Back to Journals » Pathology and Laboratory Medicine International » Volume 14
Overcoming Infections Including COVID-19, by Maintaining Circulating 25(OH)D Concentrations Above 50 ng/mL
Authors Wimalawansa SJ
Received 22 July 2022
Accepted for publication 29 November 2022
Published 16 December 2022 Volume 2022:14 Pages 37—60
DOI https://doi.org/10.2147/PLMI.S373617
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Paul Zhang
Sunil J Wimalawansa
Department of Medicine, CardioMetabolic and Endocrine Institute, North Brunswick, NJ, USA
Correspondence: Sunil J Wimalawansa, Email [email protected]
Abstract: The elderly and those with underlying chronic diseases (i.e., comorbidities) such as pulmonary, cardiovascular, metabolic, and renal diseases, increase their susceptibility to sepsis, including COVID-19. The SARS-CoV-2 virus damages pulmonary cells, causing acute respiratory distress syndrome (ARDS) and hypoxia. It further damages endothelial cells, altering clotting mechanisums causing intravascular hemolysis, microvascular thrombosis, and micro-embolization, contributing to the risk of death. Approximately 75% of the immune system functions of humans depend on vitamin D and the availability of sufficient amounts of vitamin D metabolites [vitamin D and 25(OH)D] concentrations to enter immune cells from the bloodstream. Such concentrations are achievable through sun exposure, targeted food fortification programs, and adequate daily or weekly vitamin D supplements. That would allow for generating 1,25(OH)2D (non-hormonal form of calcitriol) intracellularly in peripheral target cells like immune cells. This enables immune cells’ physiological functions, including intracrine/autocrine and paracrine signaling processes. This initiates and maintains robust immune functions, such as forming antibodies and antimicrobial peptides, suppressing inflammation, and increasing the expression of anti-inflammatory and antioxidant genes, thus, strengthening immune functions. The opposite occurs in hypovitaminosis D, increasing vulnerability to infections and dying from it. Therefore, governments should make the population sufficient with immunoceuticals—micronutrients, especially vitamin D, and other micronutrients: the most cost-effective intervention to keep the population healthy. The cost of such interventions are minuscule compared to the expenses related to increased hospitalizations and premature deaths. Supposed such a program was implemented in mid-2020 as the author proposed, we estimated that 50% of hospitalizations (and the associated healthcare costs) and a third of deaths from COVID could have been prevented. Described herein are cost-effective strategies using vitamin D to achieve and sustain serum D3 and 25(OH)D concentrations crucial for maintaining a robust immune system, improving general health, minimizing disease severities and deaths, and reducing healthcare costs.
Keywords: angiotensin-converting enzyme, 1, 25(OH)2D, coronavirus, endocrine system, renin-angiotensin, hypovitaminosis D, innate immune system, SARS.CoV-2, vitamin D, Autocrine and paracrine, cost-effetive therpies
Introduction
The original SARS-CoV-2 virus and early variants, including the Delta, predominantly affected the lower respiratory tract.1 Whereas, its recent mutants, especially Omicron and its subvariants, are up to ten-fold more infectious than the original SARS-CoV-2 but less lethal.2 In addition, a a higher percentage of children are getting affected.3,4 However, unlike SARS-CoV-2, Omicron affects both the lower and upper airway,5 impacts more on children and young adults,4,6 and presents with less severe symptomatology even in those with co-morbid conditions.7 Through multiple gene mutations, Omicron variants have acquired sufficient changes of amino acids in Spike protein region, allowing immune evasion—resistance to COVID vaccines.8–10
Individuals over 70 with pre-existing chronic pulmonary, cardiac, or renal diseases, hypertension, diabetes, or obesity, and those immune-suppressed, have a higher vulnerability to developing symptomatic COVID-19, experiencing complications, and a higher risk of death.11,12 Most of those who develop complications have a weaker immune system, primarily due to chronic hypovitaminosis D. Characteristically, those who are vulnerable have low serum 25(OH)D and angiotensin-receptor coverting enzyme-2 (ACE-2) receptor concentrations.13 Vitamin D sufficiency rectifies the low concentrations of ACE-2.
Meanwhile, 20 to 40% of those who recover from COVID-19 develop longer-term complications.14,15 These manifest as diverse syndromes, predominantly related to the central nervous system,16 with a high likelihood of dormant viruses.17 This post-COVID syndrome, also known as long-COVID, affects multiple systems and is a chronic process.16 Evidence suggests that those with low circulating 25(OH)D have a higher risk of acquiring post-COVID syndrome.14,18
Progress in Managing COVID-19
Worldwide, people experienced varied COVID-19 pandemic-related situations, such as lockdowns and curfews in some countries to no restrictions in others, yet with similar clinical outcomes. Ironically, countries with the highest vaccine-booster uptakes like the USA, UK, and Isreal had higher hospitalization and death rates and outbreaks from SARS-CoV-2 than African countries with less than 10% vaccination. Populations were subjected to unprecedented contradictory advice from leading health organizations like the World Health Organization (WHO) and quasi-experts with conflicts of interest. Such confusion eroded people’s trust and confidence, and the consequent lack of compliance hampered the development of sound national policies and effective control of local outbreaks.
In mid-2020, despite uncertainties and unknowns, the United States government initiated by then president, Mr. Trump, a ten billion-dollar COVID-support program targeted free for larger pharmaceutical companies. This unique initiative of public funding subsidy, prioritized and kick-started the designing and manufacturing of COVID-19 vaccines.19 Several pharmaceutical companies leveraged this free-funding opportunity to develop COVID vaccines and antiviral agents against SARS-CoV-2, some of which effectively reduced the risks of severe complications and deaths from the original SARS-CoV-2 (https://www.nejm.org/coronavirus) but not for Omicron or the disease spread.
Meanwhile, the United States Food and Drug Administration (FDA) and regulatory bodies in other countries approved several diagnostic tests for SARS-CoV-2, such as polymerase chain reaction (PCR) and rapid antigen test (RAT) kits.20 However, many of these diagnostic tests had high false positivity and negativity, reducing their diagnostic reliability. While the combination of PCR and RAT tests has improved specificity, but their usefulness remains questionable.21
Despite these advances, controversies arose about ways to prevent the spread of the disease, including wearing facemasks. Meanwhile, the regulator’s refusal to approve early preventative and therapeutic options using repurposed, cost-effective therapies for COVID-19 (eg, ivermectin and vitamin D), prevented effective control of COVID-19. In addition, contradictory advice from the WHO, the Centers for Disease Control (CDC), and governments, enfosed prolonged and unnecessary lockdowns that compounded the failures of health authorities. In parallel, pharma’s refusal to share COVID vaccine technology and provide raw material to manufacture vaccines to impoverished developing countries from generating and accessing vaccines. This was likely led to increased hospitalizations and deaths in early 2021 in middle-income and developing countries and prolonge the pandemic. This perspective focuses on the use of vitamin D for prevention and as an adjunct therapy for SARS-CoV-2 infection, complications, and to overcome cytokine storms.
Bioavailability and the Role of Vitamin D
Vitamin D deficiency is widespread in most populations. However, there is a high prevalence of hypovitaminosis D among underprivileged ethnic minorities, especially in those with darker skin color who live in temperate countries, and residents in nursing homes, disability centers, and prisons.22–24 Multiple mechanisms related to benefts from vitamin D were reported to affect all body systems, especially the immune system. Considering the complexity, vitamin D deficiency should be defined as a convoluted clinical syndrome resulting from the “inability of circulatory D3 and 25(OH)D to enter target cells due to inadequate circulatory concentrations”, preventing its intended biological functions.
The higher the fat mass, the higher the likelihood of more sequestration of vitamin D and 25(OH)D and increased catabolism of inactive metabolites—vitamin D and 25(OH)D,25 resulting in a lower bioavailability of active component, 1,25(OH)2D.26 Chronic vitamin D deficiency contributes to or causes a variety of disorders. Examples include obesity, preventing weight loss, metabolic syndrome, autoimmune disease, and increased vulnerability to infections. Others have speculated reverse causation, as with infections like SARS-CoV-2, but there is no convincing evidence to support that.27,28
Oral Doses of Vitamin D to Raise Serum 25(OH)D Concentrations
The relationship between doses of vitamin D and the serum 25(OH)D concentrations achieved is curvilinear. Increasing doses lessen the cumulative increases of serum 25(OH)D concentration. Figure 1 illustrates the relationships between the dose of vitamin D and serum 25(OH)D concentrations achieved in several studies. It demonstrates that the higher the intake lesser the percentage increase of circulatory 25(OH)D concentrations, reflecting lesser absorption and increased catabolism. Whereas those with higher serum 25(OH)D concentrations gained proportionately smaller increases than at lower range (despite some sequestration, but lasting for a shorter period), making it safer to take higher doses.
 |
Figure 1 Illustrates the curvilinearity of the dose-response relationships between oral vitamin D and serum 25(OH)D concentrations in 22,215 healthy volunteers. It highlights the importance of not relying upon the administered vitamin D doses but on measured serum 25(OH)D concentration to make conclusions in clinical studies. A similar but exaggerated relationship exists between obesity (body weight/fat mss or BMI) and the serum 25(OHD concentrations achieved. Notes: Reproduced with modifications, from Ekwaru JP, Zwicker JD, Holick MF, Giovannucci E, Veugelers PJ. The importance of body weight for the dose response relationship of oral vitamin D supplementation and serum 25-hydroxyvitamin D in healthy volunteers. PLoS One. 2014. 9(11): p. e111265. Open Access29. |
The above illustrations do not necessarily apply to the lower and higher circulatory 25(OH)D concentrations. These are, however, confounded by the concentrations of vitamin D binding protein (i.e., bioavailability) and sequestration of circulating D3 and 25(OH)D into fat and muscle storage sites.27,30,31 Consequently, the doses needed to raise serum 25(OH)D concentrations are higher with conditions associated with increased vitamin D binding protein (VDBP), the rate of catabolism of vitamin D metabolites, interfering components in the circulation, and in overweight and obese.
In contrast to, as seen with iodine, iron, and calcium supplementations, due to many reasons, food-based and fortification strategies for vitamin D have not been successful in preventing vitamin D deficiency. Besides bioavailability issues of vitamin D, the doses recommended by governments are up to ten times less than that needed to achieve the intended goals. In the case of food fortification, it is necessary to use three to four times higher than recommended. Therefore, using the correct quantities of vitamin D3 and other immunoceuticals–micronutrients32 are necessary for fortifying dairy products, breakfast cereals, and other staple foods is a cost-effective strategy for preventing vitamin D micronutrient deficiencies.
Targeted supplementation or food fortifications are particularly relevant and effective for ethnic minorities with darker skin color, those in residential facilities, the elderly, and those with comorbidities.22,33 As mentioned, the currently recommended amounts for food fortification globally, are insufficient: these should be increased by two-to-four-fold to achieve meaningful population-based intakes for disease prevention and improved clinical outcomes. Tangible increases and maintenance of serum 25(OH)D concentrations as mentioned above will significantly benefit vulnerable populations.
The Importance of Vitamin D on the Immune System
Formation of Vitamin D Metabolites and Their Significance
Vitamin D is generated from 7-dehydrocholesterol following exposure to solar UVB rays in the skin.34,35 Skin-generated D3, dietary (D2 and D3), and supplemental vitamin D3 are primarily hydroxylated in mitochondria in the liver by the enzyme CYP2R1 to form 25(OH)D. Peripheral target cells, like immune cells, also have the 25-hydroxylase enzyme, which converts vitamin D to 25(OH)D. However, compared to the liver, the capacity is small.36 Most vitamin D and 25(OH)D in the circulation are bound to VDBP and transported to the liver, renal tubular cells, storage tissues, and peripheral target tissue cells. In these cells, the enzyme CYP27B1 further hydroxylates 25(OH)D to form its most active metabolite, calcitriol [1,25(OH)2D].36
In proximal renal tubular cells, parathyroid cells, fat and muscle cells, vitamin D, and 25(OH)D are transported via an active megalin-cubilin-based endocytosis system.37 This functional entry is essential for actively transporting both vitamin D and 25(OH)D from the circulation into fat, muscle, and renal tubular cells for either 1α-hydroxylation and/or for storage process. For example, renal tubular cells generate the hormonal form of calcitriol for its endocrine functions,38,39 and muscle and fat cells sequestrate for storage.37 The three most standard forms of vitamin D compounds and their circulatory concentrations are illustrated in Figure 2.
