Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Paediatric Pyodermatitis-Pyostomatitis Vegetans Without Underlying Inflammatory Bowel Disease: A Case Report of a 3-Year-Old African Girl
Received 27 July 2022
Accepted for publication 8 October 2022
Published 3 November 2022 Volume 2022:15 Pages 2363—2367
DOI https://doi.org/10.2147/CCID.S383926
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Frans Maruma, Harriet Makuru
Department of Dermatology, Faculty of Health Sciences, University of the Free State, Bloemfontein, South Africa
Correspondence: Frans Maruma, Department of Dermatology, Faculty of Health Sciences, University of the Free State, Bloemfontein, South Africa, Tel +2751 401 7504/9045, Email [email protected]; [email protected]
Abstract: Pyodermatitis-Pyostomatitis Vegetans (PD-PSV) remains a rare disorder of unknown etiology that is often associated with underlying inflammatory bowel disease (IBD). It is also worth noting that PD-PSV is particularly rare in the paediatric population with 15.4 years as the average age of onset in reported cases. Although adults and children with IBD may exhibit similar clinical features, children often manifest with additional features of failure to thrive and delayed puberty due to the background chronic inflammatory process. In this case report, we present a case of paediatric PD-PSV without underlying inflammatory bowel disease in a 3-year-old African girl. This case presents a unique opportunity of reminding clinicians that not all cases of paediatric PD-PSV may be associated with underlying IBD at presentation.
Keywords: paediatric pyodermatitis-pyostomatitis vegetans, azathioprine, Crohn’s disease, ulcerative colitis, case report
Introduction
Pyodermatitis-pyostomatitis vegetans (PD-PSV) is a chronic inflammatory disease affecting the skin and mucous membranes. The aetiology of PD-PSV remains unknown. Clinically, the initial skin lesions are papulopustular whereas the mucous membrane’s lesions are vesiculopustular. PD-PSV less commonly affects genital and groin areas in most of the reported cases. In addition, this condition is often associated with an underlying inflammatory bowel disease (IBD) namely Crohn’s disease (CD) and ulcerative colitis (UC).1,2
There is considerable evidence that the paediatric cases of IBD are on the rise with the peak incidence of IBD occurring in patients between the ages of 15 and 30 years.1–5 In patients below 20 years of age, CD constitutes nearly 20% of the cases, whereas 12% of them develop UC.5,6 Children with PD-PSV that is associated with IBD may exhibit similar clinical features but often they may manifest with additional features of failure to thrive and delayed puberty due to the background chronic inflammatory process. It is therefore crucial to rule out underlying IBD in all cases of PD-PSV.
In this case report, we present a case of paediatric PD-PSV without underlying inflammatory bowel disease in a 3-year-old African girl. This case presents a unique opportunity of reminding clinicians that not all cases of PD-PSV may be associated with underlying IBD at presentation.
Case Report
A 3-year-old girl presented with a 4-month history of skin lesions involving the scalp, oral cavity, eyes, neck and genital areas of her body. The child was neither acutely nor chronically ill, but she had obvious limb abnormalities (bilaterally absent forearms with distal split deformities) as well as history from a paediatrician who documented findings of an imperforate hymen with normal growth and development. The limb deformities could not be attributed to any genetic syndrome after consultation with medical genetics specialists and paediatricians. The medical genetics reported that the constellation of history, clinical, and laboratory findings were not indicative of a particular syndrome of note. Notably, the genetic testing service in our setting remains a scarce resource and thus, no specific genetic tests were performed.
