Back to Journals » Clinical Interventions in Aging » Volume 17
Paraspinal Muscle Degeneration: A Potential Risk Factor for New Vertebral Compression Fractures After Percutaneous Kyphoplasty
Authors Si F, Yuan S , Zang L , Fan N, Wu Q , Wang T, Wang A
Received 16 May 2022
Accepted for publication 2 August 2022
Published 13 August 2022 Volume 2022:17 Pages 1237—1248
DOI https://doi.org/10.2147/CIA.S374857
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Zhi-Ying Wu
Fangda Si,* Shuo Yuan,* Lei Zang, Ning Fan, Qichao Wu, Tianyi Wang, Aobo Wang
Department of Orthopedics, Beijing Chaoyang Hospital, Capital Medical University, Beijing, 100043, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lei Zang, Department of Orthopedics, Beijing Chaoyang Hospital, Capital Medical University, Beijing, 100043, People’s Republic of China, Tel +86-13601252787, Email [email protected]
Background: The paraspinal muscle is essential for maintaining normal spine function and structure, which degeneration is closely related to various spinal diseases. The main objective of this study was to identify the potential role of paraspinal muscle degeneration in the occurrence of new vertebral compression fractures (NVCF) and develop a clinically applicable nomogram for prospective NVCF risk prediction.
Methods: A total of 202 patients with single-level osteoporotic vertebral compression fractures (OVCF) who underwent percutaneous kyphoplasty treatment between January 2016 and March 2019 were included in this study. Demographic, clinical, radiological, and treatment data were collected and analyzed. The paraspinal muscle cross-sectional area (CSA) and fat signal fraction (FSF) were measured to quantify the extent of muscle degeneration. Multivariate binary logistic regression analysis was performed to select risk factors to build a nomogram that predicted the occurrence of NVCF. The concordance index (C-index) and calibration curve were used to evaluate the discriminative capacity and predictive accuracy of the nomogram.
Results: NVCF occurred in 54 of 202 patients (26.7%). The erector spinae FSF (OR = 1.064; P = 0.001), psoas major FSF (OR = 1.326; P < 0.001), and the difference index of the muscle CSA between multifidus and psoas major (OR = 1.048; P < 0.001) were independent risk factors for the occurrence of NVCF. The nomogram performance was good after evaluation using the calibration curves and C-index (95% confidence interval, 0.854– 0.943).
Conclusion: Paraspinal muscle degeneration is a potential risk factor for NVCF occurrence. A nomogram was designed to precisely predict the risk of NVCF. This predictive nomogram may help clinicians to make better clinical decisions and provide more accurate functional exercise protocol for OVCF patients.
Keywords: paraspinal muscle degeneration, new vertebral compression fractures, percutaneous kyphoplasty, risk factors, nomogram
Introduction
Osteoporotic vertebral compression fracture (OVCF) is the most common form of osteoporotic fracture and is a major cause of pain and disability in older adults.1 In the USA alone, there are approximately 750,000 new vertebral fractures each year, surpassing the summation of hip and ankle fractures per year.2 For the benefit of patients, a technique termed percutaneous kyphoplasty (PKP) was introduced in 1998 and has been proven to rapidly and effectively alleviate symptoms caused by vertebral fractures.3 However, this approach still faces significant challenges in maintaining long-term efficacy and safety. As one of the postoperative complications, new vertebral compression fractures (NVCF) following augmentation have attracted tremendous attention due to their high incidence and severe clinical consequences.4
Over the past several decades, numerous studies have elucidated many risk factors that may cause NVCF occurrence, such as older age, lower bone mineral density, lower body mass index, intradiscal cement leakage, number of pre-existing vertebral fractures and vertebral height restoration.5 However, most of these studies may have overlooked the potential role of the paraspinal muscles. The paraspinal muscle is tightly attached to the spine and is essential for sustaining normal spinal function and structure. Previous studies have demonstrated that the paraspinal muscle is involved in maintaining natural anterior pelvic tilt and lordotic curve.6,7 Meanwhile, the paraspinal muscle is also an essential regulator of posture balance and spinal stability.8–10 Based on these lines of evidence, paraspinal muscle degeneration, including atrophy and fat infiltration, may have profound negative effects. In fact, the occurrence and development of lumbar disc herniation, spinal stenosis and scoliosis have been closely related to paraspinal muscle degeneration.11–13 Moreover, paraspinal muscle degeneration has already been a significant predictor of low back pain.14 Paraspinal muscle degeneration is associated with various spinal diseases. Hence, it is reasonable to suspect a connection between paraspinal muscle degeneration and NVCF occurrence. However, to the best of our knowledge, this hypothesis has not been conclusively demonstrated. To evaluate this issue, we analyzed data from patients with OVCF treated by PKP to identify the role of paraspinal muscle degeneration in the occurrence of NVCF and develop a nomogram to predict the probability of NVCF.
