Back to Journals » Drug Design, Development and Therapy » Volume 17
Oliceridine for the Management of Moderate to Severe Acute Postoperative Pain: A Narrative Review
Authors Daksla N, Wang A , Jin Z, Gupta A, Bergese SD
Received 2 November 2022
Accepted for publication 11 March 2023
Published 22 March 2023 Volume 2023:17 Pages 875—886
DOI https://doi.org/10.2147/DDDT.S372612
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Neil Daksla,1 Ashley Wang,1 Zhaosheng Jin,1 Abhishek Gupta,1 Sergio D Bergese1,2
1Department of Anesthesiology, Stony Brook University Health Science Center, Stony Brook, NY, 11794-8480, USA; 2Department of Neurosurgery, Stony Brook University Health Science Center, Stony Brook, NY, 11794-8480, USA
Correspondence: Sergio D Bergese, Department of Anesthesiology, Stony Brook University School of Medicine, Health Sciences Center, Level 4, Room 060, Stony Brook, NY, 11794, USA, Tel +1 631 444-2979, Fax +1 631 444-2907, Email [email protected]
Abstract: Despite current advances in acute postoperative pain management, prevalence remains high. Inadequate treatment could lead to poor outcomes and even progression to chronic pain. Opioids have traditionally been the mainstay for treatment of moderate to severe acute pain. However, their use has been associated with opioid-related adverse events (ORAEs), such as respiratory depression, sedation, nausea, vomiting, pruritus, and decreased bowel motility. In addition, their liberal use has been implicated in the current opioid epidemic. As a result, there has been renewed interest in multimodal analgesia to target different mechanisms of action in order to achieve a synergistic effect and minimize opioid usage. Oliceridine is a novel mu-opioid receptor agonist that is part of a new class of biased ligands that selectively activate G-protein signaling and downregulate β-arrestin recruitment. Since G-protein signaling has been associated with analgesia while β-arrestin recruitment has been associated with ORAEs, there is potential for a wider therapeutic window. In this review, we will discuss the clinical evidence behind oliceridine and its potential role in acute postoperative pain management. We have systematically searched the PubMed database using the keywords oliceridine, olinvyk, and trv130. All articles identified were reviewed and evaluated, and all clinical trials were included.
Keywords: postoperative pain, opioids, multimodal analgesia, opioid-related adverse events, biased agonism
Introduction
Postoperative pain is one of the most common complaints following surgery. Despite current advances and a greater understanding of the various pain pathways, results from national surveys in the United States show that incidence could be as high as 86%.1,2 A 2016 cross-sectional observational study including over 15,000 patients undergoing surgery in the United Kingdom revealed that 37.2% and 11.0% of patients reported moderate and severe pain at the surgical site, respectively, within 24 hours of their procedure.3 Such results are also replicated around the world, such as in Germany,4 Sweden,5 Thailand,6 and Kenya.7 Postoperative pain management remains a common challenge in modern day medicine, and adverse outcomes of inadequate treatment have been well documented. Poorly controlled pain causes physiologic stress in the body that compromises various organ systems, resulting in adverse events ranging from myocardial ischemia and infarction, pulmonary infections, paralytic ileus, urinary retention, thromboembolism, and impaired immunity.8 In addition, it also induces psychologic stress responses such as anxiety and fear—all of which lead to increased morbidity and mortality.9 Undertreatment may also impair the rehabilitation process, prolong a patient’s length of stay in the hospital, increase the risk of readmission, and increase the overall cost of care.2,10 Finally, in certain cases, acute postoperative pain may even develop into persistent pain lasting more than 3–6 months after surgery in 10–50% of patients.11
Opioid analgesia is considered the mainstay of postoperative pain management, including morphine, hydromorphone, and fentanyl. Opioids bind to receptors in the central nervous system and peripheral tissues. The three main types of opioid receptors are mu, delta, and kappa; all three are seven transmembrane G protein-coupled receptors (GPCRs). The binding of a ligand triggers conformational changes in the opioid receptor, which causes its C terminus to couple to the heterotrimeric Gi protein.12 When GTP is replaced by GDP at the Gɑ subunit, the trimeric G protein complex dissociates into Gɑ and Gβγ subunits. The Gɑ subunit in turn inhibits adenylyl cyclase from producing cAMP, reduces the conductance of voltage-gated Ca2+ channels, and opens inwardly rectifying K+ (GIRK) channels to prevent neuronal excitation and stop the propagation of action potentials. In addition, the Gβγ subunit activates the phospholipase C (PLC)/phosphokinase C (PKC) pathway to modulate Ca2+ channel activity in the plasma membrane, which may reduce the release of pronociceptive neuropeptides. Overall, activation of the G-protein signaling pathway results in analgesia. On the other hand, GPCR kinases can phosphorylate intracellular portions of opioid receptors, allowing the binding of β-arrestin molecules. The resulting arrestin-opioid receptor complex prevents G protein coupling and promotes receptor internalization via clathrin-dependent pathways, which desensitizes the opioid receptor. In contrast to the analgesia-inducing G-protein pathway, the β-arrestin pathway contributes to ORAEs, notably respiratory depression and gastrointestinal complications, while simultaneously weakening analgesic effects.13
The use of opioid analgesics for postoperative pain management remains popular due to its potent effect, rapid onset, and broad range of formulations.14 However, there are also limitations to opioids due to the notable and prevalent adverse events, including respiratory depression, sedation, nausea, vomiting, pruritus, and decreased bowel motility.15 Without careful monitoring of respiration and oxygen saturation, opioid-induced respiratory depression (OIRD) may lead to hypoxia and respiratory distress in patients receiving opioids. In a 2018 retrospective study, it was shown that among 135,000 adult patients who were given opioids after surgical and endoscopic procedures, over 10% experienced at least one ORAE. In addition, the study found that ORAEs resulted in a 2.9% increase in absolute mortality as well as a 1.6-day increase in length of stay and $8225 increase in cost for the index hospitalization.16 Similarly, in the PRODIGY trial, Khanna et al used continuous capnography and oximetry on 1335 patients receiving parenteral opioids in the general care floor in order to develop a risk prediction tool for OIRD. They found that 46% of patients experienced one or more episodes of respiratory depression and had a mean hospital length of stay three days longer compared to patients without episodes of respiratory depression.17 ORAEs may disproportionately affect