Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 19
A Low Eosinophil to Platelet Ratio as a Worse Prognostic Index for Emergency Department Attendance in Acute Exacerbation of COPD
Authors Hu D , Huang J , Zhao W, Xu M, Ma Y, Gong Z, Zhang Q, Zhao H
Received 24 October 2023
Accepted for publication 7 January 2024
Published 16 January 2024 Volume 2024:19 Pages 139—147
DOI https://doi.org/10.2147/COPD.S442715
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Dapeng Hu,1,2 Junwen Huang,1 Wenqu Zhao,1 Maosheng Xu,1 Yanyan Ma,1 Zhaoqian Gong,1 Qian Zhang,1 Haijin Zhao1
1Chronic Airways Diseases Laboratory, Department of Respiratory and Critical Care Medicine, NanFang Hospital, Southern Medical University, Guangzhou, 510515, People’s Republic of China; 2Department of Emergency Medicine, Dalian Municipal Friendship Hospital, Dalian, Liaoning, 116001, People’s Republic of China
Correspondence: Haijin Zhao, Chronic Airway Diseases Laboratory, Department of Respiratory and Critical Care Medicine, Nanfang Hospital, Southern Medical University, Guangzhou, Guangdong, 510515, People’s Republic of China, Tel/Fax +86-2062787112, Email [email protected]
Purpose: Identifying prognosis for patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD) is challenging. Eosinophils and platelet are involved in the development of COPD, which may predict adverse events. The objective of this study was to determine the effect of the eosinophil to platelet ratio (EPR) in predicting adverse events in patients with AECOPD who visited the emergency department.
Patients and Methods: The records of patients with AECOPD treated at Dalian Municipal Friendship Hospital from January 2018 to December 2020 were retrospectively reviewed. The relationship between the clinical characteristics and EPR, as cut-off value of 0.755, was evaluated.
Results: A total of 508 patients with an AECOPD (316 male, 192 female) were included. An optimal AUC cutoff of 0.755 for the EPR segregated the patients into 2 groups with significantly different mortality (25.3% vs 5.5%, P < 0.001). The same mortality risk with lower EPR was observed among the patients with emergency room attendance (35.6% vs 11.1%, P < 0.001). A model including EPR < 0.755, exacerbation history, PaO2 < 60mmHg, PaCO2 > 50 mm Hg, hypoalbuminemia and age ≥ 80 was developed to predict death risk and showed good performance.
Conclusion: During severe COPD exacerbation, an EPR < 0.755 preceding therapy can predict worse outcomes in patients with an AECOPD.
Keywords: eosinophil, COPD, eosinophil to platelet ratio, emergency department
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of death and disability worldwide.1 Acute exacerbation of COPD (AECOPD) is commonly described as a rapid increase in COPD symptoms outside of the normal daily variation, and requires changes to daily medication. Such exacerbations are often linked to poor health status, and high rates of hospitalization, readmission, and disease progression.2
The neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) in peripheral blood are being increasingly studied as systemic inflammatory markers. Notably, they can be rapidly determined, are widely available, and are relatively inexpensive through routine blood count analysis. Xiong et al3 in a retrospective study found that baseline NLR values were significantly higher in COPD patients who died than patients who survived, indicating that the NLR might predict mortality in hospitalized COPD patients. Kumar et al4 reported that the PLR was associated with 90-day mortality in patients with COPD. In a recent retrospective study, Yao et al5 reported that NLR and PLR values were significantly higher in non-survivor patients with COPD, and the NLR might be a useful prognostic marker for hospital mortality in patients with an AECOPD. As such, the NLR and PLR, which are convenient and simple to calculate may be used to predict the prognosis of patients with COPD.
Studies have shown that a low levels of blood eosinophils may be a predictor of a poor prognosis in patients with COPD.6–8 In addition, an eosinophil count <50 / μL may be a useful biomarker for detecting sepsis and be a predictor of mortality in patients with a critical illness.6 MacDonald et al9 found that a low eosinophil count may predict higher mortality in hospitalized patients with an AECOPD. Holland et al7 reported that a blood eosinophil count <0.04×109/L was associated with higher in-hospital mortality in patients with an AECOPD. Chan et al10 found that blood eosinophilia was associated with higher exacerbation risk in COPD patients. Prins et al11 reported that blood eosinophilia ≥2% was associated with an increased risk of relapse.
