Chongqing, People’s Republic of China; 3Department of Anesthesiology, Chongqing University Cancer Hospital, Chongqing, People’s Republic of China; 4Department of Thoracic Surgery, Chongqing University Three Gorges Hospital, Chongqing, People’s Republic of China; 5Department of Anesthesiology, Guangdong Hospital of Traditional Chinese Medicine, The Second affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ke Wei, Youyi Road, Yuzhong District, Chongqing, 400042, People’s Republic of China, Tel +86 15902360621, Email [email protected]
Purpose: We investigated the effects of intraoperative intravenous lidocaine or dexmedetomidine infusion on inflammatory factors and cognitive function in patients undergoing thoracoscopic surgery.
Patients and Methods: Patients aged > 65 years undergoing elective thoracoscopic lobectomy or segmentectomy were randomly grouped as dexmedetomidine group (group D), lidocaine group (group L), and control group (group C). The plasma cortisol, interleukin-6, and tumor necrosis factor-α concentrations were measured before anesthesia (T0) and immediately (T1), 24 h (T2), and 48 h postoperatively (T3). Postoperative delirium (POD) was assessed by 3D-CAM on days 2 and 7.
Results: The cortisol concentrations decreased for all groups at T1 from T0 although they were significantly higher at T2. Group L had significantly lower interleukin-6 concentrations at T1 and T2 than the other groups (P< 0.05). The interleukin-6 concentrations were significantly higher at T1, T2, and T3 than at T0 for all the groups, significantly lower for groups D and L than for group C at T1 and T2 (P< 0.05), and significantly lower for group L than for group D at T2 (P< 0.05). The tumor necrosis factor-α concentrations were significantly higher at T1, T2, and T3 than at T0 for all the groups and significantly lower for groups D and L than for group C at T1 and T2 (P< 0.05), although they were not statistically significantly different for groups D and L. There were no statistically significant differences in the postoperative incidence of POD between the three groups on days 2 and day 7.
Conclusion: Intraoperative continuous intravenous lidocaine or dexmedetomidine infusion reduced surgical stress and inflammatory responses. The inhibitory effect of lidocaine on surgical stress remained significant for up to 24 h postoperatively without affecting patient awakening. However, the administration of either drug failed to prevent postoperative POD.
Keywords: lidocaine, dexmedetomidine, inflammation, delirium, thoracoscopic surgery, elderly
Plain Language Summary
Intravenous infusion dexmedetomidine or lidocaine in the elderly in thoracic surgery can help patients recovery, but there is no definite evidence for improving postoperative cognitive function.
Introduction
Thoracoscopic lobectomy or segmentectomy is the primary treatment option for lung tumors.1 Thoracoscopic surgery is advantageous over traditional thoracotomy because it is less invasive and less painful, which helps accelerate postoperative recovery.2,3 Although thoracoscopic surgery is less invasive than thoracotomy, acute intraoperative inflammation and potentially harmful alveolar mechanical stress due to one-lung ventilation (OLV) can lead to the release of pro-inflammatory cytokines. This can cause inflammation and immunosuppression, affect postoperative recovery, and even lead to postoperative delirium (POD).4,5
POD occurs within 7 days postoperatively and is a common but severe cerebral complication. Some research has shown that the incidence of POD after major surgery is approximately 20–40% in the adults older than 60 years.6 Current theories on the induction of POD include anesthesia-, surgery- and patient-related factors and the inflammatory state. In addition to reducing trauma and providing multimodal analgesia, various drugs are also used during the perioperative period to improve inflammation.