patients with certain risk factors, such as sleep apnea, cognitive impairment, history of substance abuse, disease states that alter drug metabolism (eg, renal failure, hepatic failure), and the elderly.8,18 Furthermore, long-term opioid use may result in dependence and addiction. In a 2016 survey of patients receiving chronic opioid therapy (>90 days), the study showed that 27.0% identified post-operative pain as the reason they were started on opioid treatment. Among the same participants, 21.7% self-reported addiction to some type of substance and 32.5% have documented aberrant drug-related behavior, such as self-increased dose, misuse of medication, or use of other non-prescribed drugs.19 Many of these findings shed light on factors contributing to the recent rise of the opioid crisis in the United States. According to the Center for Disease Control and Prevention (CDC), the rate of opioid-involved deaths has increased by 38% while that of prescription opioid-involved deaths increased by 17% from 2019 to 2020.20 This in turn places profound economic burden, with estimates from 2017 showing $471 billion on opioid use disorder and $550 billion on fatal opioid overdose, totaling $1021 billion in cost.21
The concept of multimodal analgesia was first introduced in 1993. The rationale of the strategy for postoperative pain is to leverage the additive and/or synergistic effects of different classes of analgesics to attack pain from multiple angles. The combined use of various drugs should thereby reduce the dosage of medication while minimizing side effects.22 Options in the arsenal of analgesics include acetaminophen, non-steroidal anti-inflammatory drugs (NSAIDs), N-methyl-D-aspartate (NMDA)-receptor antagonists, and gabapentinoids, with each carrying certain risks and benefits.10 For example, acetaminophen and NSAIDs are popular and relatively safe choices that provide acute pain relief after major surgery.23 Acetaminophen and NSAIDs have been shown to decrease opioid consumption as well as postoperative nausea and vomiting in surgery patients who use patient-controlled analgesia (PCA) morphine. However, excessive acetaminophen use is associated with hepatotoxicity, particularly in the elderly or those with chronic alcoholism,14,15 whereas NSAIDs may increase the risk of gastrointestinal bleeding, damage kidney function, and impair wound healing.14 Nevertheless, regional anesthesia and non-opioid analgesics may not be sufficient for all procedures. A recent survey of practicing physicians showed that although there were concerns over ORAEs, opioids were still the most likely IV analgesic to be prescribed for moderate to severe acute postoperative pain. Furthermore, they identified the availability of effective analgesics associated with fewer side effects as the top unmet need in acute postoperative pain management.24 In this review, we will discuss oliceridine, a novel mu-opioid receptor agonist, and its potential role in acute postoperative pain management. This includes a comprehensive overview of the clinical findings, and although preclinical studies will be discussed, further detail can be found in other reviews such as Azzam et al.25 We have systematically searched the PubMed database for all studies related to oliceridine conducted between 2013 and 2023. All articles identified were reviewed, and all clinical trials were included. The search flowchart and risk of bias assessment are provided in Figures 1 and 2. These findings are then narratively described.
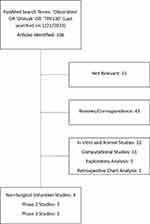 |
Figure 1 Flowchart summarizing literature search results for all studies related to oliceridine. |
 |
Figure 2 Risk of bias assessment for phase II and III randomized clinical trials. |
Basic Knowledge on Oliceridine
Oliceridine is a novel mu-opioid receptor agonist that is part of a new class of biased ligands that preferentially activate one intracellular signaling pathway over another. By selectively stimulating G-protein coupling and downregulating β-arrestin recruitment, there is potential to limit the development of ORAEs and widen the therapeutic window, fulfilling the need for an analgesic with the efficacy of a conventional opioid but with a reduced side effect profile.26 Bohn et al27 found that β-arrestin 2 knockout mice had a more potentiated and prolonged response to morphine compared to wild-type mice undergoing a hot-plate test. Nevertheless, Raehal et al28 showed that unwanted side effects were actually diminished. In particular, β-arrestin 2 knockout mice had less constipation, assessed by bead expulsion test and amount of fecal boli over a 6-hour period, and respiratory depression, assessed by whole-body plethysmography. Subsequently, after screening a collection of small molecules, Chen et al29 identified a promising starting compound with low β-arrestin activity. This would be further optimized for potency and ligand bias by investigating structure–activity relationships, leading to the discovery of TRV130. This compound would later be renamed oliceridine and will be referred to as such for the remainder of this review. DeWire et al30 then found that oliceridine had a G-protein coupling efficacy of 71% of a full synthetic agonist, which was comparable to morphine (92%). Furthermore, oliceridine showed significantly less mu-opioid receptor phosphorylation and subsequent internalization, resulting in only 14% of the efficacy of morphine for β-arrestin 2 recruitment. In vitro studies using human embryonic kidney cells showed that oliceridine possesses higher potency (8 nM for oliceridine versus 50 nM for morphine).30 Using rodent models to assess the analgesic effect of oliceridine, in vivo studies showed that oliceridine is 4 to 10 times more potent than morphine in achieving robust analgesia in rats.30,31
In addition to its demonstrated efficacy, oliceridine was shown to be safe for use. In preclinical toxicology studies, no specific oliceridine-induced toxicity besides the typical side effects of opioids (eg, decreased activity, lower blood pressure and body temperature) had been found after 28 days of continuous IV oliceridine infusion in rats or 14 days in monkeys. No adverse events were observed in rats and monkeys at doses of 0.5 mg/kg/hr and 1 mg/kg/hr respectively, which are 3-fold and 22-fold the maximal daily exposure of 40 mg/day for humans. Moreover, findings suggest that oliceridine caused less adverse effects.32 By comparing oliceridine to morphine in rodent models, DeWire et al30 found that a subanalgesic dose of subcutaneous oliceridine caused no gastrointestinal dysfunction (ie, colonic immotility and subsequently delayed fecal output), whereas a similar subanalgesic dose of subcutaneous morphine led to significant gastrointestinal issues. In addition, administering oliceridine at 8 times the equianalgesic dose did not lead to severe levels of respiratory suppression, while only a 4-fold equianalgesic dose of morphine led to a statistically significant increase in pCO2. In summary, these results indicate that oliceridine may be a safer therapy compared to traditional opioids.
