Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
New Perspectives on Chronic Obstructive Pulmonary Disease
Authors Celli BR, Singh D, Vogelmeier C, Agusti A
Received 10 March 2022
Accepted for publication 2 August 2022
Published 6 September 2022 Volume 2022:17 Pages 2127—2136
DOI https://doi.org/10.2147/COPD.S365771
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Bartolome R Celli,1 Dave Singh,2 Claus Vogelmeier,3 Alvar Agusti4
1School of Medicine, Harvard University, Boston, MA, USA; 2Division of Infection, Immunity & Respiratory Medicine, University of Manchester, Manchester University NHS Hospital Trust, Manchester, UK; 3Department of Medicine, Pulmonary and Critical Care Medicine, German Center for Lung Research (DZL), University of Marburg, Marburg, Germany; 4Respiratory Institute, Hospital Clinic, University of Barcelona, IDIBAPS, CIBERES, Barcelona, Spain
Correspondence: Bartolome R Celli, School of Medicine, Harvard University, 25 Shattuck St, Boston, MA, USA, 02115, Tel +1-617-678-0177, Email [email protected]
Abstract: Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality worldwide; many recent advances have been made in many aspects of the disease. The aim of this article is to illustrate and discuss some of these advances in the management of different types of patients. Large-scale trials have confirmed that long-acting bronchodilator therapy, particularly using the combination of LABA/LAMA, remains the mainstay of COPD treatment, with special attention being paid to careful selection of inhaler devices. The initial choice of pharmacological therapy is based on the GOLD ABCD grouping of patients. It is very important to stress that there is a need to implement a management cycle because COPD is a chronic disease with varying clinical course and a high number of potential comorbidities that may affect morbidity and mortality. Therefore, regular reevaluation of the patient is mandatory. This allows identification of characteristics aimed at maximizing the benefits for a specific patient or a subset of patients. Within this context, the role of the blood eosinophil count as a marker of inhaled corticosteroids response to prevent future exacerbations in patients who, despite appropriate bronchodilator therapy, still suffer from them has been proven to be a useful simple biomarker in medication selection. These advances support the concept of precision medicine, with the goal that patients get the right medicine at the right time for the right reason. Finally, recent studies have shown that early life events may be of critical relevance for the development of COPD. With this as a background, concepts to identify individuals at risk and early identification of cases have become an important objective of current research with the hope of maximizing the effects of therapy and the possibility of impacting disease progression.
Keywords: COPD, ABCD grouping, management cycle, eosinophil, LABA/LAMA
Introduction
Chronic obstructive pulmonary disease (COPD) is a major cause of illness and death worldwide.1,2 It is characterized by persistent respiratory symptoms and airflow limitation and is associated with parenchymal destruction (emphysema), the relative contributions of which vary from person to person.3 The present article provides relevant new evidence on the treatment of COPD and discusses new perspectives to ensure more effective management. The data reported are based on the Industry Evening Symposium entitled “ABCD in COPD”, which was organized by the Menarini Group and held during the European Respiratory Society (ERS) International Congress in Madrid on 29th September 2019.
COPD is a Complex and Heterogeneous Disease
COPD is a complex and heterogeneous disease.4 In this setting, “complex” means that COPD has several elements with non-linear relationships between them (eg, FEV1, exacerbations, symptom perception, comorbidities, …); this means that one component cannot be predicted from another one. Alternatively, “heterogeneous” indicates that not all these elements are present in all patients or, even in a given patient, at all time-points.4 For decades, however, the prevailing paradigm of the pathogenesis of COPD has been that, in susceptible persons, exposure to particulate matter—especially tobacco smoke—leads to clinical disease through acceleration of the age-related decline in lung function, as assessed by the forced expiratory volume in 1 second (FEV1).5 This long-shared view has been the starting point of many clinical trials evaluating therapy for COPD, whose common aim was to reduce the rapid decline in FEV1.6–13 Contrary to expectations, the decline in FEV1 observed in these trials and in observational cohorts of patients with COPD has been