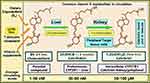 |
Figure 2 The molecular structures of three clinically relevant vitamin D metabolites, vitamin D, 25(OH)D, and 1.25(OH)2D, involved in the immune system, their circulatory half-lives, mean circulating concentrations, and respective cytochrome P450 (CYP) hydroxylating enzymes are illustrated. Most of the 25-hydroxylations of D2 and D3 occur in hepatocytes to form calcifediol (calcidiol). In contrast, calcitriol formed in the renal tubular cells and in peripheral target tissue cells as illustrated (note: serum concentration of calcitriol is about 900-fold less than calcifediol). Notes: Adapted from Wimalawansa, (S)J. Skeletal benefits, endocrine functions, and toxicity of vitamin D. J Endocrinol Diab, 2016. 3(3): p. 1–540. |
In contrast, many other peripheral target cells do not have an active transportation system. Since the concentration of hormonal form—circulatory calcitriol is in pmol [in contrast to 25(OH)D and D3 concentrations in nmol/l], thus, it is incapable of diffusing into peripheral target cells accross a concentration gradient. Therefore, targets like immune cells rely on the mentioned two precursor molecules—free D3 and 25(OH)D in a concentration-dependent diffusion from the circulation and endocytosis of VDBP-bound components41 (see the section on genomic effects of calcitriol for more details). Whereas the musculoskeletal, calcium/phosphate metabolism, and parathyroid functions depend on hormonal calcitriol synthesized in renal tubular cells and secreted into the circulation, in conjuction with magnesium.
The circulatory 25(OH)D concentration above 15 ng/mL was reported sufficient for entry into renal tubular and parathyroid cells for 1α-hydroxylation of 25(OH)D to form calcitriol.42,43 This is compatible with its hormonal functions on the musculoskeletal system.44 In contrast, peripheral target cells like immune cells require serum 25(OH)D concentrations over 50 ng/mL (also a similar concentration of D3) to enter immune cells to generate 1.25(OH)2D intracellularly for their autocrine and paracrine signaling.45–48
Therefore, a regular supply of vitamin D is necessary to maintain an adequate and stable circulatory D3 and serum 25(OH)D concentrations above 50 ng/mL. Transfer of these precursors from the circulation into immune cells is vital for intracrine/autocrine and paracrine signaling, which is necessary for robust immune functions.48–51 The circulatory half-life of vitamin D is about one day. However, following its hydroxylation into 25(OH)D, the half-life increased to 12 to 21 days, based on the individual’s vitamin D status. This is another scientific reason why a regular vitamin D supply is necessary to provide peripheral target cell activity with substrates.
Despite vast amounts of published data, many governments websites, such as NIH, NICE and SCAN, etc., and government-appointed bodies in UK and USA,42,52 continue to recommend outdated vitamin D doses, between 400 and 800 IU/day [such as, Vitamin D - Health Professional Fact Sheet (nih.gov), last updated on April 28, 2022] that are unhelpful to the public.53 These trivial doses fail to raise serum 25(OH)D concentrations meaningfully and have no tangible benefit on extra-renal targets like the immune cells and human physiological systems.
A 70 kg (non-obese), vitamin D sufficient or insufficient adult could raise their circulatory 25(OH)D to a steady above 50 ng/mL by consuming approximately 5000 IU/day (4000 to 7000 IU/day). However, in vitamin D deficiency, achieving this recommended therapeutic level would take a few months.48 The latter is necessary to elicit robust immune responses.48,54 Older adults, overweight and obese, those on anti-epileptic or retroviral therapies, or those with gastrointestinal absorption problems, require two to four times higher doses of vitamin D to maintain the mentioned 25(OH)D concentration.
In contrast to taking a few months to increase circulating 25(OH)D concentration, an upfront loading or a bolus dose administration of vitamin D can raise the circulatory 25(OH)D concentrations within three to four days.36,55 However, in acutely ill patients, as in ICUs, increasing circulating 25(OH)D could take more than a week. Therefore, administering vitamin D, even with high doses, is unlikely to benefit severely ill patients in less than a week.48 Because of this, such approaches should not be used in clinical studies or practice. Maintaining adequate serum D3 and/or 25(OH)D concentrations provide the basis to enhance immune cell activity and reduce the risks of infections, especially from viral respiratory illnesses like COVID-19.56
Sun Exposure Stimulates the Immune System
Casual sun exposure (or application of sunscreens before sun exposure) is insufficient to generate the daily requirement of vitamin D. On the other hand, daily sun exposure between 45 to 60 minutes with one-third of the skin surface open to direct summer-like sunlight, between 10.30 AM and 1.30 PM, can generate meaningful amounts of vitamin D: the quantity generated could vary between 2000 and 10,000 IU, dependent on the type of exposure, age, and the skin darkness—the melanin contents of the skin.
For some, exposure to the sun with 10-minute intervals is more practical. Times of the day outside mentioned above, early spring, late fall, and winter periods, the application of sunscreens prematurely, indoor sun exposure throug windoors, and for those with scarred skin (e.g., following burns) will not generate sufficient D3 to raise vitamin D and 25(OH)D in the circulation, necessary to contribute to better immunity.50,51
When one’s shadow is shorter than the height (a pratical guide) is the optimum time of the day for sun exposure. During this period, UVB rays reach the earth at an acute angle (zenith angle of sunrays), providing better skin penetration to generate vitamin D. The goal is to maintain individuals circulating 25(OH)D concentration above 50 ng/mL for better health, via sun exposure and/or vitamin D supplementation.36–39,48
During viral epidemics, as with the current COVID-19 pandemic, it is recommended that non-obese adults (60 to 70 kg) take vitamin D supplementation between 4000 and 7000 IU/day or 50,000 IU once a week (preferably every 10th day).50,51 While persons with bodyweight less than 60 kg could take lesser amounts (eg, <4000 IU daily, 25,000 IU once a week, or 50,000 IU once in two weeks), the obese and the elderly need two to four times more than the doses mentioned above. To raise circulatory D3 and 25(OH)D concentrations within a few days for disease prevention and to reduce complications, one could use upfront loading or a bolus dose of vitamin D3 between 100,000 and 400,000 IU.1,54,57
Genomic Effects of Calcitriol
Calcitriol circulates in the blood in a concentration approximately 900-time less than D3 and 25(OH)D (pmol vs nmol. Therefore, in the abscence of an energy-dependent mechanism, the concentration of circulatory calcitriol is too low (approximately 0.045 ng/mL) to enter immune cells. Therefore, hardly any hormonal form of calcitriol or orally administered calcitriol enters immune cells; consequently, there are no measurable effects on immunity. Besides its adverse effects from pharmacological doses of calcitriol, this is another reason why calcitriol should not administer as a therapeutic agent to overcome infections. The estimated intracellular concentration of calcitriol necessary for immune cells’ autocrine and paracrine signaling is approximately 1.0 ng/mL. This is more than 20 times higher than the average circulatory calcitriol concentration.58,59
Intracellular calcitriol interacts with its receptors and migrates into the nucleus. These calcitriol/receptor complexes regulate the transcription of over 1000 genes. These enhance the transcription and the release of anti-inflammatory and antioxidant cytokines and suppresses the expression of inflammatory cytokines.47,60–63 In addition, calcitriol initiates the synthesis of neutralizing antibodies and stabilizes epithelial and endothelial cells and gap-junctions, thus minimizing pathogen spread and fluid leakage into soft tissues.59,64–66
Most peripheral target tissue cell functions, including immune responses, depending on locally synthesized calcitriol (eg, within immune cells) for their biological activity. Longer-term maintenance of circulating 25(OH)D concentrations above 50 ng/mL allows a constent supply of sufficient concentration of D3 and calcifediol diffuse and endocytose into immune cells. This intracellularly generated calcitriol in target cells initiates intracrine/autocrine and paracrine signaling and related physiological functions.
As descrbed abive, most peripheral target cell-derived, end-organ-directed vitamin D functions are reliant on generating calcitriol intracellularly, as in the case with immune cells. Considering its broader benefits, lack of adverse effects, wide availability, and economical to use, healthcare workers should consider using vitamin D as a primary line of therapy for disease prevention and treatment and use it as an adjunct therapy to prevent infections/ sepsis—infections like COVID-19.49,54,67,68
Vitamin D deficiency has been associated with many adverse clinical outcomes, such as increased risks of septicemia, and COVID-19-associated complications and the severity of infections.69 Many studies have reported the importance of maintaining serum 25(OH)D concentration above 50 ng/mL, which is necessary to stimulate the immune system to overcome infections.47,63,70 Figure 3 ilustrates summary data by Borsche L, et al,70 from seven hospital studies. It showed a median baseline vitamin D3 level of 23.2 ng/mL (range, 14.5–30.9 ng/mL) at their hospital admissions. These data are plotted with clinical outcomes (mortality coefficients), after correcting patient characteristics and combining data points. In Figure 3, the regression line of combined data crossed the zero 25(OH)D concentrations, baseline approximately 50 ng/mL (range 45–55 ng/mL).
 |
Figure 3 Illustrates the Scatter plot and regressions of the individual and combined datasets. The data from seven hospital studies were plotted after correcting for patient characteristics. The correlation for the combined datasets intersects the axis at approximately 50 ng/mL, which suggests that this vitamin D3 blood level likely prevent excess mortality. Notes: Adapted from Borsche L, Glauner B, von Mendel J. COVID-19 mortality risk correlates inversely with vitamin D3 Status, and a mortality rate close to zero could theoretically be achieved at 50 ng/mL 25(OH)D3: results of a systematic review and meta-analysis. Nutrients 2021;13(10):3596. Open Access.70 |
These data are consistent with the circulatory vitamin D3 concentrations of unsupplemented traditional hunter-gatherers reported to be between 45–50 ng/mL.59,71,72 In Borsche L, et al study, mathematical regressions suggested that the minimum effective serum 25(OH)D concentrations is about 50 ng/mL (125 nmol/L).70 Taken together, this and other data59,71,72 suggest that when serum 25(OHD concentration is maintained above 50 ng/mL provides optimal immune protection against infections,47,48,63 which would save lives from pathogenic microbes.
The above conclusions are further supported by a larger veteran administration study evaluating approximately two million patients, with 351,999 COVID-related deaths through 2020. Those who were vitamin D supplemented had a significant 28% reduction in symptomatic COVID-19 and a 33% decrease in 30-day mortality from COVID-19, compared to controls.73 In this database, there were 343,094 cases and 14,981 known deaths from COVID, up to February 2021, with a Hazard Ratio (HR) = 0.67; 95% CI 0.59, 0.75. The authors estimated that proper doses of vitamin D would have reduced 69,000 cases and saved 4,900 lives of veterans from COVID-19.
As illustrated above, clinical studies overwhelmingly support the beneficial effects of vitamin D supplementation in reducing SARS-CoV-2 risk and preventing severe complications and deaths.74–76 As illustrated in Figures 2 and 3, a robust immune system secondary to having a sustained serum 25(OH)D concentration above 50 ng/mL (125 nmol/L) minimizes infections to background levels of around 2.5%.77
Immune Modulation by Calcitriol Generated Within Immune Cells
Vitamin D has profound, beneficial effects on the immune system’s activation and multiple mechanisms for stimulating the body’s immune defenses.49,78 Higher circulatory D and 25(OH)D concentrations are associated with better immune functions and lesser inflammatory and oxidative activities.79 These include the production of cathelicidin and defensins, which neutralize microbes, reduce microbial replication, prevent viruses from becoming attached to epithelial cells, and increase the rate of virus elimination.49,80 A summary of the effects and the calcitriol-driven pathways of CD4—derived Th-immune cells are illustrated in Figure 4.
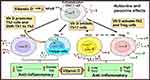 |
Figure 4 Illustrates the intracrine/autocrine pathways of immune modulation following calcitriol’s interactions with its receptors (CTR) in immune cells (eg, CD4+ T cells). Illustrates the effects of vitamin D (calcitriol) on immune cells, Th1, Th2, Th17, and Treg cells, and balances inflammation and anti-inflammatory in immune functions. The presence of adequate intracellular production of calcitriol suppresses the excess inflammatory reactions by immune cells while maintaining a healthy balance between the expression of inflammatory cytokines, such as IL-2, −8, −12, −17, −22, and TNFα, and the anti-inflammatory cytokines IL-1, −4, −5, −6, −10. Notes: Adapted from Bishop, (E) et al, Vitamin D and immune regulation: antibacterial, antiviral, anti-inflammatory.JBMR Plus. 2020;5(1):e10405. Open Access81. |
Figure 4 above illustrates that the Th1 (inflammatory) phenomenon further accentuates inflammation and autoantibody formation but promotes the lymphocytes’ switch to the Th2 and Treg cells (anti-inflammatory) phenotype in response to intracellular intracrine signaling by calcitriol. With compliments, it modulates cell behavior, enables control of inflammation, and enhances autocrine functions. COVID-19 prevents the transformation of pro-inflammatory Th1 lymphocytes and Th17 to the anti-inflammatory Th2 and Treg status, maintaining their inflammatory status.47,60,61,63 This also impairs the generation of protective antibodies by B-cells. In response to infections, the innate immune system releases cytokines to combat invading pathogens.
