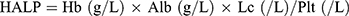Back to Journals » Cancer Management and Research » Volume 14
Predictive Value of the Hemoglobin-Albumin-Lymphocyte-Platelet (HALP) Index on the Oncological Outcomes of Locally Advanced Cervical Cancer Patients
Authors Leetanaporn K, Hanprasertpong J
Received 9 March 2022
Accepted for publication 4 June 2022
Published 14 June 2022 Volume 2022:14 Pages 1961—1972
DOI https://doi.org/10.2147/CMAR.S365612
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Kittinun Leetanaporn,1 Jitti Hanprasertpong2
1Department of Biomedical Science and Biomedical Engineering, Faculty of Medicine, Prince of Songkla University, Songkhla, 90110, Thailand; 2Department of Obstetrics and Gynecology, Faculty of Medicine, Prince of Songkla University, Songkhla, 90110, Thailand
Correspondence: Jitti Hanprasertpong, Department of Obstetrics and Gynecology, Faculty of Medicine, Prince of Songkla University, Songkhla, 90110, Thailand, Tel +66 74 45 1201, Fax +66 74 429 617, Email [email protected]; [email protected]
Purpose: The oncological outcomes of locally advanced cervical cancer (LACC) patients after treatment are poor and heterogeneous. This study aimed to determine the role of the hemoglobin-albumin-lymphocyte-platelet (HALP) inflammatory index in predicting oncological outcomes in LACC patients.
Patients and Methods: A total of 1588 LACC patients who received radiation therapy or concurrent chemoradiation were divided into training and test sets. Characteristics, survival, and a HALP cutoff determined by X-tile software were used to build predictive survival models on the training data. Validation of the model was performed on both sets.
Results: Patients with a HALP score ≤ 22.2 tended to have lower age (p < 0.001), lower comorbidity rate (p = 0.016), lower body mass index (p < 0.001), higher stage (p < 0.001), larger tumor size (p < 0.001), and higher likelihood to receive radiation alone than concurrent chemoradiation (p < 0.001). Survival analysis demonstrated that HALP > 22.2 was independently associated with better progression-free survival (PFS; hazard ratio; HR 0.55) and overall survival (OS; HR 0.43). Validation of survival prediction by receiver-operating characteristics demonstrated a significantly improved area under the curve of survival prediction in both sets (p < 0.001) after the addition of the HALP index to the model.
Conclusion: A lower HALP score was an independent predictive factor for poorer oncological outcomes. The addition of the HALP index can improve the accuracy of predicting the oncological outcomes of LACC patients.
Keywords: cervical cancer, hemoglobin-albumin-lymphocyte-platelet index, survival, radiotherapy, predictive model
Introduction
Cervical cancer (CC) is one of the most burdensome malignancies worldwide. Despite the global downward trend owing to the availability of vaccinations and pap smears, the prevalence of CC is still high in many countries, especially in developing regions.1 In locally advanced CC (LACC), radiation alone (RT) or concurrent chemoradiation (CCRT) is the mainstay treatment,2 with a wide survival range of 20.9–71.5%.3–5 Thus, additional parameters that can help stratify CC oncological outcomes are still desirable.
Following this research in recent years, several novel biomarkers have been discovered, which assist in improved prognosis determination after treatments, such as the squamous cell antigen (SCC-Ag) and HPV-DNA.6,7 However, these biomarkers have relatively high costs and are not part of a routine examination. Therefore, a combination of pre-treatment laboratory investigations with relatively high accuracy and low cost is still in demand for determining the prognosis of an individual CC patient.
There has been evidence reported on the linkage between inflammation, nutritional status, and cancer progression.8–11 Chronic inflammation accelerates the release of various cytokines that enhance cancer growth by inhibiting apoptosis and accommodating a neovascularization process leading to cancer progression.8 It is also understood that poor nutritional status in cancer patients causes impaired immune function, increased inflammatory response, and leads to increased treatment side effects.9,10 Additionally, malnutrition status can reflect high tumor metabolic activity since cancer supplies itself with extra energy by taking it from the surrounding environment.11 For this reason, several inflammatory indices have been established to identify the clinical association between inflammation and cancer prognosis, such as C-reactive protein, lactate dehydrogenase, neutrophil-to-lymphocyte ratio (NLR);12–14 nutritional status indices such as body mass index (BMI) and prognostic nutrition index.15,16 The hemoglobin-albumin-lymphocyte-platelet (HALP) index is a novel score based on a combination of inflammatory and nutritional deficiency concepts. This index has been found to enhance the prediction accuracy of various cancer prognoses.17–19 However, there are, to date, no studies on the effect of this index on CC prognoses.