Prolonged opioid exposure may lead to the development of tolerance and dependence. Tolerance refers to the reduced analgesic effects when given the same amount of medication, whereas dependence is a maladaptive mechanism to tolerance-prone drugs that manifests as disruptive physical symptoms when a medication is withdrawn. In a mice-model-based preclinical study, Liang et al31 found that the medium effective dose (ED50) of oliceridine before and after 4 days of chronic dosing remained similar, while the ED50 for morphine was higher (ie, less effective) after chronic treatment. This suggests that tolerance is less likely to be observed after prolonged use of oliceridine compared to morphine. In the same study, naloxone-induced jumping in mice, considered an opioid withdrawal symptom, was used as a measure for physical dependence. No significant difference was found in jumping frequency caused by naloxone in oliceridine- versus morphine-treated mice, demonstrating that the level of physical dependence created by oliceridine is similar to or no different from that generated by morphine.31 Similar results have been replicated in other studies. Mice that underwent a 3-day treatment of oliceridine continued to experience maximal antinociception with additional doses of oliceridine, which indicates the lack of tolerance development. In contrast, those with 3-day treatment of morphine ended up developing tolerance to morphine.33
Another complication to consider in opioid usage is opioid-induced hyperalgesia (OIH), a state of increased sensitivity to pain and decreased pain threshold in which higher doses of opioid may paradoxically worsen one’s pain.8 In preclinical testing, OIH can be assessed by measuring mechanical paw withdraw thresholds in mice before and after treatment with opioid. Liang et al31 found that a 4-day treatment of either oliceridine or morphine produced lower mechanical paw withdraw thresholds compared to the baseline thresholds. However, morphine treatment lowered the threshold significantly more than oliceridine, thereby demonstrating that the extent of sensitization or OIH generated by oliceridine not as severe as that induced by morphine.
Pharmacokinetics and Pharmacodynamics
Oliceridine (brand name Olinvyk) was approved for intravenous use by the FDA in 2020 for the treatment of acute pain severe enough to require an intravenous opioid when alternative treatment is inadequate.34 Its development was comprised of 17 clinical studies. This included 10 different Phase I studies that looked at pharmacokinetics and safety in healthy volunteers, as well as patients with and without hepatic impairment and end stage renal disease (ESRD), 1 human abuse liability study, 1 Phase II open-label study in patients with long bone fractures that was terminated due to lack of enrollment, 2 phase II placebo-controlled studies, 2 Phase III placebo-controlled studies, and 1 phase III open-label study.32 The results of the completed phase II and III studies are summarized in Table 1. Oliceridine is an amine compound and available as a fumarate salt with molecular formula C22H30N2O2S·C4H4O4 dissolved in a clear, colorless, sterile, and preservative-free solution. Each milliliter of the solution contains 1mg of the oliceridine free base, along with L-histidine and mannitol.34 It can be administered either via intermittent bolus or PCA with a recommended starting dose of 1.5mg and a maximum total daily dose of 27mg. This can be followed up with PCA demand doses of 0.35mg, or up to 0.5mg in certain patients, with a 6-minute lockout interval. Supplemental doses of 0.75mg can then be given hourly as needed.34 Oral bioavailability is low (5.77%), and there are currently no plans for development of an oral formulation.32 Its plasma protein binding is estimated to be 77%, and volume of distribution ranges from 90–120L.34 Onset of analgesia occurs in 1–2 minutes and peaks at 6–12 minutes.32 It has a half-life of 1.3–3 hours and metabolized primarily in the liver by CYP3A4 and CYP2D6 P450 into inactive metabolites via oxidation and glucuronidation. These inactive metabolites have a longer half-life of approximately 44 hours and are excreted primarily in the urine (around 70%) with the remainder in the feces. Only 0.97–6.75% of an unchanged oliceridine dose can be found in the urine.34 In addition, a Phase I, open-label, single dose study by Nafziger et al35 found that there was no significant difference in clearance between healthy volunteers and subjects with either ESRD or mild or moderate hepatic impairment. However, patients with decreased CYP2D6 function or severe hepatic impairment may have significantly longer clearance and may require less frequent dosing.