In contrast, maintaining circulatory 25(OH)D concentrations above 50 ng/mL facilitate favorable modulation of innate and adaptive immune responses. In addition, it reduces the expression of pro-inflammatory cytokines such as TNFα and IFNγ by Th1 cells, aiding the reduction of the hyper-immune status. Therefore, it is not surprising that vitamin D sufficiency reduces vaccination-associated adverse effects from viral Spike protein, autoimmune reactions, and increase vaccine’s efficacy. This is partly by preventing over-activations of the renin-angiotensin, controlling inflammation, and providing a better antibody response following vaccination.76,82
Vitamin D, Viruses, and the Respiratory System
Hypovitaminosis D impairs immunity and increases vulnerability, particularly to respiratory viral infections,54,68,83–86 and increases the severity of infections.87,88 The highest incidences and the severity of respiratory tract illnesses such as colds and influenza occur during winter when people’s serum 25(OH)D concentrations are the lowest.89–91 All coronaviral diseases also exacerbate during the winter with the need for hospitalization.92–94 Since the countries far from the equator have a higher prevalence of vitamin D deficiency, they have a higher incidence of winter-associated respiratory viral diseases (Figure 5).
Without effective UV rays, particularly in cold and dry climatic conditions, respiratory viruses remain infectious longer outside human bodies.56,95,96 Despite these, during viral epidemics, maintaining a serum 25(OH)D concentration in individuals and communities greater than 50 ng/mL45 would significantly reduce the risk of symptomatic infections, complications, and deaths.68,97,98 This strategy is highly cost-effective. Children and younger adults have stronger innate immunity and cell-mediated acquired immunity (ie, the second line of defense) than the elderly, making them less vulnerable to developing complications from viral infections, including SARS-CoV-2, unless they are severely vitamin D deficiency. Therefore, vaccination cannot be justified in healthy children and younger males because of unacceptably high rates of adverse effects but minimum benefits.
Younger children with vitamin D deficiency are at a higher risk for developing symptomatic colds, partly due to close social interaction in schools and childcare centers. Children with severe vitamin D deficiency are at high risk for developing life-threatening conditions like Kawasaki-like syndrome, multi-system inflammatory syndrome, and COVID-19.99
Associations Between Hypovitaminosis D and Known Risk Factors for COVID-19 Severity
Epidemiological, observational, and randomized controlled clinical studies (RCTs) have reported evidence of the protective effects of vitamin D against several common diseases, such as obesity, diabetes mellitus, asthma, and autoimmune diseases.100–103 Different mechanisms have been proposed to explain the associations between hypovitaminosis D and the above disorders.100,102
Common factors leading to vitamin D deficiency include insufficient exposure to sunlight due to predominant indoor work, sun avoidance behavior, impairment of the ability of the skin to generate vitamin D (eg, aging, scarred skin), decreased intestinal absorption of fat (impairing the absorption of fat soluble vitamins), impaired hydroxylation of D into 25(OH)D in the liver and to 1.25(OH)2D in renal tubular cells and peripheral target cells. Supporting the above data, many studies reported that vitamin D supplementation has beneficial effects in mentioned groups.101,102
Hypovitaminosis D Increases the Severity of Complications and Deaths
In most community-dwelling individuals, serum 25(OH)D concentrations range between 15 and 30 ng/mL;104,105 thus, they are less protected from viral infections. Per person basis, countries with a higher percentage of people with vitamin D deficiency have higher healthcare costs. Their risks can be mitigated cost-effectively by maintaining the population serum 25(OH)D concentrations above 50 ng/mL, to sustain robust systems. Individuals with the mentioned therapeutic circulatory 25(OH)D concentrations have reduced incidence, severity, and complications from COVID-19.50,106–108 Despite the availability of vast scientific literature, no government acknowledges or has implemented proactive approaches or issued guidelines on the above fundamentals to prevent infections and deaths from COVID-19 or other infections.
Therefore, in sepsis and acute infections like COVID-19, physicians need to take prompt, proactive actions to raise serum 25(OH)D concentration in their patients to activate their immune systems.76,109,110 Intracellularly generated calcitriol in immune cells interacts with its receptor, enters the nucleus, and interacts with genes to modulate genes. These genomic interactions enhance the expression of immune protective functions, such as generation of antimicrobial peptides and antibodies, stimulate immune cells to destroy microbes, and decrease cytokines that cause inflammation and oxidation. Figure 6 illustrates pathways that calcitriol initiates its intracrine/autocrine and paracrine signaling in immune cells that cause immune protective actions.
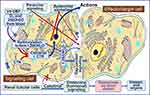 |
Figure 6 When vitamin D and 25(OH)D enter peripheral target cells, like immune cells, these compounds are hydroxylated into 1.25(OH)2D (calcitriol) via abundant intracellular CYP2R1 and CYP27B1 enzymes.111 Immune cells also have ample CYP27B1 to covert 25(OH)D to,25(OH)2D and synthesize calcitriol receptors (CTR). The calcitriol-CTR complex translocates into the nucleus to modulate over 1000 biologically crucial genes after the interaction of calcitriol with CTR. Some expressed genes include anti-inflammatory and oxidant cytokines, antimicrobial peptides, antibodies, etc. The figure illustrates simplified calcitriol-mediated mechanistic pathways, leading to immune cells’ autocrine and paracrine signaling processes. Note that the renal tubular cell-derived hormonal form of calcitriol (the last row) has little or no effect on immune cells. |
Prevention of Complications from Infections via Vitamin D
A cascade of events starts once calcitriol binds to its receptors (CTRs) in the cell cytosol. It migrates to the nucleus and combines with retinoid receptor RXR, where the complex interacts with target genes to modulate their expression and direct autocrine and paracrine signaling. In addition, the interactions of calcitriol/CTR complexes modulate the expression of specific cofactors and histones to expose upstream DNA response elements and up- or down-regulation of target genes.112 These interactions and co-factors need adequate intracellular concentrations of magnesium and other micronutrient—components. As described below, the signaling modulates the immune system with obligatory biological and physiological actions crucial to combat pathogens and prevent autoimmunity.46,47
Vitamin D Signaling Positively Modulates the Immune System
Immune modulation is initiated through the activation of the CTR pathway. Such activation down-regulates the expression of inflammatory cytokines and the oxidative stress pathways, while upregulating anti-inflammatory cytokines, activates immune cells—T and B cells, the macrophage, and dendritic cells—and enhances the production of antimicrobial peptides, facilitating infection control.113
Low pre-pandemic (i.e., prior to getting infected) vitamin D status is inversely related to increased risks of COVID-19 and its severity and complications79,114 and other manifestations and associations between vitamin D status and COVID-19. Low serum 25(OH)D concentration on hospital admission could predict the need for intensive care use and correlate with higher COVID-related complications and deaths.61,79,115–117 In contrast, higher serum 25(OH)D concentrations lessen the severity of SARS-CoV-2 infections and reduce deaths by several folds (Figure 7).118
 |
Figure 7 Post-estimation simulation of 25(OH)D concentrations using 15 and 50 ng/mL as the cut-offs predict an excess of four-fold higher mortality from COVID-19. Data were adjusted for age, sex, BMI, C-reactive protein, D-dimer, oxygen saturation, and chronic diseases, such as type 2 diabetes and chronic kidney disease. Notes: Adapted from Vanegas-Cedillo PE, Bello-Chavolla OY, Ramírez-Pedraza N et al. Serum vitamin D levels are associated with Iincreased COVID-19 severity and mortality independent of whole-body and visceral adiposity. Front Nutr. 2022;9:813,485118. |
Not only does vitamin D improve immune functions,119 but it also substantially reduces the risk of viral and bacterial infections118,120,121 and the risk of complications and deaths from COVID-19.118 Natural immunity and the protection derived from immunization could substantially reduce the viral spread and complications from COVID-19 and possibly could have eradicated the disease. However, no health administration used this low-cost strategy to overcome the pandemic. Despite the solid evidence demonstrating that vitamin D fulfiled the Bradford Hill criteria for causality, leading health authorities and governments continue to marginalize early therapies like vitamin D and ivermectin.
Studies reported that those who are PCR-positive for SARS-CoV-2 had a higher prevalence of severe hypovitaminosis D (i.e., below 12 ng/mL; p=0.004) compared with those with serum 25(OH)D concentrations higher than 24 ng/mL.122 Besides, the group with severe vitamin D deficiency had higher rates of complications and deaths.79 Severe vitamin D deficiency is also associated with hyperinflammatory, hyper-reactive immune statuses123 and cytokine storms,124,125 causing acute respiratory distress syndrome (ARDS). It also increases the incidence and severity of autoimmune diseases.126–128 While vitamin D3 does not prevent a person from contracting COVID-19, it will significantly reduce symptomatic disease and complications,129 and reduce deaths by 14-fold,79 and the development of post-COVID syndrome.
Endothelial and Epithelial Barriers
In addition to increasing the expression of the antimicrobial peptides and promoting anti-infective functions of white blood cells and natural killer cells,130 calcitriol stabilizes tight cell junctions in respiratory epithelia and vascular endothelia through non-canonical pathways. Vitamin D3 improves epithelial and endothelial stability and reduces membrane porosity and vascular leak, independent of the calcitriol/CTR genomic path.131 These actions prevent fluid leakage, viral entry, and dissemination132,133 and enhance macrophage-mediated destruction of microbes.66
Besides, vitamin D deficiency impairs endothelial barriers leading to vascular fluid leakage into soft tissues and worsening infection.131 Similarly, the weakening of gap junctions and epithelial barriers, especially in the lung and blood-brain barrier, leads to the infiltration of viruses and the propagation of microbes.134 These non-transcriptional mechanisms improve controlling infections, reduce inflammation, and prevent endothelial cells and epithelial cell damage-related embolization.
Mechanisms by Which Vitamin D Protects Cells from COVID-19
Receptors for angiotensin-converting enzyme-2 (ACE-2) and CD209L are expressed in most human tissue, inlcuding endothelial cells, pulmonary epithelial cells, the gastrointestinal tract, the brain, adipose tissue, the kidney, and the liver.135,136 A higher concentration of ACE-2 receptors and CD209L is expressed in endothelial cells and pulmonary epithelial cells, making them a potential target for coronaviral entry. Replication of the SARS-CoV-2 virus damages endothelial cells, primarily via Spike proteins, causing microvascular thrombosis, myocarditis, and embolization137 in COVID-19.138,139
Similar adverse outcomes have been reported following vaccines due to Spike protein-related adverse interactions.140,141 In either case, these can cause epithelial cell and lung damage, altering clotting mechanisms, impairing gas exchange, and causing hypoxia, viremia, effusion, pneumonia, and death. Therefore, stabilizing endothelial and epithelial cells should be investigated as a therapeutic target for preventing complications from SARS-CoV-2, in conjunction with vitamin D and magnesium adequacy.142
Once SARS-CoV-2 enters human cells,143 it downregulates ACE-2 receptors. Consequently, reduce the catabolism and hence accumulation of vasoconstrictor peptide, angiotensin-II.137 The build-up of angiotensin-II in the circulation further stimulates the expression and release of inflammatory cytokines, increasing inflammation and oxidative stress, and cytokine storms.144 Besides, excess angiotensin-II opposes the vasodilatory effects of angiotensin(1–7), thus, increasing intravascular pressure causing pulmonary hypertension and edema, and increasing the risk of developing cytokine storm and ARDS.
