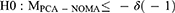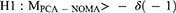Back to Journals » Journal of Pain Research » Volume 16
Comparison of a Non-Opioid Multimodal Analgesia Protocol with Opioid-Based Patient-Controlled Analgesia for Pain Control Following Robot-Assisted Radical Prostatectomy: A Randomized, Non-Inferiority Trial
Authors Lee JE, Oh J , Lee JN , Ri HS , Lee CS, Yeo J
Received 14 November 2022
Accepted for publication 1 February 2023
Published 18 February 2023 Volume 2023:16 Pages 563—572
DOI https://doi.org/10.2147/JPR.S397529
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jinlei Li
Jeong Eun Lee,1 Jinyoung Oh,1 Jun Nyung Lee,2 Hyun-Su Ri,3 Chang Sub Lee,3 Jinseok Yeo1
1Department of Anesthesiology and Pain Medicine, Kyungpook National University School of Medicine, Kyungpook National University Chilgok Hospital, Daegu, Korea; 2Department of Urology, School of Medicine, Kyungpook National University, Kyungpook National University Chilgok Hospital, Daegu, Korea; 3Department of Anesthesiology and Pain Medicine, Kyungpook National University School of Medicine, Kyungpook National University Hospital, Daegu, Korea
Correspondence: Jinseok Yeo, Department of Anesthesiology and Pain Medicine Kyungpook National University School of medicine, Kyungpook National University Chilgok Hospital, Daegu, Korea, Tel +82-53-200-2644, Fax +82-53-200-2027, Email [email protected]
Background: Opioid use after surgery is a potential contributor to the opioid epidemic. An adequate pain control method after surgery while minimizing opioid exposure is needed. This study aimed to compare the effect of non-opioid multimodal analgesia (NOMA) protocol with opioid-based patient-controlled analgesia (PCA) for pain relief after robot-assisted radical prostatectomy (RARP).
Methods: This prospective randomized, open, non-inferiority trial included 80 patients scheduled for RARP. The NOMA group received pregabalin, paracetamol, bilateral quadratus lumborum block, and pudendal nerve block. PCA group received PCA. Pain scores, postoperative nausea and vomiting, opioid requirements, and quality of recovery were recorded 48 hours after surgery.
Results: We found no significant differences in pain scores. The mean difference in pain score during rest at 24 h was 0.5 (95% CI − 0.5 to 2.0). This result demonstrated the non-inferiority of NOMA protocol to PCA at our non-inferiority margin (− 1). In addition, 23 patients in the NOMA group did not receive any opioid agonist for 48 h after surgery. Recovery of bowel function was also faster in the NOMA group than in the PCA group (25.0 hours vs 33.4 hours, p = 0.01).
Limitations: We did not evaluate whether our NOMA protocol could decrease the incidence of new continuous opioid use after surgery.
Conclusion: NOMA protocol successfully controlled postoperative pain and was non-inferior to morphine-based PCA regarding patient-reported pain intensity. It also promoted recovery of bowel function and decreased postoperative nausea and vomiting.
Keywords: multimodal analgesia, opioid sparing, postoperative pain, robot-assisted radical prostatectomy
A Letter to the Editor has been published for this article.
Plain Language Summary
- We developed non-opioid multimodal analgesia (NOMA) protocol comprising pregabalin, bilateral quadratus lumborum block, bilateral pudendal nerve block, and intravenous paracetamol to reduce postoperative opioids after robot-assisted radical prostatectomy.
- Our NOMA protocol showed non-inferiority in postoperative pain control compared to opioid-based patient-controlled analgesia, and more than half of the patients did not use any opioids 48 hours after surgery.
- It also decreased opioid-related side effects such as postoperative nausea and vomiting and enhanced recovery of bowel function after anesthesia.