inconsistent and smaller than anticipated, particularly in patients with the most severe airflow limitation.6–15 In spite of this relatively slow rate of FEV1 decline noticed in the majority of the recent trial, a meta-analysis of all randomized trials comparing the active arms with placebo, showed a beneficial effect on FEV1 decline compared to active versus the placebo arms in those trials.16 In any case, it is now well accepted that factors other than decline in lung function should be taken into account to better treat our patients where each person may benefit from different treatments. Various updates to the Global Strategy for the Diagnosis, Management, and Prevention of COPD (GOLD) reports have advanced in the direction of focusing not primarily on lung function decline but patient reported parameters. The 2007 edition introduced the concept of a stepwise increase in the drugs taken as the disease worsens, while the 2017 edition introduced the ABCD grouping based on clinical features.17,18 The 2019 update, which was based on new evidence, proposed a more personalized approach to the patient with COPD; this proposal was further confirmed and developed in later reports.3,19,20 Currently, new concepts such as pre-COPD and early COPD are being introduced based on emerging knowledge that may change the timeline for diagnosis and treatment of COPD.21
Changing the Traditional Pathogenic Paradigm of COPD
According to the classic paradigm proposed by Fletcher and Peto in 1977, COPD has been traditionally understood as a self-inflicted disease caused by tobacco smoking characterized by an accelerated decline in lung function.5 This view of COPD has changed recently thanks to new knowledge obtained in epidemiological, clinical, and imaging studies. In 2015, a study of subjects followed over time in three different cohorts, found that close to 50% of patients diagnosed with COPD at 62 years of age had not experienced a rapid decline in FEV1.22 The study stratified subjects at inception according to lung function (FEV1 ≥80% or <80% of predicted). Among 675 persons with an FEV1 <80% of predicted value before 40 years of age, 26% had COPD after 22 years of observation, whereas among 2207 persons who had a baseline FEV1 ≥80% of predicted before 40 years of age, only 7% had COPD after 22 years of observation (P < 0.001). In addition, only 5% of patients with COPD after the observation period were observed to have experienced a rapid decline in FEV1 during this period. On the contrary, 6% of those who had COPD after 22 years, had an initially low FEV1, followed by a normal decline in respiratory function during the observation period. Both groups were diagnosed with COPD at the end of the observation period (Figure 1). These data suggest that spirometry may be useful not only for following up lung function but also for assessing lung function early in life for purposes of prognosis. An initially low FEV1 is a marker of risk for COPD and may identify a patient subset with specific treatment needs. Likewise, the identification of different types of lung function trajectory suggested different disease types and different responses to treatment in subgroups of patients. Finally, patients with FEV1<80% of predicted in early adulthood (25 years of age), have a higher prevalence and earlier incidence of cardiovascular and metabolic diseases, as well as die prematurely, indicating that spirometry in early life may not only identify individuals at risk of COPD but also at risk of other non-communicable diseases of the elderly, with whom COPD may share genetic and environmental risk factors.23
 |
Figure 1 Distribution of annual declines in FEV1 in four lung-function trajectories, in 2864. Participants in the Framingham Offspring Cohort (FOC) and the Copenhagen City Heart Study (CCHS). A normal FEV1 was ≥80% of the predicted value; low FEV1 <80% of the predicted value. COPD was diagnosed according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) grading system. The mean decline in FEV1 was 24 ml per year in trajectory 1 (FEV1 ≥80% at baseline and no COPD at final examination) (A), 2 ml per year in trajectory 2 (FEV1 <80% at baseline and no COPD at final examination) (B), 53 ml per year in trajectory 3 (FEV1 ≥80% at baseline and COPD at final examination) (C), and 27 ml per year in trajectory 4 (FEV1 <80% at baseline and COPD at final examination) (D). The decline in FEV1 in trajectory 3 was considered to be rapid. From Lange P, Celli B, Agust í A, et al. Lung-Function Trajectories Leading to Chronic Obstructive Pulmonary Disease. N Engl J Med.2015;373:111–122. Copyright© 2015 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.22 |
What Has Changed in COPD in Light of Recent Evidence?