Materials and Methods
Subjects
Data of 397 patients with OVCF who underwent PKP treatment at a single spine center between January 2016 and March 2019 were retrospectively reviewed. The inclusion criteria were as follows: (1) single-level OVCF treated with PKP, (2) imaging was performed within 2 weeks preoperatively, (3) magnetic resonance imaging (MRI) examinations included L4/5 intervertebral disc level, and (4) follow-up period of at least 1 year. The exclusion criteria were as follows: (1) evidence of a previous osteoporotic vertebral fracture, (2) a history of previous spinal decompression or fusion surgery, (3) combined with a spinal tumor or the presence of pathologic compression fractures, (4) poor medication compliance with anti-osteoporotic drugs during the follow-up period, and (5) presence of Parkinson’s disease, amyotrophic lateral sclerosis, or other neuromuscular system diseases.15 The Ethics Committee for the Protection of Human Subjects approved all protocols and procedures. A waiver of informed consent was granted because of the retrospective data collection.
Surgical Technique and Anti-Osteoporosis Medications
All PKP procedures were performed under local anesthesia using a unilateral transpedicular approach. An inflatable balloon (Medtronic Sofamor Danek, Memphis, TN, USA) was inserted through the working channel and positioned in the anterior three-fourths of the vertebral body in lateral X-ray fluoroscopy during the surgical procedure. Balloon inflation is stopped once maximum pressure or volume is reached, desired fracture reduction is achieved or if balloons reach cortical walls or there are any signs of a cortical breach. Subsequently, the balloon is deflated, removed, and the void created within the vertebral body is filled with viscous polymethylmethacrylate (Mendec Spine Cement; Tecres SPA, Verona, Italy) bone cement. All patients were given postoperative anti-osteoporosis medications for 6 months, which regimen consisted of salmon calcitonin nasal spray 20IU/d, oral calcium carbonate 600mg/d and oral calcitriol 0.5µg/d with monthly monitoring of serum calcium and renal function.
Follow-Up and Diagnosis of NVCF
Patients were followed up weekly during the first 3 months after discharge, then every 3 months for the first year, every 6 months for the second year, and then annually. A patient was considered to have NVCF if he/she has (1) an acute increase in back pain or daily life disability, (2) appearance of wedge deformation or any loss of vertebral body height compared with the preoperative lateral radiograph, and (3) bone marrow edema on MRI at the corresponding anatomic level.
Clinical Data
The potential risk factors that may increase the risk of NVCF were selected using information documented in the medical records, including age, sex, body mass index, bone mineral density, follow-up duration, treated vertebral level, menopausal status, cement volume, presence of Kummell disease, leakage of cement to the adjacent disc, history of hypertension, history of diabetes, history of steroid use, and smoking status. Based on the level of OVCF occurrence, the cases were categorized into the T-L junction (T11–L2) and non-T-L junction. Gas within the vertebral body or an intravertebral vacuum cleft on plain anteroposterior and lateral radiographs was identified as the presence of Kummell disease.16
Imaging Procedures
T2-weighted axial MRI is an objective method for the quantitative assessment of paraspinal muscle degeneration.7,11,12 The MRI system used in this study was a 3.0T magnetic resonance system (MAGNETOM Verio; Siemens, Erlangen, Germany). T2-weighted axial images were acquired with TR/TE times of 3,000–3,100/70–80 ms. There were five axial slices per level, and each slice thickness was 3.0 mm. After scanning, the images were saved in DICOM format for the picture archiving and communication system.