Introduction
Opioids are the primary analgesics for pain management after surgery. Opioid-based patient-controlled analgesia (PCA) allows effective and safe pain relief and has become the keystone to postoperative pain control.1 However, opioids can cause adverse drug events such as nausea, vomiting, urinary retention, delayed recovery of bowel function, sedation, delirium, and respiratory depression.2 In addition, persistent opioid use after surgery is a new problem and a potential contributor to the opioid epidemic.3
Prostate cancer is the second most common malignancy among men, with 1,276,106 new cases diagnosed worldwide in 2018.4 Radical prostatectomies are primarily employed in treating prostate cancer. Robot-assisted radical prostatectomy (RARP) reduces perioperative blood loss, length of hospital stay, perioperative complications, pain, and opioid consumption compared to open surgery.5 RARP reduces new persistent opioid use by 35% compared to open surgery. However, 6.5% of opioid-naive patients continue opioid use after RARP.6 Nevertheless, abdominal incisions and urethral catheter-related discomfort are the primary causes of pain after RARP.7 Hence, adequate pain control while minimizing opioid exposure is needed to decrease continued opioid use after surgery.
Multimodal analgesia is defined as two or more analgesic modes targeting different receptors along the pain pathway to improve analgesia while reducing side effects.8 Currently, multimodal analgesia focuses on minimizing opioid use. However, it is not yet known whether opioid-free multimodal analgesia can provide adequate pain control compared to opioid-based PCA in pain control.
Therefore, after reviewing the evidence, we developed non-opioid multimodal analgesia (NOMA) protocol comprising pregabalin, bilateral quadratus lumborum block (QL), bilateral pudendal nerve block, and intravenous paracetamol. We hypothesized that our NOMA protocol could provide adequate analgesia after surgery similar to opioid-based PCA. Therefore, we performed a randomized controlled non-inferiority trial to compare the analgesic effect of the NOMA protocol to that of opioid-based PCA after RARP based on a primary endpoint of the pain scores at 24 h postoperatively.
Methods
This study was approved by the Kyungpook National University Chilgok Hospital Review Board and registered at CRIS (cris.nih.go.kr, KCT0003589) and conducted in accordance with the Declaration of Helsinki. This trial included patients scheduled for RARP between February 2019 and February 2021. A research assistant contacted the patients, explained the study protocol, and obtained written informed consent for their participation in the study.
We included patients aged 20–75 years with American Society of Anesthesiologists Physical Statuses of I and II, who were expected to have a midline incision of <6 cm. We excluded patients with a history of allergy to analgesics, opioids, or gabapentinoids; impaired kidney or liver function; dependency on any drug; sleep apnea; obstructive pulmonary disease; coagulopathy; chronic pain syndrome; chronic use of opioids; use of analgesic medication within 24 h before surgery; and psychotic disorder. Patients who could not provide consent or use the PCA device independently were also excluded. A research assistant trained patients to record their postoperative pain scores using an 11-point numeric rating scale (NRS; “0” for “no pain” to “10” for “worst pain imaginable”).
We randomly allocated patients to the NOMA (n=40) or PCA (n =40) groups in a 1:1 allocation ratio by block randomization with a block size of 4. According to the allocation table, a research assistant assigned the patient group the day before surgery.
On the day of surgery, propofol (1–2 mg/kg) and rocuronium (0.4 mg/kg) were administered to induce anesthesia. All participants inserted an indwelling urinary catheter after an intraurethral 2% lidocaine gel injection. Anesthesia was maintained using desflurane (4–8%) and remifentanil (2–4 ng/mL using a target-controlled infusion pump) to maintain a bispectral index (BISX™ System, Medtronic, Minneapolis, MN, USA) of 40–60 and blood pressure within 20% of the preoperative value. Additional doses of rocuronium were administered as required to maintain muscle relaxation. The patients received maintenance fluid at 6–12 mL/kg/h.
RARPs were performed by two experienced surgeons using a transperitoneal approach with a 4-arm da Vinci Xi robotic system (da Vinci Xi, Intuitive Surgical, California, USA). Most patients underwent standard pelvic lymph node dissection, including dissection of the obturator and external iliac lymph nodes.