Studies have shown that a platelet count may have a role in predicting disease prognosis. Several reports found that thrombocytopenia is an independent predictor of intensive care unit (ICU) mortality, with a relative risk (RR) ranging from 1.9 to 4.2.12–14 In addition, recent studies have shown that a decrease in platelet count is associated with respiratory diseases, Lopez et al15 performed an observational prospective study, and reported that thrombocytopenia is a marker of in-hospital mortality in ICU patients with respiratory failure due to H1N1 influenza. Ashraf et al16 reported that in patients with stable COPD, a decreased platelet count was associated with increased risk of 3-year all-cause mortality. Conversely, Harrison et al17 reported that thrombocytosis was associated with increased mortality in patients with COPD.
Taken together, the aforementioned studies indicate that eosinophil and platelet counts may be predictive of COPD outcomes. However, the conclusions of using eosinophil and platelet as biomarkers to predict the prognosis of COPD are inconsistent; therefore, we hypothesize that the eosinophil to platelet ratio can possibly be used to predict prognosis of COPD; moreover, there is a paucity of studies investigating the predictive effect of the EPR on COPD prognosis, of especially for patients seen in emergency department (ED) with an AECOPD. Thus, the purpose of this study was to investigate the relation between EPR and outcomes of patients with an AECOPD.
Materials and Methods
Patient Selection and Data Collection
Patients >45 years old with an AECOPD who were hospitalized at Dalian Municipal Friendship Hospital (a tertiary-A teaching hospital in Dalian, Liaoning Province, China), from January 2018 to December 2020 were identified using the electronic database, and followed up for 12 months. Medical records were reviewed by a respiratory physician to confirm an AECOPD and exclude alternative diagnoses. The follow-up period was from January 2021 to December 2022. During the follow-up period, patients underwent telephone visits (every 3 months) to collect detailed information. Ethics approval for the study was obtained from the Ethics Committees of Dalian Friendship Municipal Hospital [code number YY-LL-2021-050]. COPD was diagnosed according to the Global Initiative for Chronic Obstructive Lung Disease 2018. Patients taking oral corticosteroids (OCS) before admission were excluded from the study. Patients with ED attendance and non-ED attendance were analysed separately.
Outcomes and Covariates
All potential factors of interest were classified as demographic information, pre-existing co-morbidities, and clinical characteristics. Demographic variables were age, sex, complication (acute respiratory failure, ARF: including hypoxemic and hypercapnic), comorbidities (heart failure, hypoalbuminemia); clinical data included the history of exacerbations in the past 12 months, the presence of emphysema, laboratory blood test results (blood eosinophils absolute count, blood platelet count, calculated eosinophil to platelet ratio, neutrophil absolute count, blood lymphocyte count, calculated neutrophil to lymphocyte ratio and platelet to lymphocyte ratio), the presence of a lower respiratory tract infection (LRTI), spirometry results: forced expiratory volume in one second (FEV1), forced vital capacity (FVC), length of hospitalization (LOH), severe exacerbations in the follow-up period, all-cause mortality, and all-cause readmission in 12-month after discharge from the index hospitalization. Severe COPD exacerbation was defined as worsening of respiratory symptoms that led to a COPD-specific hospitalization, an ED visit, or an ICU stay. All of the above-mentioned factors were reviewed and documented from the medical records. During the follow-up period, patients received telephone calls to collect detailed information about exacerbations and other clinical outcomes.
Statistical Analysis
Normally distributed data are expressed as the mean ± standard deviation and nonnormally distributed data as the median (interquartile range [IQR], 25%, 75%). Categorical variables are summarized as numbers and percentages (n [%]). An independent t-test, χ2 test, Yates continuity correction test, Fisher exact test, or Wilcoxon rank-sum test was used to assess the differences according to EPR groups, as appropriate. The predictive values of EPR were analysed with receiver operating characteristic (ROC) curve analysis, and area under the curves (AUCs) was calculated and compared. We used the least absolute shrinkage selection operator (LASSO) regression to screen the most useful variables associated with mortality. Multivariate logistic regression was employed to build a prediction model of prognosis by incorporating predictors selected from LASSO analysis. Time-to-event survival analyses were conducted using Kaplan–Meier methods and Log rank tests. These analyses were conducted using R version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria) and GraphPad Prism 8. In all statistical analyses, two-tailed values of P < 0.05 were considered statistically significant.
Results
The records of 858 patients treated in the study period were identified and reviewed. After excluding those patients discharged from ED directly (n = 178), receiving preadmission OCS use (n = 122) and lost followed up (n = 58). Thus, 508 patients with an AECOPD (316 male, 192 female) were included in the analysis. The median age of patients was 74 years (range, 46–97 years). In our data, 35.4% (n = 180) patients were hospitalized with ED visits, shown in Figure 1, others were admission without ED attendance (n = 328, 64.6%). The ROC analysis of EPR to predict mortality resulted in a cut-off point of 0.755 (AUC = 0.718, 95% CI: 0.585–0.785, Figure 2A). The patients with ED attendance were divided into 2 groups based on the EPR value: EPR ≥ 0.755 (n = 135, 75%) and EPR < 0.755 (n = 45, 25%).