Therefore, this study aimed to examine the prognostic role of the HALP index on oncological outcomes in CC patients who received RT/CCRT treatment. In addition, a predictive model based on the HALP index and other significant parameters was constructed to validate the applicability of this index.
Materials and Methods
Study Selection
This was a retrospective study conducted after approval from the Ethics Committee of the Faculty of Medicine, Prince of Songkla University (REC.64-557-12-1), which waived the requirement for written informed consent due to the retrospective nature of the study. The confidentiality of patient data was guaranteed, as required by the Ethics Committee, and the study was conducted in accordance with the Declaration of Helsinki. CC patients diagnosed from January 2008 to December 2020 who received RT or CCRT at Songklanagarind Hospital were included in the study. The inclusion criteria were as follows: 1 stage IB2-IVA according to the International Federation of Gynecology and Obstetrics (FIGO) 2009 classification, 2 squamous cell carcinoma (SCC), adenocarcinoma (AD), or adenosquamous carcinoma (ASC) histological types, and 3 receiving a platinum chemotherapy regimen for the CCRT group. The exclusion criteria were as follows: 1 receiving neoadjuvant treatment, 2 two primary cancers, 3 inadvertent cancer (accidental diagnosis of cervical cancer after simple hysterectomy for a benign condition), 4 pregnancy, 5) HIV infection, 6 inadequate radiation, and/or 7 incomplete HALP data.
Treatment Protocol
Evaluation before treatment comprised complete physical and pelvic examinations, complete blood count, renal and liver function tests, and serologic tests (HBsAg, VDRL, and HIV status). The radiation protocol consisted of external beam radiation (EBRT) with 6–15 megavolt photon beams with anterior-posterior fields or a four-field box using conventional (2D), 3D conformal RT, occasionally with intensity-modulated radiation therapy (IMRT). The EBRT was delivered to the whole pelvis with lymph node/parametrium/pelvic sidewall boosts of up to 50–60 Gy, divided into 2030 Fr with a rate of 2 Gy/day. Platinum chemotherapy (cisplatin 40 mg/m2 or carboplatin AUC 2) was given concurrently once a week during the EBRT. Additionally, 6.5 Gy × 3–4 times of high-dose-rate intracavity brachytherapy was delivered to point A with an Iridium-192 remote after-loading technique during the 3rd to 5th week of treatment. After the treatment, the patients were followed-up according to a protocol as previously described.20
Data Collection
The following characteristics were collected from the hospital database: age, BMI (kg/m2), comorbidities (hypertension, diabetes, dyslipidemia), stage, histological type according to the World Health Organization (WHO) criteria, tumor size, and treatment modality (RT or CCRT). BMI was categorized into 3 groups following a previous study.21 For laboratory investigations, pre-treatment hemoglobin (Hb), albumin (Alb), lymphocyte count (Lc), and platelets (Plt) were measured. The HALP index was calculated with the following equation:
Oncological outcomes were defined as: 1 progression-free survival (PFS): time since the diagnosis to discovery of disease progression or last follow-up, and 2 overall survival (OS): time from the diagnosis to death or last follow-up. Patients who were lost to follow-up were censored at the last follow-up date.
Statistical Analysis
The whole cohort was split into training (70%) and test (30%) set. All model generation processes were done on the training set only. The optimal HALP cutoff was determined based on PFS by the X-tile program 3.6.1 (Yale University).22 Then, the clinicopathologic characteristics were analyzed according to frequency and percentage, categorized by the HALP cutoff. Univariate survival analysis was performed by constructing a Kaplan–Meier estimate and log-rank analysis. Multivariate analysis was performed using the Cox proportional hazards regression model. The significant p-value was set at ≤0.05. A Receiver Operating Characteristic (ROC) curve was constructed and used to calculate the AUC on both the training and testing sets. The AUC comparison between the models was made using the Wilcoxon rank-sum test. Data analysis in addition to HALP cutoff determination was done using the R program 4.1.2 (R Foundation for Statistical Computing).