Lidocaine is a commonly used local amide anesthetic with analgesic, anti-inflammatory, and anti-hyperalgesic properties and can serve as a prophylactic analgesic during surgery.7 Previous studies have shown that intravenous lidocaine inhibits pulmonary inflammation and apoptosis and reduces ventilation-related lung injury due to OLV by reducing local and systemic tumor necrosis factor (TNF)-α expression; it also reduces the inflammatory response by inhibiting high-mobility group box 1 and nuclear factor-kappa B expression.8,9 However, the efficacy of lidocaine in reducing POD among older patients lacks empirical support, and the results of two ongoing randomized controlled trials are awaited.10,11 Dexmedetomidine is an α2-adrenoceptor agonist with sedative, analgesic, sympatholytic, and hemodynamic stabilizing properties, and recent studies have shown that intravenous infusion of dexmedetomidine can exert anti-inflammatory effects. However, its ability to reduce POD has not been established.12 Duan conducted a meta-analysis of 18 clinical trials and found that intraoperative and postoperative dexmedetomidine administration significantly reduced the risk of POD (c). However, some studies have reported otherwise.13
This study aimed to investigate the effects of intravenous lidocaine and dexmedetomidine on inflammatory response and POD in older patients undergoing thoracoscopic surgery.
Materials and Methods
This study was registered in the Chinese Clinical Registry (ChiCTR2000039478) and approved by the ethics committee of the First Hospital of Chongqing Medical University (approval no. 2020–586) in accordance with the tenets of the Declaration of Helsinki. Informed consent was signed by patients and their family members. Ninety older patients were enrolled (regardless of sex) to undergo elective unilateral thoracoscopic lobectomy in the morning from May 1, 2020, to February 10, 2022. They were aged 65–75 years, weighed 48–72 kg, and had American Society of Anesthesiologists grade II or III and New York Heart Association grade I or II. The exclusion criteria were as follows: (1) sinus bradycardia or atrioventricular block; (2) local allergy to anesthetics; (3) previous use of immunosuppressants and recent chemoradiotherapy; (4) current use of nonsteroidal anti-inflammatory drugs or steroids, angiotensin converting enzyme inhibitors, or bronchodilators; (5) liver and kidney insufficiency; (7) epilepsy and associated mental and cognitive dysfunction, long-term stress stimulation, or psychological disorders; and (8) history of alcoholism, analgesic drug dependence, and long-term use of sedatives.
The patients were divided into three groups (n=30 each) using random numbers generated by a computer: control group receiving conventional general anesthesia (group C), group receiving lidocaine treatment combined with general anesthesia (group L), and group receiving dexmedetomidine treatment combined with general anesthesia (group D). The results of the grouping will follow the patient to the operating room in a sealed envelope. Specialized researchers select drugs based on grouping results, but the anesthesiologists in the operating room did not know the grouping of patients.
The electrocardiograms, heart rates (HRs), and oxygen saturation (SPO2) of all the patients were monitored on admission. Invasive arterial blood pressure was monitored using radial artery puncture and catheterization. Anesthesia was induced by infusion of 0.01–0.03 mg·kg−1 midazolam, 0.2–0.4 μg·kg−1 sufentanil, 1–2 mg·kg−1 propofol, and 0.6 mg·kg−1 rocuronium via peripheral intravenous access. After double-lumen tracheal intubation, the anesthesia machine was connected for mechanical ventilation with a tidal volume of 6 mL/kg, respiratory rate of 14–16 breaths/min, inspiratory-expiratory ratio of 1:2, and end-expiratory carbon dioxide partial pressure of 35–45 mmHg. A single-lumen central venous catheter was placed in the right internal jugular vein to monitor central venous pressure (CVP).
For anesthesia maintenance, 1–1.3 minimum alveolar concentration sevoflurane was administered intraoperatively. Rocuronium bromide (0.3 mg·kg−1) was intermittently injected intravenously, and sufentanil (0.1–0.3 μg·kg−1) was intravenously injected before skin incision, during surgery, and before chest cavity closure according to the condition of the patient. The index of consciousness (IoC), an anesthesia depth-monitoring indicator, was monitored intraoperatively: IoC1 (40–50) and IoC2 (30–50). Lactated Ringer’s solution and hydroxyethyl starch were intraoperatively infused to maintain CVP at 6–10 cm H2O. In group L, 1.0 mg·kg−1·h−1 lidocaine was infused intravenously at the induction of anesthesia for 10 min, followed by continuous infusion at a rate of 1.0 mg·kg−1·h−1 until end of surgery. (kg: ideal body weight). In group D, 1.0 μg·kg−1·h−1 dexmedetomidine was infused intravenously at the induction of anesthesia for 10 min, followed by continuous infusion at a rate of 0.5 μg·kg−1·h−1 (kg: ideal body weight). In group C, 0.2mL·kg−1·h−1 saline was infused intravenously from the beginning of anesthesia induction until end of surgery. The study staff performed drug pumping, and grouping information was delivered via a sealed envelope after the patients were admitted to the room.