Sufficient Vitamin D Doses for Improved Clinical Endpoints
Calcitriol is also involved in other regulatory and physiologic homeostatic activities. This depends on the adequate availability of D3 and 25(OH)D steady concentrations in circulation.46,47,70,128 Most importantly, intracellularly generated calcitriol in immune cells, through their intracrine mechanisms, reduces the risks of cytokine storms and acute viral-mediated lung injury in persons with COVID-19.145,146
Population-wide vitamin D supplementation could prevent generalized inflammation and symptomatic disease, including cytokine storm,138,139 and significanlty lessen the need for hospitalization. It reduces the severity and deaths from SARS-CoV-2 by more than 50%.147 Therefore, considering the minimal costs and wide availability, lack of adverse effects at the recommended doses, and vitamin D intervention in the community and hospital admissions is a highly cost-effective.51
Since hypovitaminosis D significantly increases the risks of COVID-19-related complications, it is logical to raise individuals and the population’s serum 25(OH)D concentration to greater than 50 ng/mL. This can be achieved through adequate daily sun exposure and vitamin D supplementation. This level of vitamin D sufficiency will maintain a robust immune system, reduce the severity of infections, and improve associated health conditions.1,76,148
Vitamin D on Renin-Angiotensin-Aldosterone Hormonal (RAS) Axis
Activating the renin-angiotensin-aldosterone axis (RAS) (especially production of renin and ACE1), leads to excess production of angiotensin-II, enhances the production of cytokines, worsens morbidity and mortality associated with sepsis-induced lung damage.143,149 The actions related to RAS are intimately involved with the immune system. Excess angiotensin-II enhances inflammation and oxidative stress. In contrast, sufficient vitamin D suppresses RAS, increases ACE-2 enzyme and reduces angiotensin-II; thus, it reduces inflammation and oxidative stress and improves clinical outcomes from infections.
Coronaviral infections independently increase RAS activity that further propagates SARS-CoV-2 infection and survival in the body. Calcitriol regulates the RAS activity primarily by suppressing the expression of renin, the rate-limiting step of synthesis of angiotensin-II, working through cyclic AMP (cAMP)-dependent PKA signaling. Calcitriol also increases ACE-2 and, therefore, angiotensin(1–7), reduces angiotensin-II concentration, and blocks the formation of the CRE-CREB-CBP complex.150 These actions keep the RAS activity under control. In comparison, increased RAS activity in vitamin D deficiency, worsens complications and deaths from coronaviruses like SARS-CoV-2.151 In summary hypovitaminosis D negatively affects the RAS system, leading to complications, need for hospitalization, and increased risks of death.81
Hypovitaminosis D and low ACE-2 concentrations significantly increase symptomatic SARS-CoV-2-infections and synergistically increase vulnerability, severe complications, and higher mortality.79,147,152 Coronaviral infections reduces ACE-2 concentration indirectly (as a viruses’ survival mechanism) via reducing the synthesis of ACE-2 and directly by utilization of ACE-2 in circulation. Prevention of catabolism of Angiotensin-II due to low ACE-2 enzyme creates multiple adverse effects. Therefore, via several mechanisms, vitamin D defiiency increases the vulnerability to SARS-CoV-2, its complications, and death. Whether higher concentrations of angiotensin II modulate, the cell-membrane-bound ACE-2 concentrations are unclear.
Complementary Immune Mechanisms
Low circulating D3 and 25(OH)D cause a deficiency of the substrate, for the intracellular generation of calcitriol within immune cells. This impairs intracrine and paracrine signaling, thus, weakening the immune system.153,154 The latter leads to overt pro-inflammatory responses, as seen in cytokine storms in COVID-19 and other severe infections. Children with serum 25(OH)D concentrations less than 10 ng/mL (i.e., severe vitamin D deficiency) infected with SARS-CoV-2, through similar mechanisms, causes Kawasaki-like disease and/or multi-system inflammatory syndrome that is associated with high mortality.155–157
The presence of adequate D3 and 25(OH)D in the circulation enables peripheral target cells to increase the synthesis of calcitriol and the expression of 1α-hydroxylase (CYP27B1) and 25-hydroxylase (CYP2R1) enzymes and the CTR (also known as VDR). This creates a conducive setting to synthesize both calcitriol and CTR within these cells. In contrast, vitamin D deficiency reduces the intracellular generation of calcitriol and CTR in immune cells, thus disrupting vital autocrine and paracrine signaling and weakening the innate and adaptive immune responses.
COVID Complications are Enhanced by Weakened Immunity
Weakened adaptive immune responses reduce the generation of antibodies and the effectiveness of memory cells and macrophages and impair the cytotoxic action of immune/killer cells against viruses. Consequently, exaggerated adverse responses could occur in hypovitaminosis D, following SARS-CoV-2 infection-associated over-activity of RAS and following COVID-immunization. This scenario diminishes the ability to form neutralizing antibodies and impairs the adaptive immune response. Consequently, adverse events are intensified in those with severe vitamin D deficiency following SARS-CoV-2 infection and immunization, including overt inflammation, autoimmune reactions, and pathological oxidative stress leading to systemic complications.
In addition, immune dysregulation further exacerbates the expression of pro-inflammatory cytokines and autoimmune reactions, host cell destruction, and vulnerability to hyper-inflammatory syndromes. This vicious cycle damages pulmonary epithelial cells causing hypoxia, fluid extravasation, pneumonia, and endothelial damage leading to coagulopathies, thrombocytopenia, and micro-embolisms.158 These data illustrate the importance of vitamin D adequacy for preventing infection-related complications.
The deaths caused by SARS-CoV-2 are attributed to, among others, cytokine-driven storms causing ARDS,135,159,160 primarily occurring in those with severe vitamin D deficiency (< 10 ng/mL). The latter initiates a vicious cycle, RAS-driven large quantities of angiotensin-II and expressing excessive amounts of inflammatory cytokines, causing tissue damage.135,136 Such incidences and complications are highest among those with severe vitamin D deficiency.147
The widespread inflammation and oxidative stress injure the pulmonary epithelial and vascular endothelial cells and their basement membranes. The endothelial abnormalities lead to micro-thrombosis and embolization, intravascular thrombosis, and dissemination of viruses and fluid leakage into soft tissues, including the lungs and brain.138,139 Figure 8 illustrates broader pathways causing a vicious cycle by SARS-CoV-2 infection, and the sites and locations where vitamin D is likely to have favorable immune mechanisms to overcome infections like COVID-19.81 8B illustrates the site of impacts from active vitamin D (calcitriol) in preventing and minimizing complications and adverse clinical outcomes from SARS-CoV-2.
Correlation Between Vitamin D Status and COVID-19 Clinical Outcomes
The age-specific serum 25(OH)D concentrations in persons with COVID-19 in Italy, Spain, and France have revealed that severe complications and higher deaths were associated with those with severe vitamin D deficiency (<12 ng/mL).79 The highest percentage ofdeaths occured in those with the lowest serum 25(OH)D concentration (e.g., less than 10 ng/mL), especially among those who are older than 70 years) with comorbidities. This groups had the highest number of severe complications and death.57,161
Systematic reviews and meta-analyses have reported an inverse relationship between serum 25(OH)D concentrations (also supplemented vitamin D doses) and rates of symptomatic SARS-CoV-2 infections, severity, and mortality.101,102,162 D’Ecclesiis et al163 and other researchers concluded that vitamin D supplementation reduces the risk of severity and mortality by more than 50%. Larger daily doses and hence higher serum 25(OH)D concentrations are significantly associated with better clinical outcomes from SARS-CoV-2 infection:101,102,164,165 they could have been protected with vitamin D supplementation, as also illustrated in Figure 9.
 |
Figure 9 Forest plot of risk estimates of severity for low vs high vitamin D levels. The association between serum 25(OH)D concentration and the severity of COVID-19 were evaluated regarding ICU admission (ventilated or intubated). Serum 25(OH)D was measured on admission to the hospital. Data demonstrated, on average, doubling of the risk of severity in subjects with hypovitaminosis D (SRR = 2.38, 95% CI: 1.53–3.70). Notes: Adapted from D’Ecclesiis O, Gavioli C, Martinoli C et al. Vitamin D and SARS-CoV2 infection, severity and mortality: A systematic review and meta-analysis.PLoS One. 2022; 17(7): e0268396. Open Access163. |
Figure 9 is one of four graphs from D’Ecclesiis et al, which demonstrated that subjects with low serum 25(OH)D concentrations had doubled the risk of SARS-CoV-2 infection, higher severity and mortality from COVID-19, compared to a group with higher vitamin D, as reported previously by others.67,166–168
COVID-19 is a Pandemic of Hypovitaminosis D
COVID-19 has become a pandemic among those with severe vitamin D deficiency, which causes the weakening of the immune systems, rendering them susceptible to symptomatic disease, complications, and death. This is also likely to be the case with other infectious epidemics. In these situations, data supports maintaining serum 25(OH)D concentrations above 50 ng/mL (125 nmol/L), significantly reducing infections and complications from viral disease, especially from respiratory viruses.97,169 Beneficial effects from vitamin D supplementation are most visible in those with severe hypovitaminosis D.170 No additional benefits are expected in those who are vitamin D sufficient.
In addition to sun exposure and vitamin D supplementation, food fortification programs are economical and practical for rectifying micronutrient efficiencies in specific groups and communities.32 It is particularly cost-effective when such measures target deficient populations and ethnic minority groups. Examples of similar successful targeted nutrient approaches include iodine supplementation to prevent goiters and folic acid and iron supplements during pregnancy.
The addition of other immunoceuticals–micronutrient supplementation, such as vitamins A, C, and K2, magnesium, zinc, and selenium, essential fatty acids (eg, omega-3), nitric oxide donors, and resveratrol are likely to have a significant impact on the population, aiding to maintain a more robust immune system. Such cost-effective strategies would lead to better health, fewer absentees, higher productivity, and lesser healthcare cost.171,172
Why is It Necessary to Target Serum 25(OH)D Concentration Above 50 ng/mL?
Good public health policies aim to minimize diseases, their complications, and the spread, cost-effectively. However, during the COVID pandemic, some of these principles were ignored by leading health authorities and governments, which led to chaos. Those who develop symptomatic disease and complications and die from infections have feeble immune systems. Therefore, maintaining a robust immune system is not only essential to protect the population during infectious pandemics like SARS-CoV-2 but also the most cost-effective way to control it.
Convincing evidence has been published that rapidly raising and maintaining vitamin D and/or serum 25(OH)D concentrations above the minimum required level of 50 ng/mL (125 nmol/L) would minimize infections-related adverse clinical outcomes.46–48 Therefore, in such situations, in addition to preventing disease spread (eg, wearing effective face masks and social distancing), a broader goal should be to maintain mentioned circulatory 25(OH)D concentration in the population that would significantly improve clinical outcomes, including fewer hospitalizations and deaths, while mimizing healthcare costs.50
If the goal is to achieve a population minimum serum 25(OH)D concentration of “40” ng/mL, 60% of people will be below the required serum 25(OH)D concentration of 50 ng/mL. Whereas, if the targeted minimum concentration is set for 30 ng/mL, more than 80% of the population will have serum 25(OH)D concentration below 50 ng/mL, due to the scatter of representation in the community. Consequently, such approaches are ineffective and unwise especially during infectious epidemics and pandemics. It would fail to maintain a robust immunity in the population that needs to overcome infections. This is a crucial reason for the community spread of SARS-CoV-2, its, severe complications and deaths from the current COVID pandemic.
Therefore, keeping individuals or the populations’ serum 25(OH)D concentration below 50 ng/mL as recommended by some as the lower limit, is unwise and undesirable.48 During infectious epidemics or pandemics, there is no scientific justification for maintaining minimum serum 25(OH)D concentrations at 20, 30, or even 40 ng/mL, suggested as precautionary (and theoretical) principles by some. It would lead to serum 25(OH)D concentration of over two thirds of the population under the minimum necessary level of 50 ng/mL—disadvantage them by contracting infectious pathogens—both bacteria and viruses—therefore, it is counterproductive. Such a policy would enhance the spread of the viral illness, increase complications, hospitalizations, deaths, and associated costs.
Supporting Scientific Data
Three publications have addressed the issues of how to raise and maintain the needed physiological circulatory concentrations of 25(OH)D. Veugelers et al29,173 and Huang (2021).174 While the former recommended using body-weight-based dose calculations, the latter used a physiological-based pharmacokinetic model using baseline 25(OH)D concentration levels to obtain the desired dose of vitamin D.