Results
Patient Characteristics
Over the 13 years of the recruitment period, a total of 2143 patients were identified according to the inclusion criteria. The excluded cases were as follows: neoadjuvant treatment 52 (2.4%), two primary cancers 55 (2.6%), inadvertent cancer 19 (0.9%), pregnancy 9 (0.4%), HIV infection 77 (3.6%), inadequate treatment 160 (7.5%), and incomplete HALP data 187 (8.7%). Thus, the remaining 1588 patients were eligible for the final analysis. The overall median age of the patients was 52 (interquartile range [IQR] 45–61) years. The median HALP was 34.18 (IQR 21.32–46.8). The cohort was then divided into a training set (70%) and a test set (30%). There were no significant differences in the clinicopathologic characteristics between the two groups after splitting (Table 1).
 |
Table 1 Clinicopathologic Characteristics of Overall Cohort Divided into Sets |
Establishment of HALP Cutoff
The optimal cut-point was 22.2 following X-tile’s two categorical variables chi-square test from the training data (Figure 1). Then, the patients were divided into ≤22.2 and >22.2 groups. The clinicopathologic characteristics according to the HALP index showed that patients with HALP ≤22.2 tended to have lower age (p < 0.001), lower comorbidity rate (p = 0.016), lower BMI (p < 0.001), higher stage (p < 0.001), larger tumor size (p < 0.001), and were more likely to receive RT than CCRT (p < 0.001). However, the difference in histological type between the HALP categories was not significant (Table 2).
 |
Table 2 Characteristics According to HALP Group in Training Data |
 |
Figure 1 HALP index cut point determination by X-tile program: (A) HALP histogram (B) Cut-point value. |
Survival Analysis
The median follow-up time was 2.96 (IQR1.39–5.47) years. From all patients, 456 cases (28.71%) had tumor recurrence. The 5-year PFS and 5-year OS of all patients were 65% (95% confidence interval [CI] 0.63–0.68) and 87.5% (95% CI 0.86–0.90). The univariate analysis in the training set (Table 3) demonstrated that the significant factors associated with worse PFS were HALP ≤22.2 (p < 0.001; Figure 2A), lower BMI (p = 0.01), higher stage (p < 0.001), non-SCC histology (p = 0.007), larger tumor size (p < 0.001), and RT (p < 0.001), while the important factors associated with worse OS were HALP ≤ 22.2 (p < 0.001; Figure 2B), higher stage (p < 0.001), larger tumor size (p < 0.001), and RT (p < 0.001).
 |
Table 3 Univariate Analysis of Factors Associated with Altered PFS and OS on Training Data |
 |
Figure 2 Kaplan-Meier analysis of HALP according to HALP cut-point: (A) Progression-free survival (B) Overall survival. |
Further multivariate analysis showed that HALP index (>22.2 vs ≤22.2; hazard ratio [HR] 0.55; 95% CI 0.43–0.70), stage (III vs I+II; HR 1.88; 95% CI 1.46–2.42 and IVA vs I+II; HR 5.18; 95% CI 3.18–8.44), histology (AD vs SCC; HR 1.74; 95% CI 1.32–2.31 and ASC vs SCC; HR 1.79; 95% CI 1.02–3.13), tumor size (>8 vs <4 cm; HR 1.90; 95% CI 1.12–3.24), and treatment modality (CCRT vs RT; HR 0.55; 95% CI 0.41–0.75) were independently associated with worse PFS, while only HALP cut off (>22.2 vs ≤22.2; HR 0.43; 95% CI 0.27–0.67), stage (III vs I+II; HR 1.96; 95% CI 1.22–31.7 and IVA vs I+II; HR 5.45; 95% CI 2.58–11.50), and treatment modality (CCRT vs RT; HR 0.38; 95% CI 0.21–0.67) were independently associated with worse OS (Table 4). We also tested for the time-varying effect of HALP and found that the detrimental effect of HALP ≤22.2 on both PFS and OS diminished after more than 5 years and 3 years survival, respectively (data not shown).