During surgery, an appropriate amount of atropine was administered intravenously if the HR was ≤50 beats/min, and intraoperative hypotension was managed with ephedrine (5–10 mg) or phenylephrine (30–50 μg); all were administered by the same experienced anesthesiologist blinded to the grouping. All patients were sent to the emergency room for tracheal extubation. An intravenous analgesia pump (4 μg·kg−1 sufentanil + 40 mg azathioprine/200 mL normal saline) was used with no background dose, a 2 mL bolus, and a 15 min locking time.
Central venous blood samples were collected before the induction of anesthesia (T0), immediately after surgery (T1), and at 24 h (T2) and 48 h postoperatively (T3) by the same physician blinded to the grouping.
The primary outcome were plasma cortisol, interleukin (IL)-6, and TNF-α concentrations which measured using immunoluminescence. The secondary outcome were delirium assessment at the ward that performed postoperatively (days 2 and 7), twice a day (08:00 and 20:00), using the 3D-CAM by our trained study staff and the occurrence of intraoperative hypoxemia (SPO2<90%), postoperative extubation time, incidence of nausea and vomiting over postoperative 24 h, and number of analgesic pump compressions.
The normally distributed variables (height, weight, blood loss, operative duration, postoperative extubation time, intraoperative sufentanil use, IL-6 level, cortisol level and TNF-α level) were expressed as means±standard deviations (x±s) and analyzed using one-way analysis of variance (ANOVA). Inflammatory factor indicators were analyzed using repeated-measures ANOVA. The measurement data of the groups that did not conform to a normal distribution (number of analgesic pump compressions) were expressed as medians (interquartile ranges) and compared using the Kruskal–Wallis H-test. Post hoc multiple comparisons were performed using the Mann–Whitney U-test and Bonferroni correction. Count data (incidence rate of POD and hypoxemia and incidence rate of postoperative nausea and vomiting) were expressed as number (percentages %) and compared using the Fisher’s exact test. All statistical analyses were performed using SPSS software (version 21.0; IBM Corporation, Armonk, NY, USA), with P<0.05 indicating statistical significance.
The sample size was calculated according to a pilot study.14 To evaluate the reduction in serum cortisol concentrations following video-assisted thoracoscopic surgery procedures by 20% difference in the post anesthesia care unit discharge time at a significance level of 0.05 and power of 0.8, a minimum of 27 patients were required in each group. To account for possible dropouts at a rate of 10%, 30 patients were recruited for this randomized study.
Results
A total of 90 patients were included in this study and randomly divided into three groups (C, D, and L). One patient in group D was excluded since he refused to be interviewed postoperatively (Figure 1). There were no significant inter-group differences in the general condition, duration of surgery, and intraoperative blood loss (Table 1).