The recent dose recommendations pulished (July 2022 in Nutrients) used practical clinical aspects, multiple data sets, and over two decades of extensive clinical experience and provided both bodyweight/BMI-based and serum 25(OH)D concentrations-based recommendations of appropriate vitamin D and calcifediol doses for children and adults.48 These studies conclusively illustrated that the currently recommended vitamin D dosage of 400 to 800 IU/day for adults by most governments and some appointed bodies is grossly inadequate to raise serum 25(OH)D concentration to therapeutic levels and worthless for counteracting infections.29,173–175
However, minimizing too-low and too-high circulatory 25(OH)D concentrations is necessary. Regarding this, Veugelers et al173 have examined doses of 1885, 2802, and 6235 IU per day administered to those with average body mass index (BMI) and overweight and obese individuals (maintained between 23 and 68 ng/mL)-(defined as 58 to 171 nmol/L). They concluded that the standard recommended RDA is undesirable and recommended body-weight-based doses of optimal intakes.29,173
Physiological studies with ED50 confirmed that both the baseline concentration, the daily or weekly oral doses, and the frequency of administration, influence the achievement of circulatory 25(OH)D concentration plateaus. They investigated 400 IU (10 μg) up to 50,000 IU (1250 μg) doses/day for 2 to 100 weeks of administration. Figure 10 illustrates the increase in serum 25(OH)D concentration vs baseline concentrations at each dose. A normalized increase in serum 25(OH)D was plotted against 25(OH)D baseline levels.29,173
 |
Figure 10 Illustrates mean ± SD and 95% (of 2.5th and 97.5 th percentiles are prepsented) prediction interval of serum 25(OH)D concentrations following vitamin D supplementation. The vertical axes provide serum 25(OH)D concentrations in nmol/L (left side) and ng/mL (right side). As the red arrow indicates, with a 5000 IU/day dose, serum 25(OH)D concentrations achieved were between 38 and 68 ng/mL, with a mean of 53 ng/mL with longer-term administration. The equation for The mean response for healthy individuals reported as Y = 51.9 + 17.7 *(1 - e-7.4*X) + 6.3 *X, in which Y denotes serum 25(OH)D (in nmol/L) and X, vitamin D supplementation (in 1000 IUs). Notes: Adapted from: Veugelers, PJ, Pham TM, Ekwaru JP. Optimal vitamin D supplementation doses that minimize the risk for both low and high serum 25-hydroxyvitamin D concentrations in the general population. Nutrients. 2015;7(12): 10,189–10,208173. |
These data confirmed that for a 70 kg person consuming an average dose of 7000 IU/day, 97% maintain serum 25(OH)D concentration above 30 ng/mL, but 30% would be less than 50 ng/mL. Similarly, with a dose of 25,000 IU per week, only 50% of them would reach a serum 25(OH)D concentration above 50 ng/mL. In both scenarios, a significant proportion will have insufficient circulatory 25(OH)D concentration needed to overcome infections.29 Figure 10 also illustrates the non-linearity of serum 25(OH)D concentrations in response to oral vitamin D doses and different plateau levels reached after different vitamin D doses in an average person.
Limitations of Systematic Reviews and Meta-Analyses
Several systematic reviews and meta-analyses have been published regarding vitamin D and COVID-19, with variable, non-confirmatory conclusions. Most RCTs and other clinical studies included in recent meta-analyses had significant methodological errors: poorly designed RCTs, study selection biases, and too fewer number of studies. Others have failed to address confounding variables, had inadequate sample sizes, and dramatic study group imbalances.
Because of poor-quality data from published studies and the heterogeneity of clinical studies included, outcomes and conclusions are consdered unreliable. For example, many RCTs failed to measure the baseline characteristics, especially serum 25(OH)D concentrations, the number of days from the onset of the clinical symptoms, have not included hard endpoints, severity between the interventional and control groups, etc.176 Therefore, until data from adequate well-controlled RCTs are available, it is irrational to continue to republish meta-analyses on this subject.
Discussion
There are specific groups with a higher prevalence of vitamin D deficiency. These categories include (A) elderly with comorbidities, (B) over-weight, obese, or taking medications that enhance vitamin D catabolism, (C) darker skin persons living in temperate climatic, northern and southern hemispheres (D) institutionalized persons (e.g., prisons, nursing homes, disability centers), submarine crew members, and regular night shift workers, and (E) those who avoid sun exposure.
Consequently, they have a weaker immune system, thus, a higher risk of developing symptomatic diseases, severe complications, and death from infections like COVID-19. People belonging to these groups and those with chronic metabolic disorders such as diabetes, obesity, metabolic syndrome, chronic pulmonary hypertension, and cardiovascular and renal diseases have low serum 25(OH)D and low ACE-2 concentrations. Consequently, compared to the general public, they are at a higher risk of contracting infections, including COVID-19, and developing complications.
After excluding confounders, data from the past two years of COVID confirmed that death rates from COVID-19 of blacks, Asians, and ethnic minorities (BAEM)—those with darker skin color living in northern latitudes—were significantly higher than whites.177 Socioeconomic factors, such as disparities in access to healthcare and economic status, play a role in the reported statistics related to COVID-19, but to a lesser extent than the prevelance of hypovitaminosis D.
During the COVID pandemic, lockdowns and curfews, reduced sun exposure, insufficient intake of nutritious food, and increased sedentary lifestyles led to an increased prevalence of malnutrition and hypovitaminosis D: further weakening the immune systems.48,178 These collectively contributed to the increased prevalence of hypovitaminosis D and vulnerability to infection, complications, and deaths.1,50
The relationships between serum 25(OH)D) concentrations and clinical outcomes from COVID-19 mentioned above fulfilled Bradford Hill’s criteria for causation, thus, no further RCTs are needed. Vitamin D deficiency predictably increased SARS-CoV-2-associated complications and deaths because of its adverse effects on the immune system.147 Vitamin D supplementation breaks this vicious cycle through immune-mediated mechanisms, including suppressing inflammation, oxidative stress, and cytokine storm, as described above.160,162,179 The link between severe vitamin D deficiency and COVID-19 complications is robust with a high effect size, including increased risks for cytokine storm, ARDS, needing ICU admission, assisted ventilation, and death.180
Besides, hypovitaminosis D increases the risk of ARDS, lung damage, vascular abnormalities, blood clotting, and death.135,136 Vitamin D stabilizes epithelial cells and cell gap junctions, especially in the lungs and vascular system, preventing fluid leakage into soft tissues and disseminating viruses. Moreover, it reduces microbial entry from the blood into tissues and the extracellular space, preventing viral spread, the occurrence of pulmonary edema, effusions, pneumonia, ARDS, and post-COVID syndrome.138,159
In 2020/21, due to systematic failures—conflicting guidance and misleading policies by leading global health authorities, harmful lockdowns and enforcement of unjustifiable vaccine-mandates and worthless vaccine-passports by autocratic governments—that ignored early cost-effective therapies and disregarded the importance of vitamin D in immunity led to a “pandemic of hypovitaminosis D”. However, in 2022/23, with increased hospitalizations and deaths among those fully vaccinated and boostered surpassed unvaccinated, now it has become a “pandemic of vaccinated”.
Considering the value, cost-effectiveness, and lack of adverse effects from the recommended doses of vitamin D, each country must develop new educational and health guidelines, including safe sun exposure and vitamin D supplemental programs to strengthen immunity in individuals and the population, aiming to maintain it above 50 ng/mL (125 nmol/L). Alleviating vitamin D deficiency is the most cost-effective public health intervention one can provide to prevent any symptomatic infections and complications, and improve clinical outcomes from COVID-19 outbreaks. Astonishingly, no government or regulatory agency has yet to accept this principle and implement countrywide vitamin D supplementation programs to keep their citizens healthy. Whether this flagrant inaction is due to conflicts of interest and to maintain the “emergency use authorization” for patented medications and vaccines is yet to reveal.
Implementing vitamin D supplementation programs with community-based chronic disease management programs will positively impact health, prevent diseases and complications, reduce the need for hospitalization and intensive care admissions and reduce deaths from COVID-19 and other infectious diseases. Vitamin D supplementation initiatives to keep the population healthy are economical and potentially beneficial. This is a viable and highly cost-effective strategy for protecting vulnerable groups and populations with a high prevalence of vitamin D deficiency, such as the elderly, those with darker skin color, and institutionalized persons. This cost-effective approach reduces healthcare costs, reduces absenteeism, and minimizes opportunity costs.
Summary
Vitamin D sufficiency-associated immune cell activities heightened beneficial responses and minimized infection complications and deaths. For the healthy 70 kg non-obese adult, the recommended vitamin D doses are between 4000 to 8000 IU/day, which raises and maintains serum 25(OH)D concentrations above 50 ng/mL, the therapeutic level. However, in deficiency states, administering vitamin D doses between 100,000 and 400,000 IU as a bolus or upfront loading dose is necessary to raise circulatory 25(OH)D concentrations within three to five days. In emergencies like sepsis or COVID-19, a single oral dose of calcifediol (0.014 mg/kg) raises blood 25(OHD concentration above 50 ng/mL within four hours and boosts the immune system within one day, which would save lives. The above regimens should follow up with a suitable daily maintenance dose of vitamin D, 5000 IU/day or 50,000 IU (1.25 mg) once a week (or once in ten days), to maintain a robust immune system. Each country should set practical guidelines to maintain population vitamin D sufficiency to improve general health, minimize diseases, and reduce healthcare costs.
Acknowledgment
The author appreciates the input of Mr. Eugene L. Heyden and Dr. Athula K. Polonowita.
Disclosure
The author declares no conflicts of interest in this work.
References
1. Wimalawansa SJ. Global epidemic of coronavirus—COVID-19: what can we do to minimize risks? European J. Biomed Pharma Sci. 2020;7(3):432–438.
2. Wang L, Berger NA, Kaelber DC, et al. Comparison of outcomes from COVID infection in pediatric and adult patients before and after the emergence of Omicron. medRxiv. 2022. doi:10.1101/2021.12.30.21268495
3. Qi ZH, Bei ZF, Teng S, et al. Clinical features of 19 children infected with the Omicron variant of severe acute respiratory syndrome coronavirus 2 in Hangzhou. China Zhongguo Dang Dai Er Ke Za Zhi. 2022;24(10):1092–1097.
4. Wang X, Chang H, Tian H, et al. Epidemiological and clinical features of SARS-CoV-2 infection in children during the outbreak of Omicron variant in Shanghai, March 7- 31,2022. Influenza Other Respir Viruses. 2022;16(6):1059–1065. doi:10.1111/irv.13044
5. Suryawanshi RK, Chen IP, Ma T, et al. Limited cross-variant immunity after infection with the SARS-CoV-2 Omicron variant without vaccination. medRxiv. 2022. doi:10.1101/2022.01.13.22269243
6. Ledford H. How severe are Omicron infections? Nature. 2021;600(7890):577–578. doi:10.1038/d41586-021-03794-8
7. Torjesen I. Covid-19: omicron variant is linked to steep rise in hospital admissions of very young children. BMJ. 2022;376:o110. doi:10.1136/bmj.o110
8. Chen KK, Huang DT, Huang LM. SARS-CoV-2 variants - evolution, Spike protein, and vaccines. Biomed J. 2022;45:573–579. doi:10.1016/j.bj.2022.04.006
9. Shrestha LB, Foster C, Rawlinson W, et al. Evolution of the SARS-CoV-2 omicron variants BA.1 to BA.5: implications for immune escape and transmission. Rev Med Virol. 2022;32(5):e2381. doi:10.1002/rmv.2381
10. Zhou H, Dcosta BM, Landau NR, et al. Resistance of SARS-CoV-2 Omicron BA.1 and BA.2 Variants to vaccine-elicited sera and therapeutic monoclonal antibodies. Viruses. 2022;14(6):1334. doi:10.3390/v14061334
11. Armitage R, Nellums LB. COVID-19 and the consequences of isolating the elderly. Lancet Public Health. 2020;5(5):e256. doi:10.1016/S2468-2667(20)30061-X
12. Reyes-Ortiz CA, Williams C, Westphal C. Comparison of early versus late palliative care consultation in end-of-life care for the hospitalized frail elderly patients. Am J Hosp Palliat Care. 2015;32(5):516–520. doi:10.1177/1049909114530183
13. SeyedAlinaghi S, Mehrtak M, MohsseniPour M, et al. Genetic susceptibility of COVID-19: a systematic review of current evidence. Eur J Med Res. 2021;26(1):46. doi:10.1186/s40001-021-00516-8
14. Barrea LV, Grant L, Frias-Toral WB, et al. Vitamin D: a role also in long COVID-19? Nutrients. 2022;14:1625. doi:10.3390/nu14081625
15. Pretorius E, Venter C, Laubscher GJ, et al. Prevalence of symptoms, comorbidities, fibrin amyloid microclots and platelet pathology in individuals with Long COVID/Post-Acute Sequelae of COVID-19 (PASC). Cardiovasc Diabetol. 2022;21(1):148. doi:10.1186/s12933-022-01579-5
16. Thapa Magar S, Lokhandwala HI, Batool S, et al. A Systematic Review of neurological manifestations of COVID-19. Cureus. 2022;14(8):e28309. doi:10.7759/cureus.28309
17. Sanabria-Diaz G, Etter MM, Melie-Garcia L, et al. Brain cortical alterations in COVID-19 patients with neurological symptoms. Front Neurosci. 2022;16:992165. doi:10.3389/fnins.2022.992165
18. Boucher BJ. Vitamin D deficiency in British South Asians, a persistent but avoidable problem associated with many health risks (including rickets, T2DM, CVD, COVID-19 and pregnancy complications): the case for correcting this deficiency. Endocr Connect. 2022;11(12). doi:10.1530/EC-22-0234
19. GAO-21-319. Operation warp speed: accelerated COVID-19 vaccine development status and efforts to address manufacturing challenges. Accelerated COVID-19 Vaccine Development Status and Efforts to Address Manufacturing Challenges. U.S. GAO; 2020. Available from: www.gao.gov/products/gao-21-319.