 |
Table 4 Multivariate Analysis of Factors Associated with Altered PFS and OS on Training Data |
Construction of the Predictive Survival Model
To further investigate the predictive power of the HALP index on survival, ROC analysis of PFS and OS prediction was performed based on two constructed Cox’s hazard regression models (Figures 3 and 4): 1 independently significant factors from multivariate analysis (Table 4) without HALP and 2 independently significant factors from multivariate analysis with HALP. The results demonstrated that the addition of HALP to the model improved the AUCs for prediction of PFS at 1, 3, and 5 years from 0.70, 0.71, and 0.71 to 0.73, 0.73, and 0.72 in the training set (p < 0.001) and from 0.66, 0.66, and 0.69 to 0.71, 0.69, and 0.71 in the test set (p < 0.001), respectively (Figure 5). The AUCs for prediction of OS also improved from 0.73, 0.68, and 0.70 to 0.79, 0.70, and 0.72 in the training set (p < 0.001) and 0.64, 0.60, and 0.63 to 0.72, 0.69, and 0.72 in the test set (p < 0.001), respectively (Figure 6). We also created an interactive web application (https://hgatc.psu.ac.th/halp/ or https://github.com/LKittinun/HALP_survival_model) generated from the formula:
 |
Figure 3 Predictive accuracy of HALP model on progression-free survival in both sets. |
 |
Figure 4 Predictive accuracy of HALP model on overall survival in both sets. |
 |
Figure 5 Spot predictive accuracy of HALP model on progression-free survival (PFS) in both sets: (A) 1-year PFS (B) 3-year PFS (C) 5-year PFS. |
 |
Figure 6 Spot predictive accuracy of HALP model on overall survival (OS) in both sets: (A) 1-year OS (B) 3-year OS (C) 5-year OS. |
log(HRPFS) = 0.40−0.60(HALP > 22.2)+0.63(stage III)+1.64(stage IVA)+0.56(AD)+
0.58(ASC)−0.59(CCRT)+0.26(tsize = 4–8)+0.64(tsize > 8)
log(HROS) = 0.82−0.96(HALP > 22.2)+0.71(stage III)+1.89(stage IVA)−0.52(CCRT).
Discussion
To date, many hematologic indices or parameters have been proposed to help determine the prognosis of cancers.12–16 However, combinations of commonly used pre-treatment investigations, such as NLR, platelet-to-lymphocyte ratio (PLR), and HALP, that can help determine a patient’s prognosis at no additional cost, are still worth exploring. Among the other indices in this category, HALP has been proposed as the best prognosis predictor among the hematologic parameters in some cancers.23,24 For example, a study by Cong et al found that compared with NLR and PLR, only HALP was an independent significant prognostic factor among all indices of esophageal carcinoma.23 Another study was from Guo et al, which compared the AUCs of HALP, NLR, and PLR for the prognosis of metastatic prostate cancer. The study found that HALP and its variant had the highest AUC compared with the others.24 For this reason, HALP is one of the most interesting indices to be explored at this moment.
To our knowledge, our study is the first study to demonstrate that the association of HALP on oncological outcomes can be applied to LACC patients. Our study demonstrated that a HALP score ≤22.2 was associated with higher stage and tumor size in the CC cohort. This result was in line with studies of other cancers in which at least one feature of a more advanced stage or larger tumor size was present in the low HALP group.17,25–28 In the context of survival, our study found that a HALP score ≤22.2 was independently associated with worse PFS and OS. This result was in concordance with several other studies that demonstrated an impact of lower HALP on poorer prognosis for several cancers, such as bladder, colorectal, gastric, prostate, esophageal, and lung cancers, with varying cut-points depending on the cancer type and study setting.17–19,23,24,29,30 Additionally, we found that the deleterious effect of a low HALP diminished when the patient survived for a particular time.