Muscle Measurements
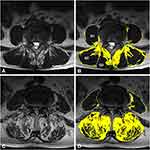
Pathological changes in the paraspinal muscle appeared most often at L4–5.17 In this study, the center slice of the five T2-weighted axial images was selected at L4–L5 to best represent the degree of paraspinal muscle degeneration. The DICOM images were displayed and analyzed using ImageJ software (version 1.40, US National Institutes of Health, Bethesda, MD, USA). Paraspinal muscle degeneration is characterized by muscle atrophy and fat infiltration, and its degree is quantified by cross-sectional area (CSA) and fat signal fraction (FSF).18 The total CSA of the multifidus (MF), erector spinae (ES) and psoas major (PM) was measured separately for the right and left sides by outlining the fascial boundary of each muscle. Then, the thresholding method was used to obtain the specific signal intensity ranges for fat and muscle tissue, to separate the intramuscular fat CSA from the original segmentation that followed fascial boundaries (Figure 1).19 Two spinal surgeons independently assessed each image. There was at least a 2-week interval between each measurement, and the average value was considered as the final result. Intra- and inter-class correlation coefficients (ICCs) were used to evaluate the intra- and inter-observer reproducibility of radiomics feature extraction.
Finally, the FSF was calculated as (intramuscular fat CSA/total CSA) × 100%. The of the CSA (CDI) was computed to demonstrate paraspinal muscle atrophy using the following formula:20
CDI between MF and ES = (1-MF muscle CSA/ES muscle CSA) × 100%
CDI between MF and PM = (1-MF muscle CSA/PM muscle CSA) × 100%
CDI between ES and PM = (1-ES muscle CSA/PM muscle CSA) × 100%
Statistical Analysis
Statistical analyses were performed using SPSS software (version 20.0; IBM Corp, 2012, Armonk NY, USA). Univariate analysis was first performed on each of the independent variables to screen for candidate variables. An independent t-test was used to compare continuous variables, and categorical variables were analyzed using the chi-square or Fisher’s exact tests. Variables exhibiting a P < 0.2 in the univariate analysis were considered for entry into the multivariate logistic regression analysis. Subsequently, a nomogram for predicting NVCF was constructed based on the multivariate binary logistic regression model using the R 4.1.1 software (R Foundation for Statistical Computing, Vienna, Austria). The discrimination performance of the nomogram was evaluated by concordance index (C-index) and receiver operating characteristic curve analysis.21 It is generally recognized that the nomogram has good discriminative ability when the C-index is >0.70. Meanwhile, the predictive accuracy of the nomogram was internally validated using calibration curves and the Hosmer–Lemeshow test by performing 1000 bootstraps.22 The 45° calibration curve represents ideal prognosis prediction. For all tests, a two-sided P < 0.05 was considered statistically significant.
Results
Basic Characteristics
Among the 397 potentially eligible patients, 202 (50.9%) were enrolled (Figure 2). There were 149 women and 53 men, the average patient age was 70.35 years, and the mean follow-up duration was 26.2 months (range, 12.7–55.4 months). NVCF occurred in 54 of 202 (26.7%) patients. Of these 54 patients, 41 (75.9%) had adjacent fractures, 5 (9.3%) had remote fractures, 8 (14.8%) had recurrence fractures of the operated vertebrae, and 28 patients (51.9%) with symptomatic NVCF underwent a second surgical treatment. Favorable inter-observer and intra-observer reproducibility of feature extraction was achieved with intra-observer ICCs ranging from 0.913 to 0.974 and inter-observer ICCs ranging from 0.824 to 0.926.