The patients in the NOMA group underwent the NOMA protocol. The NOMA protocol has four steps: 1) oral pregabalin 150 mg 2 h before anesthesia on the day of the surgery, 2) preemptive ultrasound-guided lateral QL block on both sides using 25 mL of 0.2% ropivacaine at each side, 3) preemptive pudendal nerve block on both sides using 10 mL of 0.2% ropivacaine, 4) 1.0 g intravenous paracetamol at the end of surgery and every 8 h for 48 h after surgery.
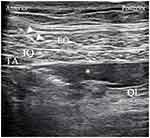
An anesthesiologist who was experienced in nerve blocks performed the bilateral lateral QL blocks and pudendal nerve blocks. QL blocks were performed using an ultrasound transducer (L11-3, Konica Minolta, Tokyo, Japan) placed on the posterior axillary line between the subcostal margin and iliac crest. The tip of the 22-gauge Tuohy needle was placed at the anterolateral border of the QL muscle. After the needle tip penetrated the transversus abdominis aponeurosis, 3 mL of saline was injected to separate the fascial layers, and the exact position of the needle tip was confirmed. After securing negative aspiration of blood, 25 mL of 0.2% ropivacaine was injected on each side (Figure 1).
Bilateral pudendal nerve blocks were performed using an anterior approach technique.9 The patients were placed in a lithotomy position, and a hockey stick ultrasound transducer (HL18-4, Konica Minolta, Tokyo, Japan) was placed medial to the bony ridge of the ischial tuberosity in an oblique sagittal or transverse position (Figure 2). First, the internal pudendal neurovascular bundle was identified using color Doppler imaging. Then, using an out-of-plane approach, a 10-cm, 22-G echogenic needle was inserted into the neurovascular bundle. Once the correct needle position was confirmed, gentle aspiration was applied to prevent intravenous injection, and 0.2% ropivacaine 5 mL was slowly injected.
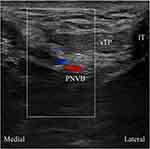 |
Figure 2 Pudendal neurovascular bundle (PNVB) in the color Doppler ultrasound image at the perineum. Abbreviations: IT, ischial tuberosity; sTP, superficial transverse perineal muscle. |
Patients in the PCA group were connected PCA device (GemStar®, Hospira Inc., Lake Forest, IL, USA) at the end of the surgery. The PCA device was set to deliver 1 mg of morphine sulfate bolus with a lockout time of 5 min, without baseline infusion.
Patients in both groups received tramadol (50 mg) as rescue analgesia if the pain score at rest was >5.
The primary outcome was NRS of pain at rest 24h after surgery. The secondary outcomes were NRS pain scores during 48h surgery at rest, during movement, and catheter-related. A research assistant monitored postoperative opioid consumption, rescue analgesic use, and postoperative nausea and vomiting (PONV) 48 h after surgery. The assistant also asked patients their NRS pain scores at rest, on movement, and catheter-related at 2, 6, 12, 24, and 48 h after surgery. We also assessed the time to first flatus by asking patients to measure the extent of the paralytic ileus. We administered the Korean version of the quality of recovery 15 questionnaires (QoR-15) the night before and 48 h after surgery to evaluate the quality of recovery.10 We converted 1mg of tramadol to 0.1mg of morphine to compare postoperative opioid consumption.11
Sample Size Calculation and Statistical Analyses
A pilot study showed a mean pain NRS score at rest of 3.0 at 24 h postoperatively in the PCA group, with a standard deviation of 1.3. We defined an acceptable non-inferiority margin of 1.0 to reflect a practical clinical perspective. Sample sizes of 36 patients per group were calculated to provide a power of 0.9 and a one-sided α of 0.025.12 We recruited 40 patients per group to account for potential dropouts. For non-inferiority evaluation, we calculated the 95% CIs of the median differences in NRS scores using the Hodges–Lehmann estimator.13 Differences in pain scores between PCA groups and NOMA groups were analyzed. We then tested the following null and alternative hypotheses as median differences in pain scores from the PCA and NOMA groups (MPCA-NOMA):
Patient characteristics, surgical values, and QoR-15 are shown as means and standard deviations, while the NRS scores are shown as medians and interquartile ranges. The incidence of PONV and rescue analgesics used were analyzed using the Wilcoxon rank-sum test. We used R statistical package (version 3.5.2, R Foundation for Statistical Computing) to assess non-inferiority. MPCA-NOMA were shown as median and 95% confidence interval (CI). Other statistical calculations were conducted using IBM SPSS Statistics for Windows, version 21.0 (IBM Corporation, Armonk, NY, USA). The analysis target was per-protocol set data, which excluded cases that dropped out after the study to compare the analgesic effect of the two analgesic methods.