20. Wolfl-Duchek M, Bergmann F, Jorda A, et al. Sensitivity and specificity of SARS-CoV-2 rapid antigen detection tests using oral, anterior nasal, and nasopharyngeal swabs: a diagnostic accuracy study. Microbiol Spectr. 2022;10(1):e0202921. doi:10.1128/spectrum.02029-21
21. Zhan Z, Li J, Cheng ZJ. Rapid antigen test combine with nucleic acid detection: a better strategy for COVID-19 screening at points of entry. J Epidemiol Glob Health. 2022;12(1):13–15. doi:10.1007/s44197-021-00030-4
22. Islamoska S, Petersen JH, Benfield T, et al. Socioeconomic and demographic risk factors in COVID-19 hospitalization among immigrants and ethnic minorities. Eur J Public Health. 2022;32(2):302–310. doi:10.1093/eurpub/ckab186
23. Aldridge RW, Lewer D, Katikireddi SV, et al. Black, Asian and Minority Ethnic groups in England are at increased risk of death from COVID-19: indirect standardisation of NHS mortality data. Wellcome Open Res. 2020;5:88. doi:10.12688/wellcomeopenres.15922.2
24. Holmes L, Enwere M, Williams J, et al. Black-white risk differentials in COVID-19 (SARS-COV2) transmission, mortality and case fatality in the United States: translational epidemiologic perspective and Challenges. Int J Environ Res Public Health. 2020;17(12):4322. doi:10.3390/ijerph17124322
25. Roizen JD, Long C, Casella A, et al. Obesity decreases hepatic 25-hydroxylase activity causing low serum 25-hydroxyvitamin D. J Bone Miner Res. 2019;34(6):1068–1073. doi:10.1002/jbmr.3686
26. Wortsman J, Matsuoka LY, Chen TC, et al. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–693. doi:10.1093/ajcn/72.3.690
27. Walsh JB, McCartney DM, Laird É, et al. Understanding a low vitamin D state in the context of COVID-19. Front Pharmacol. 2022;13:835480. doi:10.3389/fphar.2022.835480
28. Phommasone K, Xaiyaphet X, Garcia-Rivera JA, et al. A case-control study of the causes of acute respiratory infection among hospitalized patients in Northeastern Laos. Sci Rep. 2022;12(1):939. doi:10.1038/s41598-022-04816-9
29. Ekwaru JP, Zwicker JD, Holick MF, et al. The importance of body weight for the dose response relationship of oral vitamin D supplementation and serum 25-hydroxyvitamin D in healthy volunteers. PLoS One. 2014;9(11):e111265. doi:10.1371/journal.pone.0111265
30. Wimalawansa SJ. Biology of Vitamin D. J Steroids Horm Sci. 2019;198(1):1–8.
31. Vieth R. Vitamin D supplementation: cholecalciferol, calcifediol, and calcitriol. Eur J Clin Nutr. 2020;74(11):1493–1497. doi:10.1038/s41430-020-0697-1
32. Tieu S, Charchoglyan A, Lauri Wagter-Lesperance LW, et al. Immunoceuticals: harnessing their immunomodulatory potential to promote health and wellness. Nutrients. 2022;14:4075. doi:10.3390/nu14194075
33. Patel HM, Khandwala S, Somani P, et al. Determining whether ethnic minorities with severe obesity face a disproportionate risk of serious disease and death from COVID-19: outcomes from a Southern California-based retrospective cohort study. BMJ Open. 2022;12(6):e059132. doi:10.1136/bmjopen-2021-059132
34. Lucock M, Thota R, Garg M, et al. Early lifecycle UV-exposure calibrates adult vitamin D metabolism: evidence for a developmentally originated vitamin D homeostat that may alter related adult phenotypes. Am J Hum Biol. 2019;31(4):e23272. doi:10.1002/ajhb.23272
35. Del Giudice M, Indolfi M C, Strisciuglio C, Vitamin D: immunomodulatory aspects.
36. Wimalawansa SJ. Vitamin D deficiency: effects on oxidative stress, epigenetics, gene regulation, and aging. Biology. 2019;8(2):30.
37. Chlon TM, Taffany DA, Welsh J, et al. Retinoids modulate expression of the endocytic partners megalin, cubilin, and disabled-2 and uptake of vitamin D-binding protein in human mammary cells. J Nutr. 2008;138(7):1323–1328. doi:10.1093/jn/138.7.1323
38. Nykjaer A, Dragun D, Walther D, et al. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96(4):507–515. doi:10.1016/S0092-8674(00)80655-8
39. Marzolo MP, Farfan P. New insights into the roles of megalin/LRP2 and the regulation of its functional expression. Biol Res. 2011;44(1):89–105. doi:10.4067/S0716-97602011000100012
40. Wimalawansa SJ. Skeletal benefits, endocrine functions, and toxicity of vitamin D. J Endocrinol Diab. 2016;3(3):1–5. doi:10.15226/2374-6890/3/3/00152
41. Hollis BW, Wagner CL. Clinical review: the role of the parent compound vitamin D with respect to metabolism and function: why clinical dose intervals can affect clinical outcomes. J Clin Endocrinol Metab. 2013;98(12):4619–4628. doi:10.1210/jc.2013-2653
42. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–58. doi:10.1210/jc.2010-2704
43. Force USPST, Curry SJ, Owens DK, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: US preventive services task force recommendation statement. JAMA. 2018;319(15):1592–1599. doi:10.1001/jama.2018.3185
44. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi:10.1210/jc.2011-0385
45. Wagner CL, Hollis BW, Kotsa K, et al. Vitamin D administration during pregnancy as prevention for pregnancy, neonatal and postnatal complications. Rev Endocr Metab Disord. 2017;18(3):307–322. doi:10.1007/s11154-017-9414-3
46. Quraishi SA, De Pascale G, Needleman JS, et al. Effect of cholecalciferol supplementation on vitamin D status and cathelicidin levels in sepsis: a randomized, placebo-controlled Trial. Crit Care Med. 2015;43(9):1928–1937. doi:10.1097/CCM.0000000000001148
47. Quraishi SA, Bittner EA, Blum L, et al. Association between preoperative 25-hydroxyvitamin D level and hospital-acquired infections following Roux-en-Y gastric bypass surgery. JAMA Surg. 2014;149(2):112–118. doi:10.1001/jamasurg.2013.3176
48. Wimalawansa SJ. Rapidly increasing serum 25(OH)D boosts the immune system, against infections-sepsis and COVID-19. Nutrients. 2022;14(14):2997.
49. Tsujino I, Ushikoshi-Nakayama R, Yamazaki T, et al. Pulmonary activation of vitamin D3 and preventive effect against interstitial pneumonia. J Clin Biochem Nutr. 2019;65(3):245–251. doi:10.3164/jcbn.19-48
50. Wimalawansa SJ. Fighting against COVID-19: boosting the immunity with micronutrients, stress reduction, physical activity, and vitamin D. Nutr Food Sci. 2020c;3:1–4.
51. Wimalawansa SJ. Achieving population vitamin D sufficiency will markedly reduce healthcare costs. EJBPS. 2020;7:136–141.
52. Institute of Medicine. Scientific evaluation of dietary reference intakes for calcium and vitamin D; 2010. Available from: http://www.iom.edu/Reports/2010/Dietary-Reference-Intakes-for-Calcium-and-Vitamin-D.aspx.
53. Annonymus. Vitamin D: fact sheet for consumers. 2022; Available from: https://ods.od.nih.gov/factsheets/vitamind-healthprofessional/.
54. Grant WB, Hadi MA, Hasan SS, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(6):1626. doi:10.3390/nu12061626
55. Cho DH, Lee GY, An JH, et al. The effects of 1,25(OH)2 D3 treatment on immune responses and intracellular metabolic pathways of bone marrow-derived dendritic cells from lean and obese mice. IUBMB Life. 2021;74:378–390.
56. Premkumar M, Sable T, Dhanwal D, et al. Vitamin D homeostasis, bone mineral metabolism, and seasonal affective disorder during 1 year of Antarctic residence. Arch Osteoporos. 2013;8:129. doi:10.1007/s11657-013-0129-0
57. Kow CS, Hadi MA, Hasan SS. Vitamin D supplementation in influenza and COVID-19 infections comment on: “evidence that vitamin d supplementation could reduce risk of influenza and COVID-19 infections and deaths”. Nutrients. 2020;12(6):988.
58. Wimalawansa SJ. Vitamin D; What clinicians would like to know. Sri Lanka J Diabet Endocrinol Metabo. 2012;1(2):73–88. doi:10.4038/sjdem.v2i2.4776
59. Wimalawansa SJ. Non-musculoskeletal benefits of vitamin D. J Steroid Biochem Mol Biol. 2018;175:60–81. doi:10.1016/j.jsbmb.2016.09.016
60. Chauss D, Freiwald T, McGregor R, et al. Autocrine vitamin D signaling switches off pro-inflammatory programs of TH1 cells. Nat Immunol. 2022;23(1):62–74. doi:10.1038/s41590-021-01080-3
61. Chambers ES, Hawrylowicz CM. The impact of vitamin D on regulatory T cells. Curr Allergy Asthma Rep. 2011;11(1):29–36. doi:10.1007/s11882-010-0161-8
62. Zhang J, McCullough PA, Tecson KM. Vitamin D deficiency in association with endothelial dysfunction: implications for patients with COVID-19. Rev Cardiovasc Med. 2020;21(3):339–344. doi:10.31083/j.rcm.2020.03.131
63. Quraishi SA, Bittner EA, Blum L, et al. Prospective study of vitamin D status at initiation of care in critically ill surgical patients and risk of 90-day mortality. Crit Care Med. 2014;42(6):1365–1371. doi:10.1097/CCM.0000000000000210
64. Jiang Y, Chen L, Taylor RN, et al. Physiological and pathological implications of retinoid action in the endometrium. J Endocrinol. 2018;236(3):R169–R188. doi:10.1530/JOE-17-0544
65. Keane KN, Cruzat VF, Calton EK, et al. Molecular actions of vitamin D in reproductive cell biology. Reproduction. 2017;153(1):R29–R42. doi:10.1530/REP-16-0386
66. Pludowski P, Holick MF, Grant WB, et al. Vitamin D supplementation guidelines. J Steroid Biochem Mol Biol. 2018;175:125–135. doi:10.1016/j.jsbmb.2017.01.021
67. McCartney DM, Byrne DG. Optimisation of vitamin D status for enhanced immuno-protection against COVID-19. Ir Med J. 2020;113(4):58.
68. Zhou YF, Luo BA, Qin LL. The association between vitamin D deficiency and community-acquired pneumonia: a meta-analysis of observational studies. Medicine. 2019;98(38):e17252. doi:10.1097/MD.0000000000017252
69. Ali N. Role of vitamin D in preventing of COVID-19 infection, progression and severity. J Infect Public Health. 2020;13(10):1373–1380. doi:10.1016/j.jiph.2020.06.021
70. Borsche L, Glauner B, von Mendel J. COVID-19 mortality risk correlates inversely with vitamin D3 Status, and a mortality rate close to zero could theoretically be achieved at 50 ng/mL 25(OH)D3: results of a systematic review and meta-analysis. Nutrients. 2021;13(10):3596. doi:10.3390/nu13103596
71. Luxwolda MF, et al., Vitamin D status indicators in indigenous populations in East Africa. Eur J Nutr, 2013. 52(3): p. 1115–25.