Several mechanisms can explain the impact of inflammation and nutrition status on the HALP index and CC prognosis. In a chronic inflammatory state, various cytokines in the body become altered, such as NF-κB, p53, HIF-α, and VEGF. The alteration of these cytokines has been reported to dysregulate cancer apoptosis inhibition and promote neovascularization, leading to more advanced stages of CC.8 In addition, inflammation causes shortened erythrocyte survival, suppressed bone marrow function, and hypoferremia, resulting in low hemoglobin levels.31 Supporting evidence from earlier studies showed that a hemoglobin level <10 g/dL had a negative impact on CC radiation outcome, whereby the impaired DNA damage process is from insufficient oxygenation of tumor tissue.32,33 Conversely, some inflammatory cytokines, such as thrombopoietin and IL-6, stimulate platelet production, a condition known as reactive thrombocytosis, which has been shown to be associated with poor CC survival.34,35 Also, malnutrition is associated with low albumin and lymphocyte quantity since essential substrates required for production are limited by these conditions.36 Accordingly, cancer patients are prone to malnutrition due to the ability of cancers to shift adjacent cells from normal conditions to generate lactate via glycolysis for them, resulting in energy leaching from cells to tumors, a process termed the reverse Warburg effect. This catabolite transfer is thought to damage the surrounding normal tissues and favor the invasion and metastasis of cancer.37
To validate the generalizable effect of HALP on oncological outcomes, we constructed multiple predictive models based on a training set and validated on a test set. We discovered that besides other known associated factors, such as stage, histology, and treatment modality, the addition of the HALP index to the model significantly improved the model’s predictive accuracy for both sets. This method resulted in findings similar to several previously published papers, such as a study by Jiang et al,18 which created a survival model for colorectal cancer patients using a training set and validation set and obtained competent AUCs at 0.73 and 0.74. Likewise, a study by Sun et al38 found an improvement in the prediction model after incorporating HALP with other known variables, such as operation outcome and TNM staging (C-index 0.66 vs 0.63).
The strength of our study is that a large number of patients were recruited over a long period. However, some limitations have to be addressed. First, some incomplete and/or missing documentation could not be avoided due to the retrospective nature of the study. Second, all patients were recruited from a single center. Therefore, the setting of the study has to be taken into account when interpreting the result for clinical use. Finally, some potential confounders that can influence LACC oncological outcomes were not available. For these reasons, further large-scale multicenter studies with standardized patient conditions are necessary to confirm our findings.
In conclusion, our study found that a lower HALP was associated with higher stage and larger tumor size and was also an independent factor for poorer oncological outcomes. The addition of a HALP index can improve the accuracy of determining oncological outcomes of LACC patients. These results suggest that the HALP index may be useful as a clinical prognostic factor for LACC patients after receiving RT/CCRT treatment.
Acknowledgments
This study was supported by a grant from the Faculty of Medicine, Prince of Songkla University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhang X, Zeng Q, Cai W, Ruan W. Trends of cervical cancer at global, regional, and national level: data from the Global Burden of Disease Study 2019. BMC Public Health. 2021;21:894. doi:10.1186/s12889-021-10907-5
2. Network NCC. Cervical cancer (Version 1.2021); 2020. Available from: https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf.
3. Kim TE, Park BJ, Kwack HS, Kwon JY, Kim JH, Yoon SC. Outcomes and prognostic factors of cervical cancer after concurrent chemoradiation. J Obstet Gynaecol Res. 2012;38:1315–1320. doi:10.1111/j.1447-0756.2012.01871.x
4. Endo D, Todo Y, Okamoto K, Minobe S, Kato H, Nishiyama N. Prognostic factors for patients with cervical cancer treated with concurrent chemoradiotherapy: a retrospective analysis in a Japanese cohort. J Gynecol Oncol. 2015;26:12–18. doi:10.3802/jgo.2015.26.1.12
5. Kumar L, Gupta S. Integrating chemotherapy in the management of cervical cancer: a critical appraisal. OCL. 2016;91:8–17.
6. Liu Z, Shi H. Prognostic role of squamous cell carcinoma antigen in cervical cancer: a meta-analysis. Dis Markers. 2019;2019:1–10.