Starting in 2017 the GOLD report suggested that the initial treatment of COPD should be based on the ABCD grouping. In 2019, GOLD went one step further and created different treatment pathways that enabled treatment to be adjusted during follow-up based on the patient’s main treatable traits (dyspnea and exacerbations). The 2020 GOLD report stressed that ABCD grouping should just be used for initial pharmacological managements.20 After initial therapy, the treatment should be adjusted based on the presence of symptoms or exacerbations in each individual patient.24 Moreover, a management cycle was introduced where treatment is adjusted over time, with regular reappraisal of the patient’s traits (Figure 2). The GOLD reports state that inhaled therapy is fundamental in all COPD patients and recognize the importance of careful inhaler selection to improve response to treatment and outcomes.3,20 Clinicians are advised to tailor selection of the device to the patient’s abilities and needs, to provide exhaustive training in device technique, and to regularly reassess correct use of the inhaler. The importance of administering more than 1 drug by the same device has also been recognized, as the use of multiple devices requiring different skills reduces the effectiveness of therapy.25
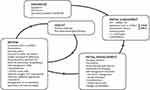 |
Figure 2 Management of COPD. Notes: Reproduced with permission from Global Strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2020. Available From: https://goldcopd.org/wp-content/uploads/2019/11/GOLD-2020-REPORT-ver1.0wms.pdf.20 |
It is also stated that the focus should not only lie on the lung but that comorbid conditions must be considered and addressed that may induce the same symptoms (ie dyspnea, cough).3 A relationship has been found between symptoms of COPD and disease burden in terms of quality of life, health status, daily activities, physical activity, sleep, comorbid anxiety, and depression, as well as the risk of exacerbations and prognosis, so improvement in symptoms should be an integral part of the overall approach to the patient.26 In addition, physical activity was found to be an excellent predictor of mortality in persons with COPD, so an active lifestyle is recommended. All these observations stress the importance of nonpharmacological treatment, which plays a key role in the optimal management of COPD.3,27
Role of Eosinophils in COPD
Precision medicine aims to use clinical and biological information to maximize treatment benefits for a specific patient or a subset of patients. Blood eosinophil count was introduced in the 2019 GOLD update as a predictive biomarker to identify COPD patients in whom inhaled corticosteroids (ICSs) better prevent exacerbations (on top of long-acting bronchodilator treatment). In several large clinical trials, a continuous relationship was found between blood eosinophil count and the effect of ICSs; the higher the eosinophil count, the higher the probability of benefiting from ICSs regarding exacerbation prevention. Available evidence shows that a threshold eosinophil blood count <100/µL can be used to identify those patients who are least likely to benefit from ICS and a blood eosinophil count >300/µL can be used to identify those who are most likely to benefit.28
The role of eosinophils in COPD physiopathology is not a new line of research. As early as in 2000, it was found that nonatopic COPD patients had a higher percentage of eosinophils in induced sputum than controls.29 More recently, a study recruiting nonatopic COPD patients demonstrated that a high blood eosinophil count (>250/μL) was associated with the absolute sputum eosinophil count, bronchoalveolar lavage eosinophil percentage, and submucosal eosinophil counts per mm2.30 The effect of steroids was related to sputum eosinophil count by a randomized, double-blind, crossover trial including patients who had COPD treated with bronchodilators only; the mean sputum eosinophil count fell significantly, and FEV1 was significantly improved, after addition of prednisolone (from 2.4% to 0.4%; mean difference, 6-fold [95% CI, 3.1–11.4]), but not after addition of placebo.31 Data from controlled trials also show that blood eosinophil count can be used to predict the clinical response to ICSs. The pooled analysis of 3 such trials of budesonide-formoterol in patients with COPD and a history of exacerbations and available blood eosinophil counts demonstrated interactions between eosinophil count and the effects of budesonide-formoterol over formoterol. Eosinophil count was an independent predictor of response to budesonide-formoterol in reducing exacerbations (eosinophil count, Pinteraction= 0.013).32 Interestingly, cohort studies have failed to define a single threshold for the blood eosinophil count with regard to exacerbation prediction.33,34 The difference seen between therapeutic trials and observational cohorts may relate to the fact that the exacerbation signal may be diluted in observational cohorts, where many patients do not exacerbate, an identification characteristic that is used as an inclusion criterion in most trials of therapies aiming to decrease the incidence of that outcome. The main use of blood eosinophil counts in clinical practice is as a biomarker to predict ICS effects in patients with a history of exacerbations. In clinical practice, it is important to remember that blood eosinophil counts tend to remain stable over time in many patients, with variations that are insufficient to impact the probability of responding to ICSs.35
Contribution of Large-Scale Randomized Clinical Trials to Treatment of COPD
Data from recent large-scale randomized clinical trials (RCTs) on triple combination therapy (long-acting ß-adrenergic agonist/long-acting muscarinic antagonist/inhaled corticosteroid [LABA-LAMA-ICS]) for COPD have improved our understanding of the correct use of available drugs, added value of ICSs, and eosinophil count as a potential biomarker.
The TRIBUTE study on 1532 patients with symptomatic COPD, severe or very severe airflow limitation, and a history of exacerbation despite maintenance therapy found that moderate-to-severe exacerbation rates were lower in the group receiving triple therapy with ICS (combination of beclomethasone dipropionate, formoterol fumarate, and glycopyrronium) and higher for the group receiving a dual bronchodilator (combination of indacaterol plus glycopyrronium) for 52 weeks. The rate ratio in favor of triple therapy was 0.848 (95% CI, 0.723–0.995, P = 0.043).36 It is noteworthy that patients recruited in TRIBUTE were not at a high risk of exacerbations, as about 80% of them had experienced only 1 episode before recruitment and previous triple therapy was not allowed.