72. Luxwolda MF, Kema KR, IP JDBD, Muskiet FA, Traditionally living populations in East Africa have a mean serum 25-hydroxyvitamin D concentration of 115 nmol/l. Br J Nutr., 2012: p. 1–5
73. Gibbons JB, et al., Association between vitamin D supplementation and COVID-19 infection and mortality. Sci Rep, 2022. 12(1): p. 193
74. Jayawardena R, Jeyakumar DT, Francis TV, et al. Impact of the vitamin D deficiency on COVID-19 infection and mortality in Asian countries. Diabetes Metab Syndr. 2021;15(3):757–764. doi:10.1016/j.dsx.2021.03.006
75. Castillo EM, Entrenas Costa LM, Vaquero Barrios JM, et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203:105751. doi:10.1016/j.jsbmb.2020.105751
76. Wimalawansa SJ, Polonowita A Boosting immunity with vitamin D for preventing complications and deaths from COVID-19. in COVID 19: impact, mitigation, opportunities and building resilience “From adversity to serendipity”, perspectives of global relevance based on research, experience and successes in combating COVID-19 in Sri Lanka. Colombo, Sri Lanka: National Science Foundation, Sri Lanka; 2021.
77. Quraishi SA, Litonjua AA, Moromizato T, et al. Association between prehospital vitamin D status and hospital-acquired bloodstream infections. Am J Clin Nutr. 2013;98(4):952–959. doi:10.3945/ajcn.113.058909
78. Carter SJ, Baranauskas MN, Fly AD. Considerations for obesity, vitamin D, and physical activity amidst the COVID-19 pandemic. Obesity. 2020;28:1176–1177. doi:10.1002/oby.22838
79. DrorI A, MorozovI N, Daoud A, et al. Pre-infection 25-hydroxyvitamin D3 levels and association with severity of COVID-19 illness. PLoS One. 2022;17:1–18.
80. Russell B, Moss C, George G, et al. Associations between immune-suppressive and stimulating drugs and novel COVID-19-A systematic review of current evidence. Ecancermedical Sci. 2020;14:1022. doi:10.3332/ecancer.2020.1022
81. Bishop E, Ismailova A, Dimeloe S, et al. Vitamin D and immune regulation: antibacterial, antiviral, anti-inflammatory. JBMR Plus. 2020;5:e10405.
82. Cao Z, Wu Y, Faucon E, et al. SARS-CoV-2 & Covid-19: key-roles of the ‘renin-angiotensin’ system/ vitamin D impacting drug and vaccine developments. Infect Disord Drug Targets. 2020;20(3):348–349. doi:10.2174/1871526520999200505174704
83. Czaja AJ. Factoring the intestinal microbiome into the pathogenesis of autoimmune hepatitis. World J Gastroenterol. 2016;22(42):9257–9278. doi:10.3748/wjg.v22.i42.9257
84. Jin D, Wu S, Zhang Y-G, et al. Lack of vitamin D receptor causes dysbiosis and changes the functions of the murine intestinal microbiome. Clin Ther. 2015;37(5):996–1009 e7. doi:10.1016/j.clinthera.2015.04.004
85. Laplana M, Royo JL, Fibla J. Vitamin D Receptor polymorphisms and risk of enveloped virus infection: a meta-analysis. Gene. 2018;678:384–394. doi:10.1016/j.gene.2018.08.017
86. Platitsyna NG, Bolotnova TV. Vitamin D deficiency as a risk factor for chronic non-infectious diseases. Adv Gerontol. 2017;30(6):873–879.
87. Pletz MW, Terkamp C, Schumacher U, et al. Vitamin D deficiency in community-acquired pneumonia: low levels of 1,25(OH)2 D are associated with disease severity. Respir Res. 2014;15:53. doi:10.1186/1465-9921-15-53
88. Ginde AA, Mansbach JM, Camargo CA. Vitamin D, respiratory infections, and asthma. Curr Allergy Asthma Rep. 2009;9(1):81–87. doi:10.1007/s11882-009-0012-7
89. Ianevski A, Zusinaite E, Shtaida N, et al. Low temperature and low UV indexes correlated with peaks of influenza virus activity in Northern Europe during 2010–2018. Viruses. 2019;11(3):207. doi:10.3390/v11030207
90. Imai CM, Halldorsson TI, Eiriksdottir G, et al. Depression and serum 25-hydroxyvitamin D in older adults living at northern latitudes - AGES-Reykjavik Study. J Nutr Sci. 2015;4:e37. doi:10.1017/jns.2015.27
91. Devaraj S, Jialal G, Cook T, et al. Low vitamin D levels in Northern American adults with the metabolic syndrome. Horm Metab Res. 2011;43(1):72–74. doi:10.1055/s-0030-1268485
92. Eroglu C, Demir F, Erge D, et al. The relation between serum vitamin D levels, viral infections and severity of attacks in children with recurrent wheezing. Allergol Immunopathol. 2019;47(6):591–597. doi:10.1016/j.aller.2019.05.002
93. Arihiro S, Nakashima A, Matsuoka M, et al. Randomized trial of vitamin D supplementation to prevent seasonal influenza and upper respiratory infection in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2019;25(6):1088–1095. doi:10.1093/ibd/izy346
94. Jolliffe DA, Greiller CL, Mein CA, et al. Vitamin D receptor genotype influences risk of upper respiratory infection. Br J Nutr. 2018;120(8):891–900. doi:10.1017/S000711451800209X
95. Reichrath J, Saternus R, Vogt T. Challenge and perspective: the relevance of ultraviolet (UV) radiation and the vitamin D endocrine system (VDES) for psoriasis and other inflammatory skin diseases. Photochem Photobiol Sci. 2017;16(3):433–444. doi:10.1039/c6pp00280c
96. Saraiva GL, Cendoroglo MS, Ramos LR, et al. Influence of ultraviolet radiation on the production of 25 hydroxyvitamin D in the elderly population in the city of Sao Paulo (23 degrees 34’S), Brazil. Osteoporos Int. 2005;16(12):1649–1654. doi:10.1007/s00198-005-1895-3
97. Zdrenghea MT, Makrinioti H, Bagacean C, et al. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev Med Virol. 2017;27(1):e1909. doi:10.1002/rmv.1909
98. Morris SK, Pell LG, Rahman MZ, et al. Maternal vitamin D supplementation during pregnancy and lactation to prevent acute respiratory infections in infancy in Dhaka, Bangladesh (MDARI trial): protocol for a prospective cohort study nested within a randomized controlled trial. BMC Pregnancy Childbirth. 2016;16(1):309. doi:10.1186/s12884-016-1103-9
99. Sabatier I, Chabrier S, Brun A, et al. Stroke by carotid artery complete occlusion in Kawasaki disease: case report and review of literature. Pediatr Neurol. 2013;49(6):469–473. doi:10.1016/j.pediatrneurol.2013.08.011
100. Di Filippo L, Allora A, Doga M, et al. Vitamin D levels are associated with blood glucose and BMI in COVID-19 patients, predicting disease severity. J Clin Endocrinol Metab. 2022;107(1):e348–e360. doi:10.1210/clinem/dgab599
101. Di Filippo L, De Lorenzo R, Giustina A, et al. Vitamin D in osteosarcopenic obesity. Nutrients. 2022;14(9):1816. doi:10.3390/nu14091816
102. Migliaccio S, Di Nisio A, Mele C, et al. Obesity and hypovitaminosis D: causality or casualty? Int J Obes Suppl. 2019;9(1):20–31. doi:10.1038/s41367-019-0010-8
103. Maddaloni E. Vitamin D and diabetes mellitus. Front Horm Res. 2018;50:161–176.
104. Cashman KD, Ritz C, Carlin A, et al. Vitamin D biomarkers for Dietary Reference Intake development in children: a systematic review and meta-analysis. Am J Clin Nutr. 2022;115(2):544–558. doi:10.1093/ajcn/nqab357
105. Cashman KD, FitzGerald AP, Viljakainen HT, et al. Estimation of the dietary requirement for vitamin D in healthy adolescent white girls. Am J Clin Nutr. 2011;93(3):549–555. doi:10.3945/ajcn.110.006577
106. Merzon E, Tworowski D, Gorohovski A, et al. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study. FEBS J. 2020;287(17):3693–3702. doi:10.1111/febs.15495
107. Grant WB, Al Anouti F, Moukayed M. Targeted 25-hydroxyvitamin D concentration measurements and vitamin D3 supplementation can have important patient and public health benefits. Eur J Clin Nutr. 2020;74(3):366–376. doi:10.1038/s41430-020-0564-0
108. Kazemi A, Mohammadi V, Aghababaee SA, Golzarand M, Clark CCT, Babajafari S. Association of vitamin D status with SARS-CoV-2 infection or COVID-19 severity: a systematic review and meta-analysis. Adv Nutr. 2021;00(p):1–23.
109. Wimalawansa SJ. Controlling COVID-19 pandemic with cholecalciferol. Heathcare Res. 2020;5(1):155–165.
110. Wimalawansa SJ. Oral calcifediol repletes blood vitamin D concentration within 4 hours; 2021. Available from: https://www.linkedin.com/posts/sunilwimalawansa_oral-calcifediol-repletes-blood-vitamin-activity-6803351558714204160-dtnd.
111. Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. doi:10.1126/science.1123933
112. Vieth R. Why “Vitamin D” is not a hormone, and not a synonym for 1,25-dihydroxy-vitamin D, its analogs or deltanoids. J Steroid Biochem Mol Biol. 2004;89(1–5):571–573. doi:10.1016/j.jsbmb.2004.03.037
113. Aygun H. Vitamin D can prevent COVID-19 infection-induced multiple organ damage. Naunyn Schmiedebergs Arch Pharmacol. 2020;393(7):1157–1160. doi:10.1007/s00210-020-01911-4
114. Kaufman HW, Niles JK, Kroll MH, et al. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One. 2020;15(9):e0239252. doi:10.1371/journal.pone.0239252
115. Alexander J, Tinkov A, Strand TA, et al. Early nutritional interventions with zinc, selenium and vitamin D for raising anti-viral resistance against progressive COVID-19. Nutrients. 2020;12(8):2358. doi:10.3390/nu12082358
116. Bakaloudi DR, Chourdakis M. A critical update on the role of mild and serious vitamin D deficiency prevalence and the COVID-19 epidemic in Europe. Nutrition. 2022;93:111441. doi:10.1016/j.nut.2021.111441
117. Akbari AR, Khan M, Adeboye W, et al. Ethnicity as a risk factor for vitamin D deficiency and undesirable COVID-19 outcomes. Rev Med Virol. 2021;32:e2291. doi:10.1002/rmv.2291
118. Vanegas-Cedillo PE, Bello-Chavolla OY, Ramírez-Pedraza N, et al. Serum vitamin D levels are associated with Increased COVID-19 severity and mortality independent of whole-body and visceral adiposity. Front Nutr. 2022;9:813485. doi:10.3389/fnut.2022.813485
119. DiNicolantonio JJ, O’Keefe JH. Magnesium and vitamin D deficiency as a potential cause of Immune dysfunction, cytokine storm and disseminated intravascular coagulation in COVID-19 patients. Mo Med. 2021;118(1):68–73. doi:10.1101/2020.04.24.20075838
120. Kumar P, Kumar M, Bedi O, et al. Role of vitamins and minerals as immunity boosters in COVID-19. Inflammopharmacology. 2021;29(4):1001–1016. doi:10.1007/s10787-021-00826-7
121. Kumar R, Rathi H, Haq A, Wimalawansa SJ, Sharma A. Putative roles of vitamin D in modulating immune response and immunopathology associated with COVID-19. Virus Res. 2021;292:198235. doi:10.1016/j.virusres.2020.198235
122. D’Avolio A, Avataneo V, Manca A, et al. 25-hydroxyvitamin D concentrations are Lower in patients with positive PCR for SARS-CoV-2. Nutrients. 2020;12(5):1359. doi:10.3390/nu12051359
123. Sims JT, et al. Characterization of the cytokine storm reflects hyperinflammatory endothelial dysfunction in COVID-19. J Allergy Clin Immunol. 2020;147:107–111.
124. Hojyo S, Uchida M, Tanaka K, et al. How COVID-19 induces cytokine storm with high mortality. Inflamm Regen. 2020;40:37. doi:10.1186/s41232-020-00146-3
125. Iannaccone G, Gul F, Ram P, et al. Weathering the cytokine storm in COVID-19: therapeutic implications. Cardiorenal Med. 2020;10:1–11. doi:10.1159/000503919
126. Amaya-Mejia AS, O’Farrill-Romanillos PM, Galindo-Pacheco LV, et al. Vitamin D deficiency in patients with common variable immunodeficiency, with autoimmune diseases and bronchiectasis. Rev Alerg Mex. 2013;60(3):110–116.