7. Sabeena S, Kuriakose S, Damodaran B, Ravishankar N, Arunkumar G. Human Papillomavirus (HPV) DNA detection in uterine cervix cancer after radiation indicating recurrence: a systematic review and meta-analysis. J Gynecol Oncol. 2019;31:e20. doi:10.3802/jgo.2020.31.e20
8. Ibeanu OA. Molecular pathogenesis of cervical cancer. Cancer Biol Ther. 2011;11:295–306. doi:10.4161/cbt.11.3.14686
9. Mueller C. Inflammation and malnutrition. Top Clin Nutr. 2011;26:3–9. doi:10.1097/TIN.0b013e318209e38b
10. Medina-Jiménez AK, Monroy-Torres R. Repurposing individualized nutritional intervention as a therapeutic component to prevent the adverse effects of radiotherapy in patients with cervical cancer. Front Oncol. 2020;10:595351. doi:10.3389/fonc.2020.595351
11. Wilde L, Roche M, Domingo-Vidal M, et al. Metabolic coupling and the reverse Warburg effect in cancer: implications for novel biomarker and anticancer agent development. Semin Oncol. 2017;44:198–203. doi:10.1053/j.seminoncol.2017.10.004
12. Polterauer S, Grimm C, Tempfer C, et al. C-reactive protein is a prognostic parameter in patients with cervical cancer. Gynecol Oncol. 2007;107:114–117. doi:10.1016/j.ygyno.2007.06.001
13. Wang H, Wang MS, Zhou YH, Shi JP, Wang WJ. Prognostic values of LDH and CRP in cervical cancer. Onco Targets Ther. 2020;13:1255–1263. doi:10.2147/OTT.S235027
14. Nuchpramool P, Hanprasertpong J. Preoperative neutrophil-lymphocyte ratio and platelet-lymphocyte ratio are not clinically useful in predicting prognosis in early stage cervical cancer. Surg Res Pract. 2018;2018:9162921. doi:10.1155/2018/9162921
15. Lee J, Meyerhardt JA, Giovannucci E, Jeon JY. Association between body mass index and prognosis of colorectal cancer: a meta-analysis of prospective cohort studies. PLoS One. 2015;10:e0120706. doi:10.1371/journal.pone.0120706
16. Hua X, Long ZQ, Huang X, et al. The value of Prognostic Nutritional Index (PNI) in predicting survival and guiding radiotherapy of patients with T1-2N1 breast cancer. Front Oncol. 2020;9:1562. doi:10.3389/fonc.2019.01562
17. Peng D, Zhang CJ, Gong YQ, et al. Prognostic significance of HALP (Hemoglobin, Albumin, Lymphocyte and Platelet) in patients with bladder cancer after radical cystectomy. Sci Rep. 2018;8:794. doi:10.1038/s41598-018-19146-y
18. Jiang H, Li H, Li A, et al. Preoperative combined hemoglobin, albumin, lymphocyte and platelet levels predict survival in patients with locally advanced colorectal cancer. Oncotarget. 2016;7:72076–72083. doi:10.18632/oncotarget.12271
19. Chen XL, Xue L, Wang W, et al. Prognostic significance of the combination of preoperative hemoglobin, albumin, lymphocyte and platelet in patients with gastric carcinoma: a retrospective cohort study. Oncotarget. 2015;6:41370–41382. doi:10.18632/oncotarget.5629
20. Jiamset I, Hanprasertpong J. Impact of diabetes mellitus on oncological outcomes after radical hysterectomy for early stage cervical cancer. J Gynecol Oncol. 2016;27:e28. doi:10.3802/jgo.2016.27.e28
21. Leetanaporn K, Hanprasertpong J. Impact of obesity on clinical outcomes in patients with early-stage cervical cancer after radical hysterectomy with pelvic node dissection. Oncol Res Treat. 2019;42:553–563. doi:10.1159/000502752
22. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–7259. doi:10.1158/1078-0432.CCR-04-0713
23. Cong L, Hu L. The value of the combination of hemoglobin, albumin, lymphocyte and platelet in predicting platinum-based chemoradiotherapy response in male patients with esophageal squamous cell carcinoma. Int Immunopharmacol. 2017;46:75–79. doi:10.1016/j.intimp.2017.02.027