 |
Table 1 Characteristic of Participants |
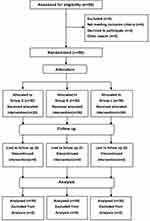 |
Figure 1 CONSORT flow diagram showing the number of patients at each phase of the study. |
Figure 2 shows that the cortisol concentrations decreased for all three groups at T1, compared with those at T0, although they increased significantly at T2. At T1 and T2, group L had significantly lower cortisol concentrations than group D (T1: 176.17±24.2 vs 201.32±30.5 nmol/L, P=0.0487; T2: 324.33±17.2 vs 388.21±17.4 nmol/L, P=0.0005 and group C (T1: 176.17±24.2 vs 220.65±35.7 nmol/L, P=0.0007; T2: 324.33±17.2 vs 398.43±22.1 nmol/L, P<0.0001). The IL-6 concentrations were significantly higher for all three groups at T1, T2, and T3 than at T0, significantly lower for groups D and L than that for group C at T1 and T2 (DT1: 50.24±6.7 vs 74.92+9.8 pg/mL, P<0.0001; DT2: 42.56±5.2 vs 48.46±6.4 pg/mL, P=0.0008; LT1: 48.58±6.1 vs 74.92±9.8 pg/mL, P<0.0001; LT2: 33.21±5.0 vs 48.46±6.4 pg/mL, P<0.0001), and significantly lower for group L than that for group D at T2 (33.21±5.0 vs 42.56±5.2 pg/mL, P<0.0001; Figure 3). The TNF-α concentrations were significantly higher for all three groups at T1, T2, and T3 than that at T0, and they were significantly lower for both groups D and L than that for group C at T1 and T2 (DT1: 23.56±4.12 vs 30.32±4.89 pg/mL, P<0.0001; DT2: 16.33±2.98 vs 23.24±3.67 pg/mL, P<0.0001; LT1: 20.18±3.26 vs 30.32±4.89 pg/mL, P<0.0001.; LT2: 14.67±2.61 vs 23.24±3.67 pg/mL, P<0.0001). Moreover, the TNF-α concentrations were significantly higher for group D than that for group L at T1 (23.56±4.12 vs 20.18±3.26 pg/mL, P=0.0014) (Figure 4).
 |
Figure 2 Changes in cortisol at different time points. Notes: *P<0.05, ***P<0.001, ****P<0.0001. |
 |
Figure 3 Changes in IL-6 at different time points. Notes: ***P<0.001, ****P<0.0001. |
 |
Figure 4 Changes in TNF-α at different time points. Notes: **P<0.01, ****P<0.0001. |
The incidence of POD in groups D and L were lower than those in group C 2 days after surgery, but there was no statistical significance (D: relative risk: 2.057, 95% CI [0.645 to 6.50], P=0.360; C: relative risk: 2.175, 95% CI [0.80 to 6.15], P=0.209). There was no statistical significance in the incidence of POD in the three groups at 7 days after surgery. Intraoperative sufentanil use was significantly lower for group L than that for groups C (35.6±4.6 vs 40.5±5.3 μg, P=0.0009) and D (35.6±4.6 vs 38.9±5.2 μg, P=0.034). However, there was no statistical difference between the three groups in the number of analgesic pump boluses at 24 h postoperatively and the incidence of intraoperative hypoxemia and analgesic remedies. The incidence of postoperative nausea and vomiting was lower for groups L than that for group C (L: 16.7 vs 43.3%, P=0.047). The postoperative duration of extubation was significantly longer for group D than that for groups C (16.8±4.6 min vs 12.4±3.1 min, P<0.0001) and L (16.8±4.6 min vs 13.5±3.5 min, P=0.003) (Table 2).
 |
Table 2 Perioperative Variables |
Discussion
This study demonstrated that the intraoperative intravenous infusion of lidocaine or dexmedetomidine may reduce the surgical inflammatory response in older patients perioperatively but not the incidence of POD. Intravenous lidocaine reduced intraoperative sufentanil use. However, opioid consumption via the postoperative analgesia pump did not differ across the three groups. Both intravenous lidocaine and dexmedetomidine reduced the incidence of postoperative nausea and vomiting, while intraoperative infusion of dexmedetomidine delayed tracheal extubation.
Clinical studies have shown that pro- and anti-inflammatory cytokines play a key role in the acute inflammatory and immune responses after surgical trauma, with TNF-α and IL-6 being the most important cytokines in this regard. TNF-α is released as a major cytokine to mediate early response to tissue injury, while IL-6 concentrations are associated with the severity of surgical trauma.15,16 Corticosterone (Cor) and catecholamines are relatively sensitive indicators of stress response. Stimulation by adverse factors in vivo can lead to the secretion of adrenal Cor, and its immediate plasma concentration can indicate the stress state of the body, as well as the degree to which anesthesia and surgery stimulate the body.17,18 CRP, IL-6, S-100β, and TNF-α were considered to be significantly associated with the occurrence of POD (k). While this study reveals the patterns of these inflammatory factors in older patients undergoing thoracoscopic surgery and the impact of intraoperative lidocaine or dexmedetomidine infusion, we did not establish a correlation between intraoperative inflammatory and the occurrence of POD.