A detailed examination of her integument revealed multiple well circumscribed, inflamed, vegetative plaques with surface erosions and pustulation on her scalp and neck areas. The lesions particularly demonstrated a characteristic border reminiscent of a snail-track appearance as illustrated in Figures 1–3.
 |
Figure 1 Lesions on scalp, before and after 8 weeks of azathioprine treatment. |
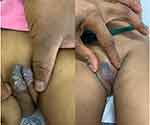 |
Figure 2 Genital vegetative plagues with yellowish pustules on surface before and after 8 weeks of azathioprine treatment. |
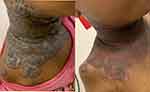 |
Figure 3 Before and after images of vegetative plagues with surface erosions as well as characteristic snail-track border. |
Evidently, another well-defined and symmetric vegetative plaque with numerous yellowish pustules on its surface mostly affecting the labia majora was present as depicted in Figure 2. Oral lesions were detected in the form of erosions and ulcerations. There were also whitish patches noted on the tongue and palatal areas. Additionally, prominent mucogingivitis, cobble-stoning and angular stomatitis were present. Ophthalmic manifestations were conjunctivitis accompanied by bilateral semi-purulent discharge. Vision was, however, unaffected. Of note, the anal mucosa with each subsequent visit remained clear.
A complete work-up was conducted to include an HIV test (negative), primary immunodeficiency screen (normal), complete blood count, thyroid (normal), liver and renal function tests, visceral radiology (normal), histopathology, and blood cultures (negative). Both upper and lower gastrointestinal scope (GI Scope) results were unremarkable. Except for pronounced eosinophilia of 21.5% (normal range 0–0.5%), hypoalbuminemia of 14g/L (normal range 29–42g/L), thrombocytosis of 826 × 109/L (normal range 180–440 × 109/L) and lymphocytosis of 20.41 × 109/L (normal range 6–18 × 109/L), no other abnormalities from the panel of all blood tests.
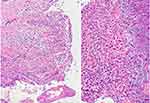
Histopathology specimens taken from scalp and neck lesions demonstrated epidermal hyperplasia with areas of ulceration, suprabasal acantholysis, marked eosinophilic infiltrate admixed with neutrophils in the papillary and superficial dermis and relatively spared deeper reticular dermis. See Figure 4. To rule out other autoimmune blistering dermatosis, a direct immunofluorescent test was done and the results were negative. With careful clinico-pathologic correlation, the above features were consistent with the diagnosis of PD-PSV. The characteristic clinical morphology of skin lesions (non-bullous, vegetative plaques with snail track border) were not consistent with the other bullous dermatosis that could very well make the diagnosis of PD-PSV prove to be difficult. Considering the negative direct immunofluorescence results, peripheral eosinophilia and careful clinico-pathologic correlation, a diagnosis of PD-PSV was made.
In light of this diagnosis, a short course of systemic corticosteroids (1mg/kg/day) was initially administered and tapered off within 3 months. Azathioprine (1mg/kg/day) was also initiated within 3 weeks of starting systemic corticosteroids and the patient responded exceptionally well to this treatment regimen with no considerable adverse effects. In our setting, there is no facility to perform thiopurine methyltransferase test in order to give an appropriate dosage of azathioprine. Therefore, we relied more on the complete blood count and normal liver function tests results in monitoring adverse drug events. The patient is currently well maintained on azathioprine, however, it is also worth noting that the mucocutaneous disease tends to flare up whenever the patient stops taking the azathioprine or miss follow-up date. During the periods of therapy interruption, the patient would also report symptoms of bloating and constipation We thus decided to continue with therapy uninterrupted and monitor the side-effects accordingly.
The GI scope evaluations as performed by paediatric surgeons were normal at baseline and again after 1 and 2 years of treatment. In order to watch out for the potential IBD, GI scopes were performed once a year concurrent with acute exacerbation of the mucocutaneous lesions along with gastrointestinal symptoms of bloating, loss of appetite and constipation as reported by the caregiver. The patient is currently well controlled and maintained on azathioprine, vitamins and mineral supplements.