The IMPACT study enrolled COPD patients with a higher risk of exacerbations.37 Patients receiving triple therapy with ICS, LAMA, and LABA (fluticasone furoate, umeclidinium, and vilanterol, respectively) had less frequently experienced moderate-to-severe exacerbations than those treated with LAMA and LABA (umeclidinium-vilanterol), with a 25% increased benefit (rate ratio with triple therapy, 0.75 [95% CI, 0.70–0.81]; 25% difference; P < 0.001).38 Similar findings were recently reported in the ETHOS trial using triple therapy combining formoterol, glycopyrrolate and budesonide used twice daily versus LABA/ICS and LABA/LAMA.39 The results of both studies provide support to the concept that in patients with frequent or severe exacerbations, triple therapy is effective.20
In contrast to the results of triple-therapy studies, the FLAME study showed that dual therapy with LABA/LAMA combination indacaterol-glycopyrronium was superior to a LABA/ICS combination (salmeterol-fluticasone) in reducing the annual rate of all COPD exacerbations; the rate was 11% lower in the indacaterol-glycopyrronium group than in the salmeterol-fluticasone group (3.59 vs 4.03; rate ratio, 0.89 [95% CI, 0.83 to 0.96]; P = 0.003).40 It is noteworthy that this population had a lower risk of exacerbation compared to participants in the IMPACT study.37
Taken together, data from these different studies suggest that dual-bronchodilator therapy (LABA-LAMA) is a good option for patients with a low risk of exacerbations, while patients with severe COPD and a history of moderate and/or severe exacerbations benefit from the addition of an ICS. In conclusion, both the risk of exacerbation and the blood eosinophil count should be taken into consideration when deciding whether ICSs are to be used. A high potential for increased benefit from ICSs is linked to a history of exacerbations, while a higher eosinophil count suggests a higher probability of response to ICSs.
Among the 3 classes of drugs generally used to treat COPD, LABAs are still the mainstay, although the results of the DYNAGITO study, which compared the LABA/LAMA combination tiotropium/olodaterol with the LAMA tiotropium only, showed that the combination studied did not reduce the exacerbation rate as much as expected compared with the LAMA in monotherapy.41
The results of the AMPLIFY study showed the LAMA/LABA combination to be highly effective in the management of COPD.42 This trial, which randomized over 1500 patients with moderate to very severe symptomatic COPD, assessed the efficacy and safety of therapy with the fixed combination of 2 bronchodilators, aclidinium bromide/formoterol fumarate (a LABA/LAMA combination), twice a day compared with single components or tiotropium alone. After 24 weeks of treatment, the aclidinium bromide/formoterol fumarate combination improved lung function compared with the other treatments assessed. The area under the curve (AUC0–3/3 h) for the FEV1 after dosing was significantly improved with respect to all comparators (p < 0.0001). Trough FEV1 was significantly better than with formoterol (p < 0.001). Remarkably, a significant decrease was observed in the overall early morning symptoms compared with the LAMA tiotropium alone (p < 0.05, Figure 3). At week 24, aclidinium bromide/formoterol fumarate significantly decreased the use of rescue medication vs tiotropium (- 0.39 puffs/day, p < 0.05). The benefits of the combination were observed in the first few hours after administration, in the post-dose period, at nighttime, and early in the morning. Finally, the data suggested that some patients may benefit from twice-daily administration of bronchodilators.42
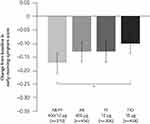 |
Figure 3 Change from baseline in early-morning symptoms after 24 weeks of treatment with the fixed combination of aclidinium bromide/fumarate formoterol, its individual components, and tiotropium. *p≤0.05 vs baseline. Notes: Reproduced with permission from Sethi S, Kerwin E, Watz H, et al. AMPLIFY: a randomized, Phase III study evaluating the efficacy and safety of aclidinium/formoterol vs monocomponents and tiotropium in patients with moderate-to-very severe symptomatic COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:667–682. Copyright 2019, Dove Medical Press.42 |
The prolonged use of aclidinium in patients with COPD has been shown not only to reduce the incidence of exacerbation but has also proven to be well tolerated in relation to cardiovascular events.43 The ASCENT-COPD trial added data to the long-standing controversy over the safety of LAMAs in COPD.43 Indeed, 3630 patients with moderate to very severe COPD and either a history of cardiovascular disease or at least 2 atherothrombotic risk factors were randomized to receive aclidinium or placebo via a dry-powder inhaler twice daily for up to 3 years. A major adverse cardiovascular event occurred in 69 patients taking aclidinium (3.9%) and in 76 patients taking placebo (4.2%) (hazard ratio [HR], 0.89 [1-sided 97.5% CI, 0–1.23]). The annual moderate-to-severe exacerbation rates were reduced in the aclidinium group compared with the placebo group (aclidinium, 0.44; placebo, 0.57; rate ratio, 0.78 [2-sided 95% CI, 0.68–0.89]; P < 0.001).43