 |
Table 1 Summary of Results for Phase II and III Studies |
Clinical Studies
Based on the promising preclinical results, Soergel et al36 conducted a crossover study of 30 healthy volunteers, who were randomized to one of five groups: oliceridine (1.5, 3, or 4.5mg IV), morphine 10mg IV, or placebo. These initial oliceridine doses were selected based on a previous trial that used pupillometry data of 74 healthy volunteers to determine the expected pharmacodynamically active range.37 Analgesic effects were assessed by the cold pain test (CPT), in which a continuous circulating cold water bath was used to measure hand removal latency and time to first perceptible pain. In addition, ventilatory response to hypercapnia (VRH) was assessed by using a gas-mixing rebreathing apparatus to deliver 95% O2 and 5% CO2 into a reservoir bag. Subjects would then breathe this hypercapnic gas mixture through a facemask for five minutes, and the ratio of minute ventilation over end-tidal CO2 would be measured at designated intervals and compared to baseline. They found that all doses of oliceridine resulted in significant increases of hand removal latency compared to placebo (81, 105, and 116 seconds vs 41 seconds latency), with the 3 and 4.5mg showing higher peak analgesia compared to morphine (75 seconds latency). Furthermore, all doses were associated with less reduction in respiratory drive compared to morphine (−7.3, −7.6, and −9.4 h*L/min vs −15.9 h*L/min). Notably, respiratory depression was not absent with oliceridine but very transient. Even though both displayed a similar peak effect at 30 minutes, the effect of morphine on VRH would last all the way through the final measurement at 4 hours. Finally, subjects taking the 1.5 and 3mg doses experienced less severe nausea compared to those taking the 4.5mg dose and morphine (n = 0, 1 vs n = 9, 7). Overall, it would appear that the 3mg dose would provide significant efficacy while still maintaining the reduction in PONV exhibited by lower doses of oliceridine.
Efficacy was further explored in phase II studies. Viscusi et al38 examined the use of oliceridine in patients undergoing bunionectomy under regional anesthesia with a popliteal sciatic nerve block. Patients reporting a numeric rating scale (NRS) greater than or equal to four within nine hours after discontinuation of the regional anesthetic infusion were included. The primary endpoint looked at the time-weighted average change in NRS over a 48-hour period. The study was split into two phases, with the first phase acting as a pilot phase to determine the dosing regimen for the second phase. In the first phase, 144 subjects were randomized to one of six groups: oliceridine (1, 2, 3, or 4mg IV q4h), morphine 4mg IV q4h, or placebo. After an interim analysis found that analgesia was not adequately maintained for the full four-hour dosing interval, the oliceridine regimen for the second phase, which randomized 195 subjects, was switched to 0.5, 1, 2, or 3mg IV q3h. They found that oliceridine 2 and 3mg significantly reduced pain intensity compared with placebo. Furthermore, the 3mg dose resulted in a significant improvement compared with morphine (−1.4 and −2.4 vs −1.3 least-squares mean reduction). Oliceridine was also rapid in onset, with both oliceridine 2 and 3mg leading to peak categorical pain relief at less than 5 minutes compared with greater than 20 minutes for morphine. No serious adverse events were reported for all treatment groups, with the most common adverse events being those expected of a mu-opioid receptor agonist, such as nausea, dizziness, headache, and vomiting. However, although oliceridine carried a lower risk of desaturation (integrated mean percent change from baseline in oxygen saturation over 0 to 24.5 hours −1.5, −0.5, −2.3, and −5.1%·hour vs −14.5%·hour), this was not clinically significant, and there was not a sufficient number of patients to determine whether oliceridine was associated with less respiratory depression events.
Another phase II study by Singla et al39 looked at 200 patients with moderate to severe pain following abdominoplasty. In contrast with the fixed bolus dosing of the previous study, oliceridine was administered via PCA to better reflect the as-needed dosing typical for postoperative patients. This would also be another solution for the problem encountered in the previous study, in which adequate analgesia was not sustained for a full four-hour dosing interval. Similar to the previous study, an interim analysis was planned since this would be the first time administering via PCA. Oliceridine regimen A consisted of two loading doses of 0.75mg separated by 10 minutes that were followed by demand doses of 0.1mg with a 6-minute lockout interval, while the morphine regimen consisted of two loading doses of 2mg separated by 10 minutes that were followed by demand doses of 1mg with a 6-minute lockout interval. After the interim analysis, oliceridine regimen B was adjusted to have demand doses of 0.35mg. Just like the previous study, they looked at the time-weighted average change in numerical pain rating scale (NPRS) but this time over a 24-hour period. Both oliceridine regimens were found to produce significant reduction in pain compared to placebo (2.3 and 2.1 points). This was comparable to the morphine regimen over the 24-hour period (2.1 points). However, oliceridine was more rapid, with regimen B showing more significant reduction when measured at 5 minutes following the loading dose and median time to meaningful pain relief of 0.3 hours compared with 1.0 hours for morphine. Unlike the previous phase II study, there were no dosing regimens associated with greater analgesia than morphine. By using a PCA, patients in both groups were able to self-titrate to a desired level of analgesia while balancing potential side effects. However, this does not eliminate the possibility of developing ORAEs, and a systematic review by McNicol et al showed that other than pruritus, both PCA and non-patient-controlled analgesia had a similar incidence of adverse events.40 Similar to the previous study, oliceridine had no serious adverse events, with the most common adverse events being nausea, vomiting, and headache. Post hoc analyses showed that patients treated with oliceridine experienced significantly less nausea (41% and 46% vs 72%), vomiting (15% for both vs 42%), and respiratory effects (15% and 31% vs 53%) compared to morphine. Here, respiratory effects included hypoxia, bradypnea, decreased respiratory effort, or hypoventilation.39