Lidocaine has significant anti-inflammatory effects, such as reducing cytokine release by inhibiting neutrophil activation. In addition, lidocaine inhibits lymphocyte proliferation and reduces the production of pro- and anti-inflammatory cytokines, thereby affecting the acute inflammatory response.19 However, the dosage and timing of intravenous lidocaine infusion during the perioperative period require special attention, as side effects due to inappropriate use can still occur. Studies have shown that the initial dose should not exceed 1.5 mg∙kg−1, the infusion should over 10 min, the subsequent infusion rate should not be more than 1.5 mg∙kg−1∙h−1, and the infusion should be completed within 24 h.20 However, the actual plasma concentration of the patient is generally 20% higher than that predicted using the actual body weight.21 Therefore, the infused lidocaine dose should be calculated based on the ideal body weight of the patient, an approach that we followed in this study.
Multimodal analgesia is currently recommended to reduce opioid use and associated complications in older patients. However, guidelines for the intraoperative infusion of lidocaine report that intravenous lidocaine should not be administered within 4 h of any nerve block, and no nerve block should be performed within 4 h after intravenous lidocaine infusion.22 Therefore, we did not perform any related nerve blocks during the perioperative period. The results showed no differences in opioid use among the three groups within 24 h postoperatively. For patients undergoing any elective surgery under general anesthesia, previous studies reported that postoperative visual analog scale scores at 1–4 h and 24 h improved for those who received perioperative intravenous lidocaine compared with those who received control treatments.23 However, no benefit in pain relief associated with this intervention was observed at 48 h postoperatively. Therefore, the effects of lidocaine may be limited to the intraoperative period and shortly after surgery. There is no definite evidence that intravenous lidocaine can prevent the development of POD. Our study also showed that lidocaine does not reduce the incidence of POD, although it can reduce the perioperative stress response.
This study showed that the effect of dexmedetomidine on inflammatory factors was similar to that of lidocaine. There was no significant difference in sufentanil use between the dexmedetomidine and control groups, although the control group exhibited prolonged postoperative extubation due to the strong sedative effect of dexmedetomidine. Dexmedetomidine is a highly effective, selective alpha-2 adrenergic agonist with intrinsic analgesic, sedative, anxiolytic, and sympatholytic properties.24 Some studies have shown that the intraoperative administration of dexmedetomidine under general anesthesia improves pain within 24 h postoperatively and has fewer side effects as compared to remifentanil.25 However, some studies have shown that intraoperative dexmedetomidine infusion fails to prevent the development of hypotension and the delay of postoperative extubation.13 The effect of dexmedetomidine on POD remains controversial. One study confirmed that low-dose dexmedetomidine did not increase the risk of delirium in older patients undergoing cardiac surgery. However, another study showed that prophylactic low-dose dexmedetomidine significantly reduced the incidence of delirium during the first 7 days after non-cardiac surgery for patients older than 65 years.26,27 The dose-related effect of dexmedetomidine on POD and its effective dose require further research to confirm.
This study has some limitations. First, the intraoperative administration of vasoactive drugs may have affected the plasma catecholamine concentrations. Second, this was a short-term study that did not analyze differences in the recovery of gastrointestinal function and hospitalization length. A larger study population and longer durations are needed in future studies.
In summary, intraoperative continuous intravenous infusion of either lidocaine or dexmedetomidine, compared with general anesthesia alone, reduced the surgical stress and inflammatory responses of patients.
Compared with dexmedetomidine, the inhibitory effect of lidocaine on surgical stress was prominent for up to 24 h postoperatively. The two drugs have been widely used and are low-cost, although the long-term effects of both and the improvement in clinical outcomes require further investigation.
Abbreviations
CVP, central venous pressure; HR, heart rate; IL, interleukin; IoC, index of consciousness; OLV, one-lung ventilation; SPO2, oxygen saturation; T0, induction of anesthesia; T1, immediately after surgery; T2, 24h postoperatively; T3, 48 h postoperatively; TNF-α, tumor necrosis factor-alpha.