Future Perspectives
More than three quarters of COPD patients in the community remain undiagnosed, hence untreated. Thus, identification of COPD patients is an important public health objective. Current research is studying “early COPD”, this is COPD in young individuals because studies have shown that younger patients with COPD may be as severely affected as the older ones including an increased risk of death.4,44 These findings point to a need to detect patients in the early phase of the disease so their outcomes can be improved. In contrast, it is currently not recommended to screen for airflow limitation in asymptomatic individuals.45 Some subjects with COPD can be asymptomatic even though significant airflow limitation might already be present.46 Individuals with undiagnosed COPD were found to have fewer symptoms and less impairment than those with diagnosed COPD, but they still represent a considerable burden to the health care system and had an increased risk of early death.47,48
A population-based prospective cohort study of 95,288 participants in Denmark investigated the prognosis of symptomatic and asymptomatic individuals with undiagnosed COPD in the general population.45 Compared with those who did not have COPD, individuals with undiagnosed, symptomatic COPD had a greater risk of exacerbations (HR, 15.5 [11.0–21.8]), pneumonia (2.8 [2.4–3.3], and death (HR 4.3 [2.8–6.7]). Individuals with undiagnosed, asymptomatic COPD had an increased risk of exacerbations (HR 5.0 [95% CI 2.8–8.9]) and pneumonia (HR 1.7 [1.3–2.2]). These observations suggest that early diagnosis of COPD, even in the absence of symptoms, can detect individuals at high risk.45
As mentioned above, mortality is increased in people with reduced lung function early in life. Consequently, spirometry may not only prove to be diagnostic but also to be a marker of risk.23 An analysis from the Framingham Offspring Cohort found that the characteristics of persons with FEV1 <80% of predicted at the age of 25–40 years were as follows: higher prevalence of respiratory, cardiovascular, and metabolic abnormalities in early adulthood; higher and earlier (about a decade) incidence of comorbidities during follow-up (39 years vs 47 years, P < 0.0001); and higher all-cause mortality than individuals with normal lung function in early adulthood (HR 2.3 [95% CI 1.4–3.7], P = 0.001), which was independent of, but additive with, cumulative smoking exposure.23
Currently, diagnosis of COPD is based on demonstration of a not fully reversible airflow limitation by spirometry. In order to increase the pre-test probability of airflow limitation, simple diagnostic tools may be helpful. The recently developed 5-item questionnaire CAPTURE (COPD Assessment in Primary Care to Identify Undiagnosed Respiratory Disease and Exacerbation Risk) was validated to assess exposure, breathing problems, tiring easily, and acute respiratory illnesses. This method has a sensitivity of 95.7% and specificity of 44.4% for differentiating cases from controls and a sensitivity of 95.7% and a specificity of 67.8% for differentiating cases from non-COPD controls. Sensitivity and specificity can be increased by adding an assessment of peak expiratory flow.49
If COPD is to be diagnosed early in life, a uniformly accepted definition of early COPD is mandatory. In addition, risk factors should be established in order to identify specific subgroups. Current research on lung function trajectories seems likely to provide information about future diseases.50,51 CADSET is a Clinical Research Collaboration launched by the European Respiratory Society with the objective of creating a consortium of investigators with access to population cohorts, birth cohorts, and cohorts of patients with chronic airway disease in order to work together to better understand how lung function trajectories influence the clinical presentation of chronic disease in general and COPD in particular.51
The Concept of Pre COPD
The idea that COPD may have a pre-obstructive phase is suggested by several studies of subjects who do not have airflow limitation on spirometry at baseline but are followed over time. A population study in the USA showed that an emphysema-like image on chest computerized scan was associated with an increased incidence of COPD in 5 years, both in smokers and in nonsmokers (for post-bronchodilator airflow limitation, HR, 4.38 [95% CI, 1.63–11.74; P = 0.003]).52 The same is true of the presence of chronic mucous secretion, which is associated with the development and progression of COPD in a large cohort followed in the United Kingdom.53 Findings from the Lovelace Smokers Cohort showed that current and ex-smokers with normal baseline spirometry but who over 18 months of follow-up demonstrated >40 ml/year drop in FEV1 were 36 times more likely to develop GOLD spirometric stage II obstruction than those subjects with no lung function decline.54 Thus, rapid lung function decline in normal subjects can be a marker of risk of COPD development.