 |
Figure 2 Flow chart for screening eligible patients. Abbreviations: PKP, percutaneous kyphoplasty; OVCF, osteoporotic vertebral compression fractures; NVCF, new vertebral compression fractures. |
No statistically significant differences in sex, treated vertebral level, menopausal status, history of hypertension, history of diabetes, history of steroid use, smoking status, presence of Kummell disease, and intradiscal cement leakage were noted between groups (Table 1). Significant differences between groups in terms of BMI, BMD, follow-up duration, cement volume, and CDI between the MF and ES were not observed (Table 2). However, statistically significant differences were found between groups in terms of age, MF fat signal fraction, ES fat signal fraction, PM fat signal fraction, CDI between the MF and PM, and CDI between the ES and PM (P < 0.001) (Table 2).
 |
Table 1 Categorical Variables of NVCF and Non-NVCF Groups |
 |
Table 2 Continuous Variables of Patients in NVCF and Non-NVCF Group |
Risk Factors of NVCF
Subsequently, all potential risk factors (P < 0.2) derived from the univariate analysis were selected for further multivariate analysis, including sex, smoking status, presence of Kummell disease, age, BMD, cement volume, MF fat signal fraction, ES fat signal fraction, PM fat signal fraction, CDI between the MF and PM, and CDI between the ES and PM. In the multivariate binary logistic regression analysis (Table 3), we found that the ES fat signal fraction (OR = 1.064; P = 0.001), PM fat signal fraction (OR = 1.326; P < 0.001), and CDI between the MF and PM (OR = 1.048; P < 0.001) were independent risk factors for NVCF occurrence.
 |
Table 3 Multivariable Binary Logistic Regression of Predictors for NVCF |
Development and Validation of the Prediction Model
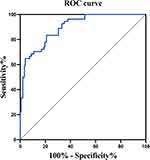
A nomogram was developed based on the results of the multivariate binary logistic regression analysis (Figure 3). The calculation method of the nomogram is described in the Figure 3 legend. By calculating the nomogram total points, we were able to predict the occurrence rate of NVCF. The C-index was 0.898 (95% CI: 0.854–0.943), indicating that the nomogram had good discrimination ability (Figure 4). Calibration curves and the Hosmer–Lemeshow test results (P = 0.756) indicated good concordance between the nomogram-predicted probability of NVCF and the actual NVCF rate (Figure 5).
Discussion
Paraspinal muscle degeneration is a complex and dynamic process with a profound negative impact on the spine. Over the past decades, studies have indicated that paraspinal muscle degeneration triggers changes in spinal structure, impairs spinal function, and is associated with various spinal diseases.6–14 The present study provides an extension to the established links between paraspinal muscle degeneration and spinal diseases. Our finding showed that the incidence of NVCF was 26.7%, in which paraspinal muscle atrophy and fat infiltration are associated with the occurrence and development of NVCF.
Muscle atrophy is a crucial feature of paraspinal muscle degeneration, which accelerates muscle aging and influences muscle function. In the current study, high CDI between the MF and PM was identified as an independent risk factor for NVCF occurrence. Specifically, heavier MF atrophy may be a causal element contributing to an increased risk of NVCF. In the paraspinal muscles, MF is the primary source of spine stability.23 It is located in the innermost portion of the spine and has the most extensive muscle attachment area, which can increase segmental tension and reduce the movement between segments, supporting the spine to maintain stability.24 Previous studies have demonstrated that the architectural design of MF, a large CSA with short muscle fiber length, acts as a stabilizer to buffer the spinal load effectively.10,25 These findings not only indicate that a decrease in attachment area, CSA, and fiber length caused by MF atrophy may result in spinal instability, but provide a potential mechanism for the occurrence of NVCF. As we previously highlighted, spinal instability suggests increased loads of the axial spine and higher relative movement between segments.23–25 Hence, the vertebrae may become less adaptive to perturbations (trauma, falling, even coughing or yawning) and are more vulnerable to fractures. However, this remains speculation and will require further biomechanical studies.