Discussion
Pyodermatitis-pyostomatitis vegetans (PD-PSV) is a rare inflammatory mucocutaneous condition that is considered to be a highly specific marker for inflammatory bowel diseases (IBD) such as ulcerative colitis and less commonly, Crohn’s disease. Other diseases reported in association with PD-PSV include T-cell lymphoma, chronic myeloid leukaemia, liver diseases, malnutrition, HIV infection, and primary immunodeficiency disorders.1,3,5,6 The skin disease is pyodermatitis vegetans and the mucosal disease is pyostomatitis vegetans. The IBD usually precedes the development of the skin and/or mucous membranes manifestations by months or years. Sometimes, however, IBD is only diagnosed when it is looked for after the skin/mucous membrane diagnosis is made.7,8
The average age of PD-PSV in paediatric population was 15.4 years as reported in 2021 by Berzin et al.2 Four of the five cases reportedly occurred in the context of extra-intestinal manifestation of inflammatory bowel disease. Accordingly, the patients responded well to systemic corticosteroids alone or combined with either immunosuppressive therapy (azathioprine and methotrexate) and/or biologic agents such as adalimumab and infliximab. There are two factors that contribute to our patient’s uniqueness: the young age of onset (3 years) and the absence of IBD at presentation and at least two years thereafter. It is still not clear whether this patient would develop the IBD at a later stage in keeping with the average age of onset. We will keep the patient on maintenance treatment (azathioprine) and follow her up into her late adolescent age. At some stage, we may consider tapering off the maintenance medication but currently the child keeps getting flares each time they run out of azathioprine.
Conclusion
In summary, this case report presents clinicians with an opportune moment to recognise that not all paediatric patients with PD-PSV have an underlying IBD at presentation. Another interesting observation that was evident in this case is that the skin lesions suggested a proclivity for affecting the keratinising more than the non-keratinizing mucosal areas. The affectation of the oral and genital areas without any involvement of the anal area was also of peculiar note.
Consent Statement
Written informed consent was obtained from the patient’s legal guardian to have the case details and associated images published. Ethical clearance was obtained from the University of the Free State’s Health Science Research Ethics Committee.
Acknowledgments
The authors would like to thank Yashica, Thabiso and Thato for their technical and administrative contribution towards this paper.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gara S, Souissi A, Mokni M. Pyodermatitis pyostomatitis vegetans. JAMA Dermatol. 2020;156(3):335. doi:10.1001/jamadermatol.2019.3515
2. Berzin D, Lahad A, Weiss B, Barzilai A, Greenberger S. Inflammatory bowel disease presenting with pyodermatitis-pyostomatitis vegetans in a pediatric patient: a case report and review of the literature. Pediatr Dermatol. 2021;38(4):868–871. doi:10.1111/pde.14625
3. Mendeloff AI, Calkins BM. The epidemiology of idiopathic inflammatory bowel disease. In: Kirsner JB, Shorter RG, editors. Inflammatory Bowel Disease. Philadelphia: Lea & Febiger; 1988:3.
4. Johnston RD, Logan RF. What is the peak age for onset of IBD? Inflamm Bowel Dis. 2008;14(Suppl 2):S4. doi:10.1002/ibd.20545
5. Benchimol EI, Fortinsky KJ, Gozdyra P, et al. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis. 2011;17(1):423–439. doi:10.1002/ibd.21349
6. Müller KE, Lakatos PL, Arató A, et al. Incidence, Paris classification, and follow-up in a nationwide incident cohort of pediatric patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2013;57(5):576–582. doi:10.1097/MPG.0b013e31829f7d8c
7. Shazib MA, Byrd KM, Gulati AS. Diagnosis and management of oral extraintestinal manifestations of pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2022;74(1):7–12. doi:10.1097/MPG.0000000000003302
8. Galbraith SS, Drolet BA, Kugathasan S, Paller AS, Esterly NB. Asymptomatic inflammatory bowel disease presenting with mucocutaneous findings. Pediatrics. 2005;116(3):e439–e444. doi:10.1542/peds.2004-2281
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.