The identification of markers for future development of COPD could herald a new concept, namely, Pre-COPD, which is similar to the concepts of prediabetes and prehypertension and may help clinicians to understand and interpret current evidence.2,21,55 Identification of at-risk persons, screening for early diagnosis, and early correction of risk are key areas in clinical research on COPD. Parenthetically, the concept of Pre-COPD (which can occur at any age) differs from “Early” COPD, which refers to the biological initiation of the process and is extremely difficult to identify in any particular patient. In turn, “Early” COPD is frequently confused with the concept of “Mild” COPD, which refers to the severity of the disease, usually graded by the degree of airflow limitation.
Conclusions
The 2019 update of the GOLD report introduced a series of novelties that were subsequently expanded in the 2020 and 2021 updates (eg, differentiation between initial and maintenance treatment). While the previous report is based on the ABCD grouping of patients, further treatment should be based on the prevailing treatable traits of the specific patient; in addition, regular reappraisal of the patient will maximize the effectiveness of therapy and limit the impact of adverse events.20,56 Based on recent large-scale trials, LABA/LAMA bronchodilator therapy is a mainstay of COPD treatment, and special attention should be paid to careful selection of inhaler devices. Patients with severe COPD and a history of multiple exacerbations despite appropriate long-acting bronchodilator treatment will benefit from the addition of an ICS, and blood eosinophil counts can be used to guide ICS treatment.3
Early diagnosis and treatment of COPD seems to be of paramount importance, and efforts are being devoted to the diagnosis of pre-COPD and COPD in young individuals. Consequently, we can no longer rely on the traditional paradigm of COPD as a disease diagnosed based on a decline in lung function and always after 60 years of age. Identification of risk factors would provide targets for preventive treatments, which may eventually pave the way for the eradication of COPD.57
Acknowledgments
This article reports on the “ABCD IN COPD”, ERS Madrid 2019 Evening Industry Symposium, organized by the Menarini Group on 29th September 2019. Editorial assistance was provided by Content Ed Net, with the helpful contribution of Laura Brogelli, M.D.; this assistance was supported by the Menarini Group. Dave Singh is supported by the National Institute for Health Research (NIHR) Manchester Biomedical Research Centre (BRC).
Funding
The Menarini group funded the “ABCD IN COPD” symposium and the editorial assistance for the preparation of the manuscript.
Disclosure
Bartolome R Celli reports having received compensation from Advisory Boards and consultation fees from Glaxo Smith Kline, Boehringer-Ingelheim, Astra Zeneca, Novartis, Pulmonx, CHIESI, Menarini and Bayer. He does not have shares or interest in any company, neither does any member of his family. He has not received or had any relationship with tobacco money.
Dave Singh has received personal fees from Aerogen, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, CSL Behring, Epiendo, Genentech, GlaxoSmithKline, Glenmark, Gossamerbio, Kinaset, Menarini, Novartis, Pulmatrix, Sanofi, Teva, Theravance and Verona
Claus Vogelmeier gave presentations at symposia and/or served on scientific advisory boards sponsored by AstraZeneca, Boehringer Ingelheim, CSL Behring, Chiesi, GlaxoSmithKline, Grifols, Menarini, Novartis, Nuvaira, OmniaMed and MedUpdate, reports personal fees from Aerogen, grants, personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, CSl Behring,
GlaxoSmithKline, Grifols, Novartis.
Alvar Agusti: Research project funds: AZ, GSK, Menarini. Lectures: AZ, Chiesi, GSK, Menarini.
AB: AZ, Chiesi, GSK, MSD, Menarini. The authors report no other conflicts of interest in this work.
References
1. Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365.
2. Celli BR, Wedzicha JA. Update on Clinical Aspects of Chronic Obstructive Pulmonary Disease. N Engl J Med. 2019;381:1257–1266.
3. Global Strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2022. Available From: https://goldcopd.org/wp-content/uploads/2021/11/GOLD-REPORT-2022-v1.0-12Nov2021_WMV.pdf.
4. Agusti A. The path to personalised medicine in COPD. Thorax. 2014;69:857–864.
5. Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J. 1977;1:1645–1648.
6. Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1: the Lung Health Study. JAMA. 1994;272:1497–1505.
7. Vestbo J, Srensen T, Lange P, et al. Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 1999;353:1819–1823.
8. Pauwels RA. Long-Term Treatment with Inhaled Budesonide in Persons with Mild Chronic Obstructive Pulmonary Disease Who Continue Smoking. N Engl J Med. 1999;340:1948.
9. Burge PS, Calverley PMA, Jones PW, et al. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000;320:1297–1303.
10. Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med. 2000;343:1902–1909.