Results
We assessed 153 patients and enrolled 80 patients. All patients followed the study protocol, and there were no dropouts. Thus, we analyzed 80 patients (Figure 3). We observed no differences in the baseline patient and surgical values (Table 1).
 |
Table 1 Patient Characteristics and Surgical Values |
 |
Figure 3 The flow of study. Abbreviations: ASA PS, American Society of Anesthesiologists physical status; NOMA, non-opioid multimodal analgesia; PCA, patient-controlled anesthesia. |
The pain scores did not differ significantly between the two groups at any time. The MPCA-NOMA at rest 24h was 0.5 (95% CI −0.5 to 2.0). The 95% CI of MPCA-NOMA at rest 24 h postoperatively was > - 1, indicating the non-inferiority of the NOMA group. The 95% CI of MPCA-NOMA at rest at 2 and 12 h, during movement at 6, 12, 24, and 48 h postoperatively, and catheter-related pain at 2, 6, 12, and 48 hours postoperatively were also > −1. The lower bound of the 95% CI of the median differences in NRS pain scores at rest 6 and 48 h after surgery, during movement at 2 h postoperatively, and catheter-related at 24 h postoperatively were on the non-inferiority margin (Figure 4).
The frequencies of rescue analgesics use did not differ significantly between the two groups. 23 patients in the NOMA group did not receive any opioid agonist for 48 h after surgery. The cumulative postoperative opioid use was significantly lower in the NOMA group (Table 2). The duration of post-anesthesia care unit stay and QoR-15 scores did not differ significantly between the two groups. However, the hours to first flatus and incidence of late PONV differed significantly between the two groups (Table 3). The groups showed no significant differences in the resumption of a regular diet. No serious adverse events were observed during the admission period.
 |
Table 2 Postoperative Opioid Use and Frequency of Rescue Analgesics |
 |
Table 3 Postoperative Recovery Profile |
Discussion
This prospective randomized clinical trial demonstrated that our NOMA protocol offered non-inferior analgesia compared to opioid-based PCA. Our protocol also showed comparable analgesic efficacy for movement-induced and catheter-related pain. In addition, our protocol minimized postoperative opioid consumption and decreased opioid-related side effects such as late PONV and delaying bowel function recovery. Furthermore, 57.5% of patients in the NOMA group did not receive any opioid agonist. Finally, the quality of recovery of the NOMA group was comparable to that of the opioid-based PCA group.
Various perioperative multimodal analgesic methods have emerged in light of the nationwide focus on reducing opioid use to combat the opioid epidemic. Multimodal analgesia protocols reportedly reduced postoperative pain and opioid use but did not eliminate postoperative opioid use in RARP.14 Therefore, we selected the components of the NOMA protocol according to their analgesic, opioid-sparing potential, and side effects.