APOLLO-1 was a Phase III, multicenter, randomized, controlled clinical trial in 389 patients undergoing bunionectomy. Similar to the first phase II study, patients were only enrolled if they experienced moderate to severe pain nine hours after discontinuation of the regional anesthetic infusion. However, just like the second phase II study, medication was administered via PCA. Loading doses of oliceridine 1.5mg, morphine 4mg, or volume-matched placebo were followed by demand doses with a 6-minute lockout interval. Clinician-administered supplemental doses of oliceridine 0.75mg, morphine 2mg, or volume-matched placebo were also permitted hourly. Subjects were randomized to one of five demand dose regimens: oliceridine (0.1, 0.35, or 0.5mg), morphine 1mg, or placebo. The primary endpoint was the percentage of treatment responders, defined as a greater than or equal to 30% improvement in time-weighted average pain intensity from baseline without the use of rescue analgesia, early discontinuation, or reaching dosing limits, for the 48-hour period. All dosing regimens of oliceridine were found to have a significantly higher responder percentage compared to placebo (50%, 62%, and 65.8% vs 15.2%). In addition, oliceridine 0.35 and 0.5mg regimens were comparable to morphine (71.1%). Adverse effects increased in a dose-dependent manner. For example, the incidence of gastrointestinal adverse events was lower than morphine in the 0.1 and 0.35mg dosing regimens (40.8% and 59.5% vs 72.4%) while similar to morphine in the 0.5mg dosing regimen (70.9%). In order to evaluate whether oliceridine reduces the risk of OIRD, they looked at the respiratory safety burden (RSB), which was designed to integrate various observed respiratory safety events into a single composite outcome. This included changes in respiratory rate, oxygen saturation, and sedation using the Moline-Roberts Pharmacologic Sedation Scale. This was calculated as the incidence of these events multiplied by their cumulative duration. Although oliceridine resulted in a reduction in RSB (0.04–0.8 hours vs 1.1 hours for morphine), it did not reach statistical significance.41
APOLLO-2 was another phase III, multicenter, randomized, controlled clinical trial in 401 patients with moderate to severe pain following abdominoplasty that used the same dosing regimens and endpoints as the previous study. Again, they found that oliceridine had a higher responder percentage compared to placebo (61%, 76.3%, and 70% vs 45.7%), with the 0.35 and 0.5mg dosing regimens being comparable to morphine (78.3%). The incidence of gastrointestinal adverse events was also lower than morphine in the 0.1 and 0.35mg dosing regimens (49.4% and 65.8% vs 79.3%) while similar to morphine in the 0.5mg dosing regimen (78.8%). The 0.35 and 0.5mg dosing regimens also led to a reduction in RSB but still not at statistical significance (1.48 and 1.59 hours vs 1.72 hours for morphine). Finally, only two serious adverse events, lethargy and syncope, were found to be related to oliceridine 0.5mg, which resolved without further sequelae.42
ATHENA was a phase III, multicenter, open-label study in 768 patients with moderate to severe acute pain requiring parenteral opioid therapy. Its goal was to better reflect how oliceridine would be used in a real-world setting. As a result, unlike the other two phase III studies, there were fewer protocol restrictions. They enrolled not only postoperative patients but also non-surgical patients with painful medical conditions. The most common surgical procedures were orthopedic, colorectal, and gynecologic. In addition, they included many patients that were excluded from previous studies, particularly those with risk factors for ORAEs, such as advanced age, obesity, and sleep apnea. Furthermore, multimodal analgesia was permitted, and oliceridine could be administered either by bolus or PCA. IV bolus dosing consisted of a loading dose of 1 to 2mg and a supplemental dose of 1mg within 15 minutes if needed. This would be followed by doses of 1 to 3mg every 1 to 3 hours as needed. PCA dosing consisted of a loading dose of 1.5mg and demand doses of 0.5mg with a 6-minute lockout interval. Supplemental doses of 1mg could be given as needed. Oliceridine was found to be effective and rapid, resulting in a 2.2-point reduction in NRS pain score within 30 minutes. The most common adverse events were nausea (31%), constipation (11%), and vomiting (10%). Serious adverse effects occurred in 3% of patients, out of which only three were considered as possibly related to oliceridine.43
Clinical studies have shown that oliceridine has efficacy that is superior over placebo and comparable to morphine in both hard and soft tissue models.38,39,41,42 However, its effect on the development of ORAEs has been mixed. At dosing regimens equianalgesic to morphine, oliceridine has been associated with a lower incidence of gastrointestinal adverse events.39,41,42 Furthermore, Beard et al44 performed an exploratory analysis of the APOLLO studies and found that when adjusted for analgesia, the odds of achieving complete GI response, defined as the proportion of patients with no vomiting or rescue antiemetic use, were 2–3 times higher with oliceridine than with morphine.
Nevertheless, its ability to reduce the risk of OIRD remains uncertain. Earlier clinical studies showed promise in regards to less reduction in VRH and lower incidence of respiratory effects.36,39 However, the APOLLO studies did not reach statistical significance when looking at RSB.41,42 Respiratory depression is a relatively rare complication, and neither study enrolled patients that would be considered high risk. As a result, it is possible that these studies were just underpowered. Furthermore, there is no standardized method of measuring OIRD. Subsequently, Ayad et al45 conducted an exploratory analysis of the APOLLO studies and looked at dosing interruption due to respiratory events and average cumulative duration of dosing interruption instead, which they felt would be a more objective surrogate marker. These were lower with all oliceridine dosing regimens compared with morphine (0.1mg: 3.2%, 0.35mg: 13.9%, 0.5mg: 15.1% vs morphine 22%). In addition, Dahan et al46 reanalyzed the pharmacokinetic-pharmacodynamic data from the healthy volunteer study by Soergel et al36 in order to develop utility functions, which were calculated as the probability of analgesia minus the probability of respiratory depression. For all clinically relevant concentrations, oliceridine had a positive utility function, whereas morphine was predominantly negative, indicating that oliceridine use leads to a greater probability of analgesia than respiratory depression.
In contrast with the APOLLO studies, ATHENA did not exclude patients with a higher risk of respiratory depression. Only 6% of patients experienced oxygen saturation less than 90%, and none required naloxone.43 However, ATHENA lacked a concurrent control group. As a result, Bergese et al47 performed a retrospective chart analysis in which a control group was identified using directed chart review of a subset of sites participating in ATHENA. These patients were admitted at the same time of ATHENA enrollment and treated with IV morphine alone or in combination with other opioids for postoperative pain. They found that patients receiving oliceridine had a significantly lower incidence of OIRD events compared to those receiving conventional opioids (8.0% vs 30.7%). Notably, this was also seen in high-risk patients (9.1% vs 34.7%). Finally, in an exploratory analysis, Brzezinski et al48 found that oliceridine use in patients with advanced age or obesity was not associated with an increased risk of OIRD (10.8% in elderly vs 15.1% in younger adults, 14% in obese vs 13.4% in non-obese, and 10.8% in patients that were both elderly and obese).