11. Decramer M, Lken M, Dekhuijzen PNR, et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (BronchitisRandomized on NAC Cost-Utility Study, BRONCUS): a randomised placebo-controlled trial. Lancet. 2005;365:1552–1560.
12. Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543–1554.
13. Celli BR, Thomas NE, Anderson JA, et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med. 2008;178:332–338.
14. Tantucci C, Modina D. Lung function decline in COPD. Int J Chron Obstruct Pulmon Dis. 2012;7:95–99.
15. Casanova C, de Torres JP, Aguirre-Jaíme A, et al. The progression of chronic obstructive pulmonary disease is heterogeneous: the experience of the BODE cohort. Am J Respir Crit Care Med. 2011;184:1015–1021.
16. Celli BR, Anderson JA, Cowans NJ, et al. Pharmacotherapy and Lung Function Decline in Patients with Chronic Obstructive Pulmonary Disease. A Systematic Review. Am J Respir Crit Care Med. 2021;203:689–698.
17. Rabe KF, Hurd S, Anzueto A. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555.
18. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. Eur Respir J. 2017;49:1700214.
19. Global Strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2019. Available From: https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf.
20. Global Strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2020. Available From: https://goldcopd.org/wp-content/uploads/2019/11/GOLD-2020-REPORT-ver1.0wms.pdf.
21. Celli BR, Agustí A. COPD: time to improve its taxonomy?. ERJ Open Res. 2018;4:132–2017.
22. Lange P, Celli B, Agustí A, et al. Lung-Function Trajectories Leading to Chronic Obstructive Pulmonary Disease. N Engl J Med. 2015;373:111–122.
23. Agustí A, Noell G, Brugada J, et al. Lung function in early adulthood and health in later life: a transgenerational cohort analysis. Lancet Respir Med. 2017;5:935–945.
24. Singh D, Barnes PJ, Stockley R. Pharmacological treatment of COPD: the devil is always in the detail. Eur Respir J. 2018;51:1800263.
25. D’Urzo A, Chapman KR, Donohue JF, et al. Inhaler Devices for Delivery of LABA/LAMA Fixed-Dose Combinations in Patients with COPD. Pulm Ther. 2019;5:23–41.
26. Miravitlles M, Ribera A. Understanding the impact of symptoms on the burden of COPD. Respir Res. 2017;18:67.
27. Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest. 2011;140:331–342.
28. Agusti A, Fabbri LM, Singh D, et al. Inhaled corticosteroids in COPD: friend or foe?. Eur Respir J. 2018;52(6):1801219.
29. Rutgers SR, Timens W, Kaufmann HF, et al. Comparison of induced sputum with bronchial wash, bronchoalveolar lavage and bronchial biopsies in COPD. Eur Respir J. 2000;15:109–115.
30. Kolsum U, Damera G. Pulmonary inflammation in patients with chronic obstructive pulmonary disease with higher blood eosinophil counts. J Allergy Clin Immunol. 2017;140:1181–1184.
31. Brightling CE, Monteiro W, Ward R, et al. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2000;356:1480–1485.
32. Bafadhel M, Peterson S. Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pulmonary disease: a post-hoc analysis of three randomised trials. Lancet Respir Med. 2018;6:117–126.
33. Singh D, Kolsum U, Brightling CE, et al.; on behalf of the ECLIPSE investigators. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J. 2014;1(44):1697–1700.
34. Casanova C, Celli BR, de-Torres JP, et al. Prevalence of persistent blood eosinophilia: relation to outcomes in patients with COPD. Eur Respir J. 2017;50:1701162.
35. Southworth T, Beech G, Foden P, Kolsum U, Singh D. The reproducibility of COPD blood eosinophil counts. Eur Respir J. 2018;52(1):1800427. doi:10.1183/13993003.00427-2018
36. Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 2018;391:1076–1084.
37. Pascoe SJ, Lipson DA, Locantore N, et al. A phase III randomised controlled trial of single-dose triple therapy in COPD: the IMPACT protocol. Eur Respir J. 2016;48:320–330.
38. Lipson DA, Barnhart F, Brealey N, et al. Once-Daily Single-Inhaler Triple versus Dual Therapy in Patients with COPD. N Engl J Med. 2018;378:1671–1680.
39. Rabe KF, Martinez FJ, Ferguson GT, et al.; ETHOS Investigators. Triple Inhaled Therapy at Two Glucocorticoid Doses in Moderate-to-Very-Severe COPD. N Engl J Med. 2020;383:35–48.
40. Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-Glycopyrronium versus Salmeterol-Fluticasone for COPD. N Engl J Med. 2016;374:2222–2234.