The incisions of RARP were located above the umbilicus.(Figure 5) To eliminate pain-associated abdominal incisions, we adopted a bilateral QL block. QL block can provide somatic and visceral analgesic effects from the thoracic 7 to the lumbar 2 dermatomes.15 The spread of local anesthetic drugs to the thoracolumbar fascia (TLF) in the QL block could provide analgesia to the incisions above the umbilicus.16
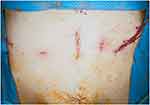 |
Figure 5 Abdominal incisions for robot-assisted laparoscopic prostatectomy. The main incision was made above the umbilicus to insert the camera. |
Bladder spasms, penile, and catheter-related pain after RARP causes discomfort and physical limitations.17 Catheter-related bladder discomfort (CRBD) is a burning sensation spreading from the suprapubic area to the penis with an urge to void.18 The urethra and bladder mucosa contain abundant nerve endings. The mucous membrane can detect stimuli and send them through the afferent nerve to the nerve center, causing bladder and urinary tract irritation. The stimulation of the urethra by the urinary catheter and the bladder trigone area by the water sac of the tube may cause CRBD.18 CRBD has been reported in 47% of men in the post-anesthesia care unit.19 CRBD is resistant to conventional opioid therapy and distressing to patients, which decreases the quality of recovery.19
The pudendal nerve is a somatic nerve derived from the ventral rami of S2–S4 that innervates the urethral muscles and the sphincter of the perineum and pelvic floor. The afferent nerves of the bladder also travel with the pudendal nerve.20 Therefore, pudendal nerve block could prevent catheter stimulation of the urinary tract of male patients and decrease the risk of CRBD after prostatectomy.20 We selected the transperineal approach to perform pudendal nerve block. This approach is performed in the lithotomy position.9 RARP is also performed in the lithotomy position, so we performed the pudendal nerve block without changing position.
Pregabalin is an antiepileptic medication to treat neuropathic pain. It binds to the α2δ subunit of voltage-gated calcium channels and blocks depolarization-induced calcium influx.21 Surgical injuries cause inflammation. Inflammation-induced pain shares the mechanism of neuropathic pain.22 For this reason, pregabalin shows an analgesic effect on postoperative pain. Pregabalin is effective for movement-evoked pain and is associated with a faster postoperative functional recovery.23 Pregabalin also showed an analgesic effect on CRBD after urologic surgery.24 Therefore, we included pregabalin in the NOMA protocol to reduce postoperative pain and CRBD. Paracetamol inhibits prostaglandin synthesis, acts as a cyclooxygenase-2 inhibitor, and directs N-methyl-D-aspartate receptor inhibition.25 Perioperative administration of paracetamol reduced postoperative pain and CRBD after RARP.26,27
Our NOMA protocol preemptively delivers pregabalin and nerve blocks. Preemptive analgesia is an antinociceptive treatment that prevents central sensitization by incisional and inflammatory injuries.28 It reduces postoperative hyperalgesia and prevents central sensitization. Intraoperative and postoperative around-the-clock intravenous paracetamol administration also reduces postoperative opioid consumption. In addition, it minimized opioid use associated with RARP and opioid-related side effects such as PONV and delayed bowel movements. Moreover, our protocol had a comparable effect on CRBD severity to that of opioid-based PCA.
This study has some limitations. First, not all protocol components have been validated for pain relief in RARP. Moreover, the analgesic effect of pregabalin is controversial.29 Therefore, the effects of each element of the NOMA protocol require verification. However, our results showed that the NOMA protocol was effective regardless of the effectiveness of each component and showed the advantage of multimodal analgesia. Second, we did not examine the sensory block area after the block. The perineal approach to the pudendal nerve block is a painful procedure that sometimes requires sedation. Therefore, we decided to perform the blocks after anesthesia. We used ultrasonography to perform QL and pudendal blocks accurately, and our results suggest that our blocks were effective. Third, our NOMA protocol did not accelerate the resumption of a regular diet. This study did not plan to enhance recovery and did not enroll enough subjects to prove the difference. Fourth, we did not evaluate whether our NOMA protocol could decrease the incidence of new continuous opioid use after surgery. Multimodal analgesia decreases opioid consumption immediately after surgery, but it is unclear whether multimodal analgesia prevents chronic opioid use after surgery.3 Further studies are needed to prove the effect of multimodal analgesia on chronic postoperative opioid use. Fourth, we used remifentanil during general anesthesia. The purpose of this study was to remove opioids after surgery, not opioid-free anesthesia. So we used remifentanil during anesthesia. Intraoperative remifentanil could produce opioid-induced hyperalgesia and removal of intraoperative remifentanil may help to reduce postoperative opioid use.30
Conclusion
Our NOMA protocol, including preoperative pregabalin, QL block, pudendal nerve block, and acetaminophen infusion, showed an equivalent effect on postoperative pain control compared to opioid-based PCA. It also showed a comparable impact on CRBD compared to opioid-based PCA. In addition, the NOMA protocol minimized postoperative opioid use and decreased opioid-related side effects.