Data Sharing Statement
The data analyzed and preserved during the current study are available from the corresponding author upon reasonable request via e-mail.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The present work was supported by grants from Chongqing Health Commission (project no. 2021MSXM146) and Chongqing medical scientific research project (Joint project of Chongqing Health Commission and Science and Technology Bureau 2023MSXM125) and Scientific and Technological Research Program of Chongqing Municipal Education Commission (KJQN202202721).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hu CG, Zheng K, Liu GH, et al. Effectiveness and postoperative pain level of single-port versus two-port thoracoscopic lobectomy for lung cancer: a retrospective cohort study. Gen Thorac Cardiovasc Surg. 2021;69(2):318–325. doi:10.1007/s11748-020-01479-z
2. Yun J, Lee J, Shin S, et al. Video-assisted thoracoscopic lobectomy versus open lobectomy in the treatment of large lung cancer: propensity-score matched analysis. J Cardiothorac Surg. 2022;17(1):2. doi:10.1186/s13019-021-01749-8
3. Chen W, Yu Z, Zhang Y, Liu H. Comparison of cost effectiveness between video-assisted thoracoscopic surgery (vats) and open lobectomy: a retrospective study. Cost Eff Resour Alloc. 2021;19(1):55. doi:10.1186/s12962-021-00307-2
4. Huang X, Li L, Feng Q, Wan C. Correlation analysis of inflammatory markers CRP and IL-6 and postoperative delirium (POD) in elderly patients: a meta-analysis of observational studies. J Environ Public Health. 2022;2022:1136386. doi:10.1155/2022/1136386
5. Zhang Y, Jiang Q, Li T. Nalbuphine analgesic and anti-inflammatory effects on patients undergoing thoracoscopic lobectomy during the perioperative period. Exp Ther Med. 2017;14(4):3117–3121. doi:10.3892/etm.2017.4920
6. Jin Z, Hu J, Ma D. Postoperative delirium: perioperative assessment, risk reduction, and management. Br J Anaesth. 2020;125(4):492–504. doi:10.1016/j.bja.2020.06.063
7. Hermanns H, Hollmann MW, Stevens MF, et al. Molecular mechanisms of action of systemic lidocaine in acute and chronic pain: a narrative review. Br J Anaesth. 2019;123(3):335–349. doi:10.1016/j.bja.2019.06.014
8. Lee IW, Schraag S. The use of intravenous lidocaine in perioperative medicine: anaesthetic, analgesic and immune-modulatory aspects. J Clin Med. 2022;11(12):3543. doi:10.3390/jcm11123543
9. Bai YX, Zhang JH, Zhao BC, Liu KX, Bai YW. Dexmedetomidine attenuates one-lung ventilation associated lung injury by suppressing inflammatory responses: a systematic review and meta-analysis. Clin Exp Pharmacol Physiol. 2021;48(9):1203–1214. doi:10.1111/1440-1681.13525
10. Buren MA, Theologis A, Zuraek A, Behrends M, Clark AJ, Leung JM. Lidocaine Infusion for the Management of postoperative Pain and Delirium (LIMPP): protocol for a randomised control trial. BMJ Open. 2022;12(6):e059416. doi:10.1136/bmjopen-2021-059416
11. Liao X, Fu B, Yun J, Lin H, Qian B, Yao Y. Efficacy of systemic lidocaine in postoperative delirium in elderly patients undergoing laparoscopic colorectal surgery: study protocol for a multicentre, prospective, double-blind, randomised, parallel-group, superiority, placebo-controlled trial. BMJ Open. 2022;12(5):e056959. doi:10.1136/bmjopen-2021-056959
12. Duan X, Coburn M, Rossaint R, Sanders RD, Waesberghe JV, Kowark A. Efficacy of perioperative dexmedetomidine on postoperative delirium: systematic review and meta-analysis with trial sequential analysis of randomised controlled trials. Br J Anaesth. 2018;121(2):384–397. doi:10.1016/j.bja.2018.04.046