41. Calverley PMA, Anzueto AR, Carter K, et al. Tiotropium and olodaterol in the prevention of chronic obstructive pulmonary disease exacerbations (DYNAGITO): a double-blind, randomised, parallel-group, active-controlled trial. Lancet Respir Med. 2018;6:337–344.
42. Sethi S, Kerwin E, Watz H, et al. AMPLIFY: a randomized, Phase III study evaluating the efficacy and safety of Aclidinium/formoterol vs monocomponents and tiotropium in patients with moderate-to-very severe symptomatic COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:667–682.
43. Wise RA, Chapman KR, Scirica BM, et al. Effect of Aclidinium Bromide on Major Cardiovascular Events and Exacerbations in High-Risk Patients With Chronic Obstructive Pulmonary Disease: the ASCENT-COPD Randomized Clinical Trial. JAMA. 2019;321:1693–1701.
44. Sanchez-Salcedo P, Divo M, Casanova C, Pinto-Plata V. Disease progression in young patients with COPD: rethinking the Fletcher and Peto model. Eur Respir J. 2014;44:324–331.
45. Çolak Y, Afzal S, Nordestgaard BG, et al. Prognosis of asymptomatic and symptomatic, undiagnosed COPD in the general population in Denmark: a prospective cohort study. Lancet Respir Med. 2017;5:426–434.
46. Soriano JB, Zielinski J, Price D. Screening for and early detection of chronic obstructive pulmonary disease. Lancet. 2009;374:721–732.
47. Labonte LE, Tan WC, Li PZ, et al. Undiagnosed COPD contributes to the burden of health care utilization: data from the CanCOLD Study. Am J Respir Crit Care Med. 2016;194:285–298.
48. Martinez CH, Mannino DM, Jaimes FA, et al. Undiagnosed obstructive lung disease in the United States. Associated factors and long-term mortality. Ann Am Thorac Soc. 2015;12:1788–1795.
49. Martinez FJ, Mannino D, Leidy NK, et al.; High-Risk-COPD Screening Study Group. A New Approach for Identifying Patients with Undiagnosed Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2017;195(6):748–756.
50. Agusti A, Faner R. Lung function trajectories in health and disease. Lancet Respir Med. 2019;7:358–364.
51. Agusti A, Faner R, Donaldson G, et al. Chronic Airway Diseases Early Stratification (CADSET): a new ERS Clinical Research Collaboration. Eur Respir J. 2019;53:1900217.
52. Oelsner EC, Smith BM, Hoffman EA, et al. Associations between emphysema-like lung on CT and incident airflow limitation: a general population-based cohort study. Thorax. 2018;73:486–488.
53. Allinson JP, Hardy R, Donaldson GC, et al. The Presence of Chronic Mucus Hypersecretion across Adult Life in Relation to Chronic Obstructive Pulmonary Disease Development. Am J Respir Crit Care Med. 2016;193:662–672.
54. Petersen H, Sood A, Polverino F, et al. The Course of Lung Function in Middle-aged Heavy Smokers: incidence and Time to Early Onset of Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2018;198:1449–1451.
55. Han MK, Agusti A, Celli BR, et al. From GOLD 0 to Pre-COPD. Am J Respir Crit Care Med. 2021;203:414–423.
56. Agusti A, Bel E, Thomas M, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J. 2016;47:410–419.
57. Dransfield M, Stolz D, Kleinert S; Lancet COPD Commissioners. Towards eradication of chronic obstructive pulmonary disease: a Lancet Commission. Lancet. 2019;393:1786–1788.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Association of Airway Eosinophilia with Small Airway Dysfunction in Patients with Mild and at Risk for COPD
Abdo M, Pedersen F, Trinkmann F, Herth FJF, Rabe KF, Kirsten AM, Watz H
International Journal of Chronic Obstructive Pulmonary Disease 2022, 17:1403-1408
Published Date: 17 June 2022
A Low Eosinophil to Platelet Ratio as a Worse Prognostic Index for Emergency Department Attendance in Acute Exacerbation of COPD
Hu D, Huang J, Zhao W, Xu M, Ma Y, Gong Z, Zhang Q, Zhao H
International Journal of Chronic Obstructive Pulmonary Disease 2024, 19:139-147
Published Date: 16 January 2024
High Blood Eosinophil Count at Stable State is Not Associated with Airway Microbiota Distinct Profile in COPD
Perotin JM, Muggeo A, Lecomte-Thenot Q, Brisebarre A, Dury S, Launois C, Ancel J, Dormoy V, Guillard T, Deslee G
International Journal of Chronic Obstructive Pulmonary Disease 2024, 19:765-771
Published Date: 18 March 2024