24. Guo Y, Shi D, Zhang J, et al. The Hemoglobin, Albumin, Lymphocyte, and Platelet (HALP) score is a novel significant prognostic factor for patients with metastatic prostate cancer undergoing cytoreductive radical prostatectomy. J Cancer. 2019;10:81–91. doi:10.7150/jca.27210
25. Peng D, Zhang C, Tang Q, et al. Prognostic significance of the combination of preoperative Hemoglobin and Albumin Levels and Lymphocyte and Platelet Counts (HALP) in patients with renal cell carcinoma after nephrectomy. BMC Urol. 2018;18:20. doi:10.1186/s12894-018-0333-8
26. Xu SS, Li S, Xu HX, et al. Haemoglobin, albumin, lymphocyte and platelet predicts postoperative survival in pancreatic cancer. World J Gastroenterol. 2020;26:828–838. doi:10.3748/wjg.v26.i8.828
27. Zhai B, Chen J, Wu J, et al. Predictive value of the Hemoglobin, Albumin, Lymphocyte, and Platelet (HALP) Score and Lymphocyte-to-Monocyte Ratio (LMR) in patients with non-small cell lung cancer after radical lung cancer surgery. Ann Transl Med. 2021;9:976. doi:10.21037/atm-21-2120
28. Feng JF, Wang L, Yang X. The preoperative Hemoglobin, Albumin, Lymphocyte and Platelet (HALP) score is a useful predictor in patients with resectable esophageal squamous cell carcinoma. Bosn J Basic Med Sci. 2021;21:773–781. doi:10.17305/bjbms.2021.5666
29. Yang N, Han X, Yu J, Shu W, Qiu F, Han J. Hemoglobin, albumin, lymphocyte, and platelet score and neutrophil-to-lymphocyte ratio are novel significant prognostic factors for patients with small-cell lung cancer undergoing chemotherapy. J Can Res Ther. 2020;16:1134. doi:10.4103/jcrt.JCRT_1066_19
30. Shen XB, Zhang YX, Wang W, Pan YY. The Hemoglobin, Albumin, Lymphocyte, and Platelet (HALP) score in patients with small cell lung cancer before first-line treatment with etoposide and progression-free survival. Med Sci Monit. 2019;25:5630–5639. doi:10.12659/MSM.917968
31. Nemeth E, Ganz T. Anemia of inflammation. Hematol Oncol Clin North Am. 2014;28:671–681. doi:10.1016/j.hoc.2014.04.005
32. Dunst J, Kuhnt T, Strauss HG, et al. Anemia in cervical cancers: impact on survival, patterns of relapse, and association with hypoxia and angiogenesis. Int J Radiat Oncol Biol Phys. 2003;56:778–787. doi:10.1016/S0360-3016(03)00123-8
33. Rockwell S, Dobrucki IT, Kim EY, Marrison ST, Vu VT. Hypoxia and radiation therapy: past history, ongoing research, and future promise. Curr Mol Med. 2009;9:442–458. doi:10.2174/156652409788167087
34. Rokkam VR, Kotagiri R. Secondary thrombocytosis. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2021.
35. Cao W, Yao X, Cen D, Zhi Y, Zhu N, Xu L. Prognostic role of pretreatment thrombocytosis on survival in patients with cervical cancer: a systematic review and meta-analysis. World J Surg Oncol. 2019;17:132. doi:10.1186/s12957-019-1676-7
36. Rocha NP, Fortres RC. Total lymphocyte count and serum albumin as predictors of nutritional risk in surgical patients. Arq Bras Cir Dig. 2015;28:193–196. doi:10.1590/S0102-67202015000300012
37. Kobayashi Y, Banno K, Kunitomi H, et al. Warburg effect in gynecologic cancers. J Obstet Gynaecol Res. 2019;45:542–548. doi:10.1111/jog.13867
38. Sun L, Guan A, Jin Y, et al. Comparison of prognostic value of red cell-related parameters of biliary tract cancer after surgical resection and integration of a prognostic nomogram: a retrospective study. Adv Ther. 2021;38:1227–1244. doi:10.1007/s12325-020-01595-5
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.