Data Sharing Statement
The data supporting this study’s findings will be available after de-identification to investigators who want to use the data. Data will be available up to 60 months from the date of issue. We will provide the data after the approval of the review committee. Data sharing proposals should be directed to [email protected] with a data access agreement.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research received no specific grant from the public, commercial, or not-for-profit funding agencies.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Grass JA. Patient-controlled analgesia. Anesth Analg. 2005;101:S44–S61. doi:10.1213/01.ANE.0000177102.11682.20
2. American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116:248–273. doi:10.1097/ALN.0b013e31823c1030
3. Hah JM, Bateman BT, Ratliff J, Curtin C, Sun E. Chronic opioid use after surgery: implications for perioperative management in the face of the opioid epidemic. Anesth Analg. 2017;125:1733–1740. doi:10.1213/ANE.0000000000002458
4. Rawla P. Epidemiology of prostate cancer. World J Oncol. 2019;10:63–89. doi:10.14740/wjon1191
5. D’Alonzo RC, Gan TJ, Moul JW, et al. A retrospective comparison of anesthetic management of robot-assisted laparoscopic radical prostatectomy versus radical retropubic prostatectomy. J Clin Anesth. 2009;21:322–328. doi:10.1016/j.jclinane.2008.09.005
6. Woldu SL, Weinberg AC, Bergman A, et al. Pain and analgesic use after robot-assisted radical prostatectomy. J Endourol. 2014;28:544–548. doi:10.1089/end.2013.0783
7. Shkolyar E, Shih I-F, Li Y, Wong JA, Liao JC. Robot-assisted radical prostatectomy associated with decreased persistent postoperative opioid use. J Endourol. 2020;34:475–481. doi:10.1089/end.2019.0788
8. Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. 1993;77:1048–1056. doi:10.1213/00000539-199311000-00030
9. Rojas-Gómez MF, Blanco-Dávila R, Tobar Roa V, Gómez González AM, Ortiz Zableh AM, Ortiz Azuero A. Regional anesthesia guided by ultrasound in the pudendal nerve territory. Rev Colomb Anestesiol. 2017;45:200–209. doi:10.1016/j.rca.2017.05.005
10. Kim D, Kim JK, Yeo J, Wang H-X. Translation and validation of the Korean version of the postoperative quality of recovery Score QoR-15. Biomed Res Int. 2020;2020:3456234. doi:10.1155/2020/3456234
11. Duthie DJ. Remifentanil and tramadol. Br J Anaesth. 1998;81:51–57. doi:10.1093/bja/81.1.51
12. Julious SA. Sample sizes for clinical trials with normal data. Stat Med. 2004;23:1921–1986. doi:10.1002/sim.1783
13. Rosenkranz GK. A note on the Hodges-Lehmann estimator. Pharm Stat. 2010;9:162–167. doi:10.1002/pst.387
14. Trabulsi EJ, Patel J, Viscusi ER, Gomella LG, Lallas CD. Preemptive multimodal pain regimen reduces opioid analgesia for patients undergoing robotic-assisted laparoscopic radical prostatectomy. Urology. 2010;76:1122–1124. doi:10.1016/j.urology.2010.03.052
15. Elsharkawy H, El-Boghdadly K, Barrington M. Quadratus lumborum block: anatomical concepts, mechanisms, and techniques. Anesthesiology. 2019;130:322–335. doi:10.1097/ALN.0000000000002524