Another crucial feature of paraspinal muscle degeneration is muscle fat infiltration. In the present study, our results revealed that a high degree of fat infiltration in ES is an independent risk factor for NVCF. Like ropes on a mast, ES plays a prominent role in maintaining sagittal alignment.6 Recently, Masaki et al26 found that an increase in thoracic kyphosis is related to a decrease in the mass of ES. Berry et al7 suggested that a stronger ES can predict decreased lordosis, lumbosacral extension, and anterior pelvic tilt. It is known that muscle fat infiltration is closely associated with reduced muscle mass and muscle strength.27 Thus, the loss of muscle mass and muscle strength caused by ES fat infiltration may exist some degree of sagittal malalignment. Previous studies suggested that sagittal malalignment may lead to potentially higher compressive forces applied to the anterior column and consequent higher incidence of vertebral fractures.28,29 Although not assessed sagittal parameters in patients, it seems that ES fat infiltration can promote sagittal malalignment, which has adverse effects on load distribution, leading to increased risk for the occurrence of NVCF.
In addition to ES fat infiltration, spine function also appeared to be affected by PM fat infiltration. Our result showed that increased PM fat infiltration was an independent risk factor for NVCF. PM is the only paraspinal muscle that connects directly from the spine to the lower limbs and plays an important role in the maintenance of posture balance. Penning8 found that the opposite action of the PM on different spine regions maintains the spine in the upright posture. Subsequently, the same author indicated that PM should also be considered an active postural muscle during walking and running.9 It can be seen that regardless of static or dynamic activities, decreased PM mass and strength are likely to challenge the maintenance of posture balance, increasing the risk of imbalance. As a joint adverse event of imbalance, falling is also a well-known factor in fractures among older adults.30 Kim et al31 suggested that a weak PM should be used as a significant predictor of falls and hip fractures in osteoporotic patients. Statistically, falling accounts for 87% of all types of fractures in the elderly, and these fractures are virtually always due to low-impact injuries in osteoporotic bones.32 These previous findings support the conceptual hypothesis that patients with increased PM fat infiltration may be at greater risk of imbalance and falls, which may be a more direct negative effect on the spine. Thus, PM fat infiltration is the strongest predictor of NVCF compared with the other two risk factors.
We developed and validated a nomogram to predict the occurrence of NVCF in patients with OVCF treated with PKP. To our knowledge, our study is the first to develop a nomogram to predict the occurrence of NVCF after PKP based on paraspinal muscle radiological parameters. Although our nomogram comes with only three parameters, its predictive performance was good after evaluation using the calibration curves and C-index. Meanwhile, all parameters included in this nomogram are easy to measure or calculate and have good reproducibility. These results strongly support that our nomogram might enable accurately predicts the incidence of NVCF. Meanwhile, patients can be classified as low or high risk based on the mean incidence of NVCF (26.7%). For high-risk patients, surgery might not be a good choice because they may face NVCF time after time. In this context, our nomogram may contribute to reducing unnecessary medical interventions, thus maximizing the cost-effectiveness of treatments.
Another advantage of our nomogram is that it provides a more targeted intervention strategy for patients. We believe that risk factors make sense only if they can be changed through evidence-based interventions. In contrast to uncontrollable parameters, such as age or sex, both parameters can be altered by functional exercise. Hides et al33 found that specific stabilization training improved MF muscle atrophy. Notably, a randomized controlled trial of older adults found that strengthening exercise can significantly increase their 1.42–1.78% MF muscle CSA per week.34 In addition, studies have increasingly recognized that increased muscle fat infiltration is closely linked to inactivity.35 Physical activity, for example, resistance training or brisk walking, can effectively reverse muscle fat infiltration that occurs in older adults.36,37 Even in elderly individuals who cannot exercise, low-magnitude vibration has been proven to reduce intramuscular adipose tissue.38 Therefore, our nomogram provides a potential strategy to prevent the occurrence of NVCF and, at the same time, make functional exercises more targeted.