13. Deiner S, Luo X, Lin HM, et al. Intraoperative infusion of dexmedetomidine for prevention of postoperative delirium and cognitive dysfunction in elderly patients undergoing major elective noncardiac surgery: a randomized clinical trial. JAMA Surg. 2017;152(8):e171505. doi:10.1001/jamasurg.2017.1505
14. Hou YH, Shi WC, Cai S, et al. Effect of intravenous lidocaine on serum interleukin-17 after video-assisted thoracic surgery for non-small-cell lung cancer: a randomized, double-blind, placebo-controlled trial. Drug Des Dev Ther. 2021;15:3379–3390. doi:10.2147/DDDT.S316804
15. Pawlik W, Pawlik J, Kozłowski M, et al. The clinical importance of IL-6, IL-8, and TNF-α in patients with ovarian carcinoma and benign cystic lesions. Diagnostics. 2021;11(9):1625. doi:10.3390/diagnostics11091625
16. He L, Qing F, Li M, Lan D. Effects of laparoscopic and traditional open surgery on the levels of IL-6, TNF-α, and Gal-3 in patients with thyroid cancer. Gland Surg. 2021;10(3):1085–1092. doi:10.21037/gs-21-60
17. Wang J, Yin Y, Zhu Y, et al. Thoracic epidural anaesthesia and analgesia ameliorates surgery-induced stress response and postoperative pain in patients undergoing radical oesophagectomy. J Int Med Res. 2019;47(12):6160–6170. doi:10.1177/0300060519866943
18. Ding W, Chen Y, Li D, et al. Investigation of single-dose thoracic paravertebral analgesia for postoperative pain control after thoracoscopic lobectomy – a randomized controlled trial. Int J Surg. 2018;57:8–14. doi:10.1016/j.ijsu.2018.07.006
19. Zhang C, Xie C, Lu Y. Local anesthetic lidocaine and cancer: insight into tumor progression and recurrence. Front Oncol. 2021;11(11):669746. doi:10.3389/fonc.2021.669746
20. Foo I, Macfarlane AJR, Srivastava D, et al. The use of intravenous lidocaine for postoperative pain and recovery: international consensus statement on efficacy and safety. Anaesthesia. 2021;76(2):238–250. doi:10.1111/anae.15270
21. Dale GJ, Phillips S, Falk GL. The analgesic efficacy of intravenous lidocaine infusion after laparoscopic fundoplication: a prospective, randomised, double-blind, placebo-controlled trial. Local Reg Anesth. 2016;9:87–93. doi:10.2147/LRA.S119483
22. Weibel S, Jelting Y, Pace NL, et al. Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery in adults. Cochrane Database Syst Rev. 2018;6(6):CD009642. doi:10.1002/14651858.CD009642.pub3
23. Weibel S, Jokinen J, Pace NL, et al. Efficacy and safety of intravenous lidocaine for postoperative analgesia and recovery after surgery: a systematic review with trial sequential analysis. Br J Anaesth. 2016;116(6):770–783. doi:10.1093/bja/aew101
24. Davy A, Fessler J, Fischler M, Le Guen M. Dexmedetomidine and general anesthesia: a narrative literature review of its major indications for use in adults undergoing non-cardiac surgery. Minerva Anestesiol. 2017;83(12):1294–1308. doi:10.23736/S0375-9393.17.12040-7
25. Grape S, Kirkham KR, Frauenknecht J, Albrecht E. Intra-operative analgesia with remifentanil vs. dexmedetomidine: a systematic review and meta-analysis with trial sequential analysis. Anaesthesia. 2019;74(6):793–800. doi:10.1111/anae.14657
26. Su X, Meng ZT, Wu XH, et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet. 2016;388(10054):1893–1902. doi:10.1016/S0140-6736(16)30580-3
27. Momeni M, Khalifa C, Lemaire G, et al. Propofol plus low-dose dexmedetomidine infusion and postoperative delirium in older patients undergoing cardiac surgery. Br J Anaesth. 2021;126(3):665–673. doi:10.1016/j.bja.2020.10.041
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