16. Shim J, Jung S, Moon HW, et al. Rectus sheath block for acute pain management after robot-assisted prostatectomy. Aparoscopic nephrectomy: a randomized, double-Blinded, controlled trial. Asian J Surg. 2022;45:1843–1848. doi:10.1016/j.asjsur.2021.10.035
17. Lepor H, Nieder AM, Fraiman MC. Early removal of urinary catheter after radical retropubic prostatectomy is both feasible and desirable. Urology. 2001;58:425–429. doi:10.1016/S0090-4295(01)01218-3
18. Agarwal A, Dhiraaj S, Pawar S, Kapoor R, Gupta D, Singh PK. An evaluation of the efficacy of gabapentin for prevention of catheter-related bladder discomfort: a prospective, randomized, placebo-controlled, double-blind study. Anesth Analg. 2007;105:1454–1457. doi:10.1213/01.ane.0000281154.03887.2b
19. Binhas M, Motamed C, Hawajri N, Yiou R, Marty J. Predictors of catheter-related bladder discomfort in the post-anaesthesia care unit. Ann Fr Anesth Reanim. 2011;30:122–125. doi:10.1016/j.annfar.2010.12.009
20. Akkaya T, Ozkan D, Karakoyunlu N, et al. Pudendal block in transurethral prostatectomy: a randomised trial. Eur J Anaesthesiol. 2015;32:656–657. doi:10.1097/EJA.0000000000000172
21. Shneker BF, McAuley JW. Pregabalin: a new neuromodulator with broad therapeutic indications. Ann Pharmacother. 2005;39:2029–2037. doi:10.1345/aph.1G078
22. Bennett GJ. Can We Distinguish between Inflammatory and neuropathic Pain? Pain Res Manag. 2006;11:11A–15A. doi:10.1155/2006/237251
23. Martins MJ, Martins CPMO, Castro-Alves LJ, et al. Pregabalin to improve postoperative recovery in bariatric surgery: a parallel, randomized, double-blinded, placebo-controlled study. J Pain Res. 2018;11:2407–2415. doi:10.2147/JPR.S176468
24. Srivastava VK, Agrawal S, Kadiyala VN, Ahmed M, Sharma S, Kumar R. The efficacy of pregabalin for prevention of catheter-related bladder discomfort: a prospective, randomized, placebo-controlled double-blind study. J Anesth. 2015;29:212–216. doi:10.1007/s00540-014-1911-x
25. Duggan ST, Scott LJ. Intravenous paracetamol (Acetaminophen). Drugs. 2009;69:101–113. doi:10.2165/00003495-200969010-00007
26. Ergenoglu P, Akin S, Yalcin Cok O, et al. Effect of Intraoperative Paracetamol on catheter-related bladder discomfort: a prospective, randomized, double-blind study. Curr Ther Res. 2012;73:186–194. doi:10.1016/j.curtheres.2012.08.001
27. Inoue S, Miyoshi H, Hieda K, Hayashi T, Tsutsumi YM, Teishima J. Postoperative around-the-clock administration of intravenous Acetaminophen for pain control following robot-assisted radical prostatectomy. Sci Rep. 2021;11:5174. doi:10.1038/s41598-021-84866-7
28. Kissin I, Weiskopf RB. Preemptive analgesia. Anesthesiology. 2000;93:1138–1143. doi:10.1097/00000542-200010000-00040
29. Sisa K, Huoponen S, Ettala O, Antila H, Saari TI, Uusalo P. Effects of pre-emptive pregabalin and multimodal anesthesia on postoperative opioid requirements in patients undergoing robot-assisted laparoscopic prostatectomy. BMC Urol. 2021;21:14. doi:10.1186/s12894-021-00785-9
30. Guignard B, Bossard A, Coste C, et al. Acute opioid tolerance: intraoperative remifentanil increases postoperative pain and morphine requirement. Anesthesiology. 2000;93:409–417. doi:10.1097/00000542-200008000-00019
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.