However, it should be noted that the odds ratios for paraspinal muscle degeneration seemed lower than other classic risk factors of NVCF reported in prior studies,5 which were almost near 1.0. There are two plausible explanations for this observation. (1) Based on our findings discussed above, we are inclined to believe that the negative effects imparted by paraspinal muscle degeneration may be slow and indirect. In other words, it may take a long time for the effects of degenerations in paraspinal muscle on NVCF to become apparent. The follow-up period of two years was not long enough to fully show the contribution of paraspinal muscle degeneration to NVCF. (2) If we examine the odds ratio for paraspinal muscle degeneration, then it is clear that these significant potential risk factors are of little clinical importance. However, by reference to the nomogram in our study, it becomes clear that the combined effect of these factors shows good accuracy and excellent discrimination in estimating the risk of NVCF. In fact, previous studies have reported that any type of movement is driven by the interaction between the groups of muscles with similar functions, namely muscle synergies.39 We speculated that the alterations in the structure and function of the spine are likely to be caused by the combination of degeneration in multiple paraspinal muscles, whereas single paraspinal muscle degeneration with a small impact on NVCF risk. Thus, it seems reasonable that the odds ratio for paraspinal muscle degeneration is small. However, caution should still be exercised, and larger studies of a longer duration are needed to confirm these findings.
The study has other limitations. First, although we built a nomogram based on paraspinal muscle MRI parameters, it did not involve intraoperative factor sufficiency and could not provide effective help for surgeons to make surgical decisions. We plan to perform this and create a more clinically meaningful model in the next step. Second, despite accounting for many important confounders, we cannot exclude residual confounding by unmeasured or unknown confounders. Finally, yet importantly, our nomogram was internally validated using bootstrap methods and achieved preferable accuracy. However, its actual effectiveness in clinical use is still unknown, and further studies are needed to externally validate the proposed nomogram.
Conclusion
In conclusion, our results confirmed that paraspinal muscle degeneration was a predictive factor for NVCF occurrence. We designed a nomogram to precisely predict the risk of NVCF. This predictive nomogram may help clinicians to make better clinical decisions and provide more accurate functional exercise protocol for OVCF patients.
Abbreviations
NVCF, new vertebral compression fractures; OVCF, osteoporotic vertebral compression fractures; CSA, cross-sectional area; FSF, fat signal fraction; C-index, concordance index; PKP, percutaneous kyphoplasty; MRI, magnetic resonance imaging; MF, multifidus; ES, erector spinae; PM, psoas major; ICCs, inter-class correlation coefficients; CDI, difference index of the cross-sectional area.
Data Sharing Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent for Publication
This study was approved by the ethics committees of the Beijing Chaoyang Hospital, with a waiver of informed consent because all data were abstracted retrospectively and anonymously without unique patient identifiers and no additional interventions were performed. (Ethic number, IRB: 2022-KE-15).
Acknowledgments
We would like to acknowledge all participants of this project and investigators for collecting data.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Beijing Municipal Science and Technology Commission (Z191100007619036, L192046).
Disclosure
All authors declare that they have no conflict of interest.
References
1. Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301(5):513–521. doi:10.1001/jama.2009.50
2. Melton LJ, Thamer M, Ray NF, et al. Fractures attributable to osteoporosis: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12(1):16–23. doi:10.1359/jbmr.1997.12.1.16