In order to further investigate the respiratory effects of oliceridine, Simons et al49 recently conducted a crossover study in 18 healthy elderly volunteers, who were randomized into either low-dose or high-dose oliceridine (0.5 or 2mg IV) or morphine (2 or 8mg IV). The primary outcome was the effect on ventilation at an extrapolated end-tidal carbon dioxide at 55mmHg. This was performed by using a face mask connected to a pneumotachograph and pressure transducer system. Subjects would first start with a 4-minute period of relaxed breathing of room air. This would then be followed by hyperventilation of a hyperoxic gas mixture for 2–3 minutes, normal breathing of a hyperoxic gas mixture for 30 seconds, and finally rebreathing from a 6L balloon containing 7% CO2 and 93% O2 for 3–4 minutes. Minute ventilation, end-tidal oxygen and carbon dioxide, and oxygen saturation would then be collected. In addition, drug concentrations would also be determined from blood samples drawn at designated intervals. They found that while low-dose oliceridine did not lead to any significant respiratory effect, high-dose oliceridine and both morphine doses led to respiratory depression that peaked at 0.5 to 1 hour after dosing. However, similar to earlier findings by Soergel et al,36 the respiratory depression caused by oliceridine was more transient and characterized by a faster return to baseline, with a blood effect-site equilibration half-life of 44.3 ± 6.1 min vs 214 ± 27 min for morphine.49
Conclusion
Despite the current advances in regional anesthesia and non-opioid analgesia, opioids have remained essential in the management of acute postoperative pain. However, their use is limited by the development of ORAEs. Oliceridine is a novel mu-opioid receptor agonist that selectively activates G-protein signaling and downregulates β-arrestin recruitment. Although it has proven to have efficacy comparable to morphine and may potentially have a reduced side effect profile, it would still be difficult to beat the cost-effectiveness of morphine. However, Simpson et al50 developed a health economic model that estimated a $96,623 greater expenditure in pain medications when using oliceridine instead of morphine for a group of 1000 patients. Nevertheless, this cost could be offset by a reduced incidence of ORAEs. When using oliceridine, the cost for managing ORAEs would be $528,424 versus $852,429 for morphine, resulting in a net savings of $324,005. Furthermore, when implementing a more targeted approach and using oliceridine only in high-risk patients and morphine for everyone else, the net savings could be increased to $363,944.51
Ultimately, whether oliceridine can become a significant component of a multimodal analgesic regimen, especially for those with risk factors, such as advanced age, obesity, and sleep apnea, depends on the reduced incidence of ORAEs, such as respiratory depression and gastrointestinal complications. Whereas oliceridine has been associated with a lower incidence of gastrointestinal adverse events,45–47 its ability to reduce the risk of OIRD remains inconclusive since later clinical trials did not reach statistical significance.41,42 However, earlier data and multiple exploratory analyses show promise and suggest that further research could be warranted.36,39,45–47,49 Because of this uncertainty, a Phase IV study called VOLITION is currently underway to further explore oliceridine’s effect on OIRD with plans to enroll 200 subjects and an estimated completion date of 2025. Respiratory compromise will be defined as a composite of end-tidal carbon dioxide <15mmHg for ≥3 minutes, respiratory rate ≤5 breaths/minute for ≥3 minutes, SpO2 ≤ 85% for ≥3 minutes, apnea episode lasting >30 seconds, and any serious respiratory event.25
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gan TJ, Habib AS, Miller TE, White W, Apfelbaum JL. Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey. Curr Med Res Opin. 2014;30(1):149–160. doi:10.1185/03007995.2013.860019
2. Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97(2):534–540. doi:10.1213/01.ANE.0000068822.10113.9E
3. Walker EMK, Bell M, Cook TM, et al. Patient reported outcome of adult perioperative anaesthesia in the United Kingdom: a cross-sectional observational study. Br J Anaesth. 2016;117(6):758–766. doi:10.1093/bja/aew381
4. Gerbershagen HJ, Aduckathil S, van Wijck AJ, Peelen LM, Kalkman CJ, Meissner W. Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiology. 2013;118(4):934–944. doi:10.1097/ALN.0b013e31828866b3
5. Svensson I, Sjöström B, Haljamäe H. Influence of expectations and actual pain experiences on satisfaction with postoperative pain management. Eur J Pain. 2001;5(2):125–133. doi:10.1053/eujp.2001.0227
6. Thienthong S, Niruthisard S, Ittichaikulthon W, et al. Clinical guidance for acute postoperative pain management 2019 The Royal College of Anesthesiologists of Thailand (RCAT) and The Thai Association for Study of Pain (TASP). Thai J Anesthesiol. 2020;46(1):47–70.