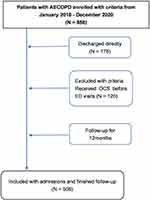 |
Figure 1 Flowchart of the cohort in the present research. |
An EPR < 0.755 is Correlated with Unfavourable Outcomes
In all patients, an EPR < 0.755 was associated with a higher frequency of acute respiratory failure (hypoxemic: 41.6 vs 13.8%, P < 0.001, hypercapnic: 29.2 vs 11.7%, P < 0.001). Notably, in patients with ED attendance, most subjects were male (58.3%) and mean (IQR) age was 78 (68, 84) years, the rate of exacerbation was 35.5% (64/180), and the 1-year all-cause readmission rate was 45% (81/180). An EPR < 0.755 was associated with a higher frequency of acute respiratory failure (hypoxemic: 46.7 vs 17.8%, P < 0.001, hypercapnic: 35.6 vs 17%, P = 0.016), heart failure (55.6% vs 28.1%, P = 0.002), lower respiratory tract infection (71.1% vs 50.4%, P = 0.024), and had a longer length of hospital stay (P = 0.013), presented in Figure 2B. Those with an EPR < 0.755 had lower PaO2 (62 [49, 78] vs 75 [65, 84], P = 0.001). However, there was no significant difference in baseline FEV1 (P = 0.876) and FVC (P = 0.927). The demographic characteristics and outcomes of patients seen in the ER are summarized in Table 1, details of all patients and those without ED attendance are presented in the Tables S1 and S2.
 |
Table 1 Demographics and Clinical Outcomes According to EPR Group in Patients with ED Attendance |
An EPR < 0.755 is Associated with a Higher Mortality Rate
The mortality rate for patients with an EPR < 0.775 was 25.8% (n = 23), and for those with an EPR ≥ 0.755 was 5.5% (n = 23, P < 0.001), and for patients with emergency room attendance the mortality rate was 11.1% (n = 15) in subjects with EPR ≥ 0.755 compared to 35.6% (n = 16) in the EPR < 0.755 group (P < 0.001). For patients without ED attendance, same results were observed, details are provided in Table S2.
Kaplan–Meier analysis confirmed that an EPR < 0.755 was associated with higher mortality in the ED. Similar results were observed in all patients and participants without ED visits, details are provided in the Figure S1.
Clinical Prediction Model
Six variables with non-zero coefficients in the LASSO regression model were included in the final multivariate logistic regression model (history of exacerbation in previous year, age ≥80, hypoalbuminemia, PaO2 <60mmHg, PaCO2 >50 mm Hg, EPR < 0.755, Table 2). A model that including the abovementioned 6 predictors was developed and visualized as a nomogram (Figure 3). The model showed excellent discrimination in predicting mortality (C-index: 0.854, 95% CI: 0.795–0.912). The goodness-of-fit of the model was evaluated using the Hosmer–Lemeshow test and bias-corrected calibration curves (Figure S2). The Hosmer–Lemeshow test yielded a non-significant value (P = 0.329), which suggested that the model fit was acceptable. Evaluation of the bias-corrected calibration curves for the prediction model indicated good agreement. On internal validation, 10-fold cross-validation (bias-corrected C-index = 0.827) indicated that the model discrimination was good. The temporal validation cohort included 114 participants, and the AUC was 0.677 (95% CI: 0.368–0.986).
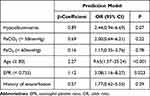 |
Table 2 Predictors of All-Cause Mortality in AECOPD Participants |
 |
Figure 3 All-cause mortality Nomogram in patients with ED attendance. |
Predictors of Mortality in Patients Seen in ED with AECOPD
In order to evaluate the predictive ability of the model, we compared it with the DCEAF score18 (including: dyspnea, eosinopenia, consolidation, acidaemia and atrial fibrillation), a predictor of mortality in patients hospitalized with an exacerbation of COPD. Compared to the DCEAF score, the new model had a higher accuracy (AUC of 0.854 vs 0.570, P = 0.012).
The AUC of the eosinophil count for predicting mortality was 0.697, with a cut-off point of 0.045. Further analysis indicated that eosinophil count less than 0.045 was associated with higher mortality.
The AUC for platelet count for predicting mortality was 0.591, with a cut-off point of 