3. Belkoff SM, Mathis JM, Fenton DC, Scribner RM, Reiley ME, Talmadge K. An ex vivo biomechanical evaluation of an inflatable bone tamp used in the treatment of compression fracture. Spine. 2001;26(2):151–156. doi:10.1097/00007632-200101150-00008
4. Lee BG, Choi JH, Kim DY, Choi WR, Lee SG, Kang CN. Risk factors for newly developed osteoporotic vertebral compression fractures following treatment for osteoporotic vertebral compression fractures. Spine J. 2019;19(2):301–305. doi:10.1016/j.spinee.2018.06.347
5. Ma X, Xing D, Ma J, et al. Risk factors for new vertebral compression fractures after percutaneous vertebroplasty: qualitative evidence synthesized from a systematic review. Spine. 2013;38(12):E713–E722. doi:10.1097/BRS.0b013e31828cf15b
6. Rath M, Vette AH, Ramasubramaniam S, et al. Trunk stability enabled by noninvasive spinal electrical stimulation after spinal cord injury. J Neurotrauma. 2018;35(21):2540–2553. doi:10.1089/neu.2017.5584
7. Berry DB, Shahidi B, Rodriguez-Soto AE, Hughes-Austin JM, Kelly KR, Ward SR. Lumbar muscle structure predicts operational postures in active-duty marines. J Orthop Sports Phys Ther. 2018;48(8):613–621. doi:10.2519/jospt.2018.7865
8. Penning L. Psoas muscle and lumbar spine stability: a concept uniting existing controversies. Critical review and hypothesis. Eur Spine J. 2000;9(6):577–585. doi:10.1007/s005860000184
9. Penning L. Spine stabilization by psoas muscle during walking and running. Eur Spine J. 2002;11(1):89–90. doi:10.1007/s005860100336
10. Ignasiak D, Valenzuela W, Reyes M, Ferguson SJ. The effect of muscle ageing and sarcopenia on spinal segmental loads. Eur Spine J. 2018;27(10):2650–2659. doi:10.1007/s00586-018-5729-3
11. Stevens S, Agten A, Timmermans A, Vandenabeele F. Unilateral changes of the multifidus in persons with lumbar disc herniation: a systematic review and meta-analysis. Spine J. 2020;20(10):1573–1585. doi:10.1016/j.spinee.2020.04.007
12. Yagi M, Hosogane N, Watanabe K, Asazuma T, Matsumoto M. The paravertebral muscle and psoas for the maintenance of global spinal alignment in patient with degenerative lumbar scoliosis. Spine J. 2016;16(4):451–458. doi:10.1016/j.spinee.2015.07.001
13. Jiang J, Wang H, Wang L, et al. Multifidus degeneration, a new risk factor for lumbar spinal stenosis: a case-control study. World Neurosurg. 2017;99:226–231. doi:10.1016/j.wneu.2016.11.142
14. Fortin M, Gibbons LE, Videman T, Battie MC. Do variations in paraspinal muscle morphology and composition predict low back pain in men? Scand J Med Sci Sports. 2015;25(6):880–887. doi:10.1111/sms.12301
15. Ding Y, Liu B, Qiao H, et al. Can knee flexion contracture affect cervical alignment and neck tension? A prospective self-controlled pilot study. Spine J. 2020;20(2):251–260. doi:10.1016/j.spinee.2019.09.008
16. Maldague BE, Noel HM, Malghem JJ. The intravertebral vacuum cleft: a sign of ischemic vertebral collapse. Radiology. 1978;129(1):23–29. doi:10.1148/129.1.23
17. Crawford RJ, Volken T, Ni Mhuiris A, et al. Geography of lumbar paravertebral muscle fatty infiltration: the influence of demographics, low back pain, and disability. Spine. 2019;44(18):1294–1302. doi:10.1097/BRS.0000000000003060
18. Parkkola R, Rytokoski U, Kormano M. Magnetic resonance imaging of the discs and trunk muscles in patients with chronic low back pain and healthy control subjects. Spine. 1993;18(7):830–836. doi:10.1097/00007632-199306000-00004
19. Niemelainen R, Briand MM, Battie MC. Substantial asymmetry in paraspinal muscle cross-sectional area in healthy adults questions its value as a marker of low back pain and pathology. Spine. 2011;36(25):2152–2157. doi:10.1097/BRS.0b013e318204b05a
20. Kim H, Lee CK, Yeom JS, et al. Asymmetry of the cross-sectional area of paravertebral and psoas muscle in patients with degenerative scoliosis. Eur Spine J. 2013;22(6):1332–1338. doi:10.1007/s00586-013-2740-6
21. Halabi S, Small EJ, Kantoff PW, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21(7):1232–1237. doi:10.1200/JCO.2003.06.100