7. Mwaka G, Thikra S, Mung’ayi V. The prevalence of postoperative pain in the first 48 hours following day surgery at a tertiary hospital in Nairobi. Afr Health Sci. 2013;13(3):768–776. doi:10.4314/ahs.v13i3.36
8. Gandhi K, Heitz JW, Viscusi ER. Challenges in acute pain management. Anesthesiol Clin. 2011;29(2):291–309. doi:10.1016/j.anclin.2011.04.009
9. Joshi GP, Ogunnaike BO. Consequences of inadequate postoperative pain relief and chronic persistent postoperative pain. Anesthesiol Clin North Am. 2005;23(1):21–36. doi:10.1016/j.atc.2004.11.013
10. Gan TJ. Poorly controlled postoperative pain: prevalence, consequences, and prevention. J Pain Res. 2017;10:2287–2298. doi:10.2147/JPR.S144066
11. Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618–1625. doi:10.1016/S0140-6736(06)68700-X
12. Stein C. Opioid Receptors. Annu Rev Med. 2016;67(433–451):433–451. doi:10.1146/annurev-med-062613-093100
13. Tan HS, Habib AS. Oliceridine: a novel drug for the management of moderate to severe acute pain - a review of current evidence. J Pain Res. 2021;14:969–979. doi:10.2147/JPR.S278279
14. Lovich-Sapola J, Smith CE, Brandt CP. Postoperative pain control. Surg Clin North Am. 2015;95(2):301–318. doi:10.1016/j.suc.2014.10.002
15. Garimella V, Cellini C. Postoperative pain control. Clin Colon Rectal Surg. 2013;26(3):191–196. doi:10.1055/s-0033-1351138
16. Shafi S, Collinsworth AW, Copeland LA, et al. Association of opioid-related adverse drug events with clinical and cost outcomes among surgical patients in a large integrated health care delivery system. JAMA Surg. 2018;153(8):757–763. doi:10.1001/jamasurg.2018.1039
17. Khanna AK, Bergese SD, Jungquist CR, et al. Prediction of opioid-induced respiratory depression on inpatient wards using continuous capnography and oximetry: an international prospective, observational trial. Anesth Analg. 2020;131(4):1012–1024. doi:10.1213/ANE.0000000000004788
18. Small C, Laycock H. Acute postoperative pain management. Br J Surg. 2020;107(2):e70–e80. doi:10.1002/bjs.11477
19. Callinan CE, Neuman MD, Lacy KE, Gabison C, Ashburn MA. The initiation of chronic opioids: a survey of chronic pain patients. J Pain. 2017;18(4):360–365. doi:10.1016/j.jpain.2016.11.001
20. Wide-ranging online data for epidemiologic research (WONDER). Atlanta. GA: CDC, National Center for Health Statistics; 2021. Available from http://wonder.cdc.gov.
21. Luo F, Li M, Florence C. State-level economic costs of opioid use disorder and fatal opioid overdose - United States, 2017. MMWR Morb Mortal Wkly Rep. 2021;70(15):541–546. doi:10.15585/mmwr.mm7015a1
22. Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. 1993;77(5):1048–1056. doi:10.1213/00000539-199311000-00030
23. Maund E, McDaid C, Rice S, Wright K, Jenkins B, Woolacott N. Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs for the reduction in morphine-related side-effects after major surgery: a systematic review. Br J Anaesth. 2011;106(3):292–297. doi:10.1093/bja/aeq406
24. Gan TJ, Epstein RS, Leone-Perkins ML, Salimi T, Iqbal SU, Whang PG. Practice patterns and treatment challenges in acute postoperative pain management: a survey of practicing physicians. Pain Ther. 2018;7(2):205–216. doi:10.1007/s40122-018-0106-9
25. Azzam AAH, Lambert DG. Preclinical discovery and development of oliceridine (Olinvyk®) for the treatment of post-operative pain. Expert Opin Drug Discov. 2022;17(3):215–223. doi:10.1080/17460441.2022.2008903
26. Gan TJ, Wase L. Oliceridine, a G protein-selective ligand at the μ-opioid receptor, for the management of moderate to severe acute pain. Drugs Today. 2020;56(4):269–286. doi:10.1358/dot.2020.56.4.3107707
27. Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286(5449):2495–2498. doi:10.1126/science.286.5449.2495
28. Raehal KM, Walker JK, Bohn LM. Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther. 2005;314(3):1195–1201. doi:10.1124/jpet.105.087254
29. Chen XT, Pitis P, Liu G, et al. Structure-activity relationships and discovery of a G protein biased μ opioid receptor ligand, [(3-methoxythiophen-2-yl)methyl]({2-[(9R)-9-(pyridin-2-yl)-6-oxaspiro-[4.5]decan-9-yl]ethyl})amine (TRV130), for the treatment of acute severe pain. J Med Chem. 2013;56(20):8019–8031. doi:10.1021/jm4010829
30. DeWire SM, Yamashita DS, Rominger DH, et al. A G protein-biased ligand at the μ-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Ther. 2013;344(3):708–717. doi:10.1124/jpet.112.201616
31. Liang DY, Li WW, Nwaneshiudu C, Irvine KA, Clark JD. Pharmacological characters of oliceridine, a μ-opioid receptor G-protein-biased ligand in mice. Anesth Analg. 2019;129(5):1414–1421. doi:10.1213/ANE.0000000000003662
32. Food and Drug Administration. Oliceridine briefing document: FDA advisory committee meeting; 2018. Available from: https://www.fda.gov/media/121230/download.
33. Altarifi AA, David B, Muchhala KH, Blough BE, Akbarali H, Negus SS. Effects of acute and repeated treatment with the biased mu opioid receptor agonist TRV130 (oliceridine) on measures of antinociception, gastrointestinal function, and abuse liability in rodents. J Psychopharmacol. 2017;31(6):730–739. doi:10.1177/0269881116689257
34. Food and Drug Administration (FDA). Highlights of prescribing information - olinvyk (oliceridine); 2021. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/210730s001lbl.pdf.