127. Broder AR, Tobin JN, Putterman C. Disease-specific definitions of vitamin D deficiency need to be established in autoimmune and non-autoimmune chronic diseases: a retrospective comparison of three chronic diseases. Arthritis Res Ther. 2010;12(5):R191. doi:10.1186/ar3161
128. Kurylowicz A, Bednarczuk T, Nauman J. The influence of vitamin D deficiency on cancers and autoimmune diseases development. Endokrynol Pol. 2007;58(2):140–152.
129. Wimalawansa SJ. COVID-19: evolution and prevention. Trends Telemed E Health. 2020;2(3):1–5.
130. Antal AS, Dombrowski Y, Koglin S, et al. Impact of vitamin D3 on cutaneous immunity and antimicrobial peptide expression. DermatoEndocrinol. 2011;3(1):18–22. doi:10.4161/derm.3.1.14616
131. Gibson CC, Davis CT, Zhu W, et al. Dietary vitamin D and its metabolites non-genomically stabilize the endothelium. PLoS One. 2015;10(10):e0140370. doi:10.1371/journal.pone.0140370
132. Aloia JF, Li-Ng M. Re: epidemic influenza and vitamin D. Epidemiol Infect. 2007;135(7):1095–1096. doi:10.1017/S0950268807008308
133. Fleming DM, Elliot AJ. Epidemic influenza and vitamin D. Epidemiol Infect. 2007;135(7):1091–1092. doi:10.1017/S0950268807008291
134. Moromizato T, Litonjua AA, Braun AB, et al. Association of low serum 25-hydroxyvitamin D levels and sepsis in the critically ill. Crit Care Med. 2014;42(1):97–107. doi:10.1097/CCM.0b013e31829eb7af
135. Hanff TC, Harhay MO, Brown TS, et al. Is There an association between COVID-19 mortality and the renin-angiotensin system-a call for epidemiologic investigations. Clin Infect Dis. 2020;71:870–874. doi:10.1093/cid/ciaa329
136. Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126:1671–1681.
137. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi:10.1016/j.cell.2020.02.052
138. Danser AHJ, Epstein M, Batlle D. Renin-angiotensin system blockers and the COVID-19 pandemic: at present there is no evidence to abandon renin-angiotensin system blockers. Hypertension. 2020;75:1382–1385. doi:10.1161/HYPERTENSIONAHA.120.15082
139. Diaz JH. Hypothesis: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID-19. J Travel Med. 2020;27(3). doi:10.1093/jtm/taaa041
140. Lim H, Kim SE, Lee YH, et al. Immunogenicity of candidate SARS-CoV-2 DNA vaccines based on the spike protein. Virology. 2022;573:118–123. doi:10.1016/j.virol.2022.06.006
141. Almehdi AM, Khoder G, Alchakee AS, et al. SARS-CoV-2 spike protein: pathogenesis, vaccines, and potential therapies. Infection. 2021;49(5):855–876. doi:10.1007/s15010-021-01677-8
142. Kong J, Zhu X, Shi Y, et al. VDR attenuates acute lung injury by blocking Ang-2-Tie-2 pathway and renin-angiotensin system. Mol Endocrinol. 2013;27(12):2116–2125. doi:10.1210/me.2013-1146
143. Liu Z, Xiao X, Wei X, et al. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2. J Med Virol. 2020;92:595–601.
144. Tomaschitz A, Pilz S, Ritz E, et al. Independent association between 1,25-dihydroxyvitamin D, 25-hydroxyvitamin D and the renin-angiotensin system: the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Clin Chim Acta. 2010;411(17–18):1354–1360. doi:10.1016/j.cca.2010.05.037
145. Xu J, Sriramula S, Xia H, et al. Clinical relevance and role of neuronal AT 1 receptors in ADAM17-mediated ACE2 shedding in neurogenic hypertension. Circ Res. 2017;121(1):43–55. doi:10.1161/CIRCRESAHA.116.310509
146. Xu J, Yang J, Chen J, et al. Vitamin D alleviates lipopolysaccharide induced acute lung injury via regulation of the renin angiotensin system. Mol Med Rep. 2017;16(5):7432–7438. doi:10.3892/mmr.2017.7546
147. Radujkovic A, Hippchen T, Tiwari-Heckler S, et al. Vitamin D deficiency and outcome of COVID-19 patients. Nutrients. 2020;12(9):2757. doi:10.3390/nu12092757
148. Wimalawansa SJ. Fighting against COVID-19: boosting the immunity with micronutrients, stress reduction, physical activity, and vitamin D. Nutr Food Sci. 2020;3(126):1–4.
149. Fedson DS. Treating the host response to emerging virus diseases: lessons learned from sepsis, pneumonia, influenza and Ebola. Ann Transl Med. 2016;4(21):421. doi:10.21037/atm.2016.11.03
150. Yuan W, Pan W, Kong J, et al. 1,25-dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J Biol Chem. 2007;282(41):29821–29830. doi:10.1074/jbc.M705495200
151. Wimalawansa SJ. ACE inhibitors and angiotensin receptor blockers reduce the complications associated with COVID-19 infection. World J Pharma Res. 2021;10(3):2579–2600.
152. Entrenas castillo M, Entrenas Costa LM, Vaquero Barrios JM, et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203:105751.
153. McGregor E, Kazemian M, Afzali B, et al. An autocrine Vitamin D-driven Th1 shutdown program can be exploited for COVID-19; 2020. Available from: https://www.biorxiv.org/content/10.1101/2020.07.18.210161v1.
154. McGregor TB, Sener A, Yetzer K, et al. The impact of COVID-19 on the Canadian Kidney Paired Donation program: an opportunity for universal implementation of kidney shipping. Can J Surg. 2020;63(5):E451–E453. doi:10.1503/cjs.012620
155. Wallis G, Siracusa F, Blank M, et al. Experience of a novel community testing programme for COVID-19 in London: lessons learnt. Clin Med. 2020;20(5):e165–e169. doi:10.7861/clinmed.2020-0436
156. Walter LA, McGregor AJ. Sex- and Gender-specific Observations and Implications for COVID-19. West J Emerg Med. 2020;21(3):507–509. doi:10.5811/westjem.2020.4.47536
157. Stagi S. Severe vitamin D deficiency in patients with Kawasaki disease: a potential role in the risk to develop heart vascular abnormalities? Clin Rheumatol. 2016;35(7):1865–1872. doi:10.1007/s10067-015-2970-6
158. Kirkham FJ, Zafeiriou D, Howe D, et al. Fetal stroke and cerebrovascular disease: advances in understanding from lenticulostriate and venous imaging, alloimmune thrombocytopaenia and monochorionic twins. Eur J Paediatr Neurol. 2018;22(6):989–1005. doi:10.1016/j.ejpn.2018.08.008
159. Kaparianos A, Argyropoulou E. Local renin-angiotensin II systems, angiotensin-converting enzyme and its homologue ACE2: their potential role in the pathogenesis of chronic obstructive pulmonary diseases, pulmonary hypertension and acute respiratory distress syndrome. Curr Med Chem. 2011;18(23):3506–3515. doi:10.2174/092986711796642562
160. Hughes DA, Norton R, Aloia JF, Li-ng M. Vitamin D and respiratory health. Clin Exp Immunol. 2009;158(1):20–25. doi:10.1111/j.1365-2249.2009.04001.x
161. Singh S, Kaur R, Singh RK. Revisiting the role of vitamin D levels in the prevention of COVID-19 infection and mortality in European countries post infections peak. Aging Clin Exp Res. 2020;32(8):1609–1612. doi:10.1007/s40520-020-01619-8
162. Ma W, Nguyen LH, Yue Y, et al. Associations between predicted vitamin D status, vitamin D intake, and risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and coronavirus disease 2019 (COVID-19) severity. Am J Clin Nutr. 2022;115(4):1123–1133. doi:10.1093/ajcn/nqab389
163. D’Ecclesiis O, Gavioli C, Martinoli C, et al. Vitamin D and SARS-CoV2 infection, severity and mortality: a systematic review and meta-analysis. PLoS One. 2022;17(7):e0268396. doi:10.1371/journal.pone.0268396
164. Chiodini I, Gatti D, Soranna D, et al. Vitamin D status and SARS-CoV-2 infection and COVID-19 clinical outcomes. Front Public Health. 2021;9:736665. doi:10.3389/fpubh.2021.736665
165. Akbar MR, Wibowo A, Pranata R, et al. Low serum 25-hydroxyvitamin D (vitamin D) level Is associated with susceptibility to COVID-19, severity, and mortality: a systematic review and meta-analysis. Front Nutr. 2021;8:660420. doi:10.3389/fnut.2021.660420
166. Santaolalla A, Beckmann K, Kibaru J, et al. Association between vitamin D and novel SARS-CoV-2 respiratory dysfunction - a scoping review of current evidence and its implication for COVID-19 pandemic. Front Physiol. 2020;11:564387. doi:10.3389/fphys.2020.564387
167. Dancer RC, Parekh D, Lax S, et al. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). Thorax. 2015;70(7):617–624. doi:10.1136/thoraxjnl-2014-206680
168. McCartney DM. Vitamin D and SARS-CoV-2 infection-evolution of evidence supporting clinical practice and policy development: a position statement from the Covit-D Consortium. Ir J Med Sci. 2021;190(3):1253–1265.
169. Arabi YM, Fowler R, Hayden FG. Critical care management of adults with community-acquired severe respiratory viral infection. Intensive Care Med. 2020;46(2):315–328. doi:10.1007/s00134-020-05943-5
170. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi:10.1136/bmj.i6583
171. Biesalski HK, Aggett PJ, Anton R, et al. 26th Hohenheim consensus conference, September 11, 2010 scientific substantiation of health claims: evidence-based nutrition. Nutrition. 2011;27(10 Suppl):S1–S20. doi:10.1016/j.nut.2011.04.002
172. Tsai F, Coyle WJ. The microbiome and obesity: is obesity linked to our gut flora? Curr Gastroenterol Rep. 2009;11(4):307–313. doi:10.1007/s11894-009-0045-z
173. Veugelers PJ, Pham TM, Ekwaru JP. Optimal vitamin D supplementation doses that minimize the risk for both low and high serum 25-hydroxyvitamin D concentrations in the general population. Nutrients. 2015;7(12):10189–10208. doi:10.3390/nu7125527
174. Huang Z, You T. Personalise vitamin D3 using physiologically based pharmacokinetic modelling. CPT Pharmacometrics Syst Pharmacol. 2021;10(7):723–734. doi:10.1002/psp4.12640
175. McKenna MJ, Lyons OC, Flynn MA, et al. COVID-19 pandemic and vitamin D: rising trends in status and in daily amounts of vitamin D provided by supplements. BMJ Open. 2022;12(8):e059477. doi:10.1136/bmjopen-2021-059477
176. Hopefl R, Ben-Eltriki M, Deb S. Association between vitamin D levels and inflammatory markers in COVID-19 patients: a meta-analysis of observational studies. J Pharm Pharm Sci. 2022;25:124–136. doi:10.18433/jpps32518
177. Rhodes JM, Subramanian S, Laird E, et al. Perspective: vitamin D deficiency and COVID-19 severity - plausibly linked by latitude, ethnicity, impacts on cytokines, ACE2, and thrombosis (R1). J Intern Med. 2020;289:97–115. doi:10.1111/joim.13149
178. Procter BC, Ross C, Pickard V, et al. Clinical outcomes after early ambulatory multidrug therapy for high-risk SARS-CoV-2 (COVID-19) infection. Rev Cardiovasc Med. 2020;21(4):611–614.
179. Gunn J, Hill MM, Cotten BM, et al. An analysis of biomarkers in patients with chronic pain. Pain Physician. 2020;23(1):E41–E49. doi:10.36076/ppj.2020/23/E41
180. Chen S, Liu G, Chen J, et al. Ponatinib protects mice from lethal influenza infection by suppressing cytokine storm. Front Immunol. 2019;10:1393. doi:10.3389/fimmu.2019.01393
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Relationship Between Serum Vitamin D and Perirenal Fat Thickness in Patients with Metabolic Syndrome in Community
Zhang HX, Zhai L, Gao Z, Yuan J
Diabetes, Metabolic Syndrome and Obesity 2022, 15:2149-2156
Published Date: 23 July 2022