22. Kramer AA, Zimmerman JE. Assessing the calibration of mortality benchmarks in critical care: the Hosmer-Lemeshow test revisited. Crit Care Med. 2007;35(9):2052–2056. doi:10.1097/01.CCM.0000275267.64078.B0
23. Wilke HJ, Wolf S, Claes LE, Arand M, Wiesend A. Stability increase of the lumbar spine with different muscle groups. A biomechanical in vitro study. Spine. 1995;20(2):192–198. doi:10.1097/00007632-199501150-00011
24. Danneels LA, Vanderstraeten GG, Cambier DC, Witvrouw EE, De Cuyper HJ, Danneels L. CT imaging of trunk muscles in chronic low back pain patients and healthy control subjects. Eur Spine J. 2000;9(4):266–272. doi:10.1007/s005860000190
25. Ward SR, Kim CW, Eng CM, et al. Architectural analysis and intraoperative measurements demonstrate the unique design of the multifidus muscle for lumbar spine stability. J Bone Joint Surg Am. 2009;91(1):176–185. doi:10.2106/JBJS.G.01311
26. Masaki M, Ikezoe T, Fukumoto Y, et al. Association of sagittal spinal alignment with thickness and echo intensity of lumbar back muscles in middle-aged and elderly women. Arch Gerontol Geriatr. 2015;61(2):197–201. doi:10.1016/j.archger.2015.05.010
27. Hamrick MW, McGee-Lawrence ME, Frechette DM. Fatty infiltration of skeletal muscle: mechanisms and comparisons with bone marrow adiposity. Front Endocrinol. 2016;7:69. doi:10.3389/fendo.2016.00069
28. Briggs AM, Wrigley TV, van Dieen JH, et al. The effect of osteoporotic vertebral fracture on predicted spinal loads in vivo. Eur Spine J. 2006;15(12):1785–1795. doi:10.1007/s00586-006-0158-0
29. Dai J, Yu X, Huang S, et al. Relationship between sagittal spinal alignment and the incidence of vertebral fracture in menopausal women with osteoporosis: a multicenter longitudinal follow-up study. Eur Spine J. 2015;24(4):737–743. doi:10.1007/s00586-014-3637-8
30. Robinovitch SN, Feldman F, Yang Y, et al. Video capture of the circumstances of falls in elderly people residing in long-term care: an observational study. Lancet. 2013;381(9860):47–54. doi:10.1016/S0140-6736(12)61263-X
31. Kim KH, Lee JH, Lim EJ. Weak psoas and spine extensors potentially predispose to Hip fracture. Hip Int. 2021;31(3):430–434. doi:10.1177/1120700020904337
32. Ambrose AF, Cruz L, Falls PG. Fractures: a systematic approach to screening and prevention. Maturitas. 2015;82(1):85–93. doi:10.1016/j.maturitas.2015.06.035
33. Hides JA, Stanton WR, McMahon S, Sims K, Richardson CA. Effect of stabilization training on multifidus muscle cross-sectional area among young elite cricketers with low back pain. J Orthop Sports Phys Ther. 2008;38(3):101–108. doi:10.2519/jospt.2008.2658
34. Shahtahmassebi B, Hebert JJ, Hecimovich M, Fairchild TJ. Trunk exercise training improves muscle size, strength, and function in older adults: a randomized controlled trial. Scand J Med Sci Sports. 2019;29(7):980–991. doi:10.1111/sms.13415
35. Brioche T, Pagano AF, Py G, Chopard A. Muscle wasting and aging: experimental models, fatty infiltrations, and prevention. Mol Aspects Med. 2016;50:56–87. doi:10.1016/j.mam.2016.04.006
36. Goodpaster BH, Chomentowski P, Ward BK, et al. Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: a randomized controlled trial. J Appl Physiol. 2008;105(5):1498–1503. doi:10.1152/japplphysiol.90425.2008
37. Marcus RL, Addison O, Kidde JP, Dibble LE, Lastayo PC. Skeletal muscle fat infiltration: impact of age, inactivity, and exercise. J Nutr Health Aging. 2010;14(5):362–366. doi:10.1007/s12603-010-0081-2
38. Frechette DM, Krishnamoorthy D, Adler BJ, Chan ME, Rubin CT. Diminished satellite cells and elevated adipogenic gene expression in muscle as caused by ovariectomy are averted by low-magnitude mechanical signals. J Appl Physiol. 2015;119(1):27–36. doi:10.1152/japplphysiol.01020.2014
39. d’Avella A, Saltiel P, Bizzi E. Combinations of muscle synergies in the construction of a natural motor behavior. Nat Neurosci. 2003;6(3):300–308. doi:10.1038/nn1010
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.