35. Nafziger AN, Arscott KA, Cochrane K, Skobieranda F, Burt DA, Fossler MJ. The influence of renal or hepatic impairment on the pharmacokinetics, safety, and tolerability of oliceridine. Clin Pharmacol Drug Dev. 2020;9(5):639–650. doi:10.1002/cpdd.750
36. Soergel DG, Subach RA, Burnham N, et al. Biased agonism of the μ-opioid receptor by TRV130 increases analgesia and reduces on-target adverse effects versus morphine: a randomized, double-blind, placebo-controlled, crossover study in healthy volunteers. Pain. 2014;155(9):1829–1835. doi:10.1016/j.pain.2014.06.011
37. Soergel DG, Subach RA, Sadler B, et al. First clinical experience with TRV130: pharmacokinetics and pharmacodynamics in healthy volunteers. J Clin Pharmacol. 2014;54(3):351–357. doi:10.1002/jcph.207
38. Viscusi ER, Webster L, Kuss M, et al. A randomized, Phase 2 study investigating TRV130, a biased ligand of the μ-opioid receptor, for the intravenous treatment of acute pain. Pain. 2016;157(1):264–272. doi:10.1097/j.pain.0000000000000363
39. Singla N, Minkowitz HS, Soergel DG, et al. A randomized, Phase IIb study investigating oliceridine (TRV130), a novel µ-receptor G-protein pathway selective (μ-GPS) modulator, for the management of moderate to severe acute pain following abdominoplasty. J Pain Res. 2017;10:2413–2424. doi:10.2147/JPR.S137952
40. McNicol ED, Ferguson MC, Hudcova J. Patient controlled opioid analgesia versus non-patient controlled opioid analgesia for postoperative pain. Cochrane Database Syst Rev. 2015;2015(6):CD003348. doi:10.1002/14651858.CD003348.pub3
41. Viscusi ER, Skobieranda F, Soergel DG, Cook E, Burt DA, Singla N. APOLLO-1: a randomized placebo and active-controlled phase III study investigating oliceridine (TRV130), a G protein-biased ligand at the µ-opioid receptor, for management of moderate-to-severe acute pain following bunionectomy. J Pain Res. 2019;12:927–943. doi:10.2147/JPR.S171013
42. Singla NK, Skobieranda F, Soergel DG, et al. APOLLO-2: a randomized, placebo and active-controlled phase iii study investigating oliceridine (TRV130), a G protein-biased ligand at the μ-opioid receptor, for management of moderate to severe acute pain following abdominoplasty. Pain Pract. 2019;19(7):715–731. doi:10.1111/papr.12801
43. Bergese SD, Brzezinski M, Hammer GB, et al. ATHENA: a Phase 3, open-label study of the safety and effectiveness of oliceridine (TRV130), A G-protein selective agonist at the µ-opioid receptor, in patients with moderate to severe acute pain requiring parenteral opioid therapy. J Pain Res. 2019;12:3113–3126. doi:10.2147/JPR.S217563
44. Beard TL, Michalsky C, Candiotti KA, et al. Oliceridine is associated with reduced risk of vomiting and need for rescue antiemetics compared to morphine: exploratory analysis from two phase 3 randomized placebo and active controlled trials. Pain Ther. 2021;10(1):401–413. doi:10.1007/s40122-020-00216-x
45. Ayad S, Demitrack MA, Burt DA, et al. Evaluating the incidence of opioid-induced respiratory depression associated with oliceridine and morphine as measured by the frequency and average cumulative duration of dosing interruption in patients treated for acute postoperative pain. Clin Drug Investig. 2020;40(8):755–764. doi:10.1007/s40261-020-00936-0
46. Dahan A, van Dam CJ, Niesters M, et al. Benefit and risk evaluation of biased μ-receptor agonist oliceridine versus morphine. Anesthesiology. 2020;133(3):559–568. doi:10.1097/ALN.0000000000003441
47. Bergese S, Berkowitz R, Rider P, et al. Low incidence of postoperative respiratory depression with oliceridine compared to morphine: a retrospective chart analysis. Pain Res Manag. 2020;2020:7492865. doi:10.1155/2020/7492865
48. Brzezinski M, Hammer GB, Candiotti KA, et al. Low incidence of opioid-induced respiratory depression observed with oliceridine regardless of age or body mass index: exploratory analysis from a phase 3 open-label trial in postsurgical pain. Pain Ther. 2021;10(1):457–473. doi:10.1007/s40122-020-00232-x
49. Simons P, van der Schrier R, van Lemmen M, et al. Respiratory effects of biased-ligand oliceridine in older volunteers: a pharmacokinetic-pharmacodynamic comparison with morphine. Anesthesiology. 2022. doi:10.1097/ALN.0000000000004473
50. Simpson KN, Fossler MJ, Wase L, Demitrack MA. Cost-effectiveness and cost-benefit analysis of oliceridine in the treatment of acute pain. J Comp Eff Res. 2021;10(15):1107–1119. doi:10.2217/cer-2021-0107
51. Simpson KN, Fossler MJ, Wase L, Demitrack MA, Wandstrat TL. Budget impact and pharmacy costs with targeted use of oliceridine for postsurgical pain in patients at high risk of opioid-related adverse events. Expert Rev Pharmacoecon Outcomes Res. 2022;22(4):671–681. doi:10.1080/14737167.2022.2038137
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
The Relationship of Postoperative Pain and Opioid Consumption to Postoperative Delirium After Spine Surgery
Sica R, Wilson JM, Kim EJ, Culley DJ, Meints SM, Schreiber KL
Journal of Pain Research 2023, 16:287-294
Published Date: 28 January 2023
Comparison of a Non-Opioid Multimodal Analgesia Protocol with Opioid-Based Patient-Controlled Analgesia for Pain Control Following Robot-Assisted Radical Prostatectomy: A Randomized, Non-Inferiority Trial
Lee JE, Oh J, Lee JN, Ri HS, Lee CS, Yeo J
Journal of Pain Research 2023, 16:563-572
Published Date: 18 February 2023
Perioperative Pain Management and Cancer Outcomes: A Narrative Review
Ramirez MF, Strang A, Roland G, Lasala J, Owusu-Agyemang P
Journal of Pain Research 2023, 16:4181-4189
Published Date: 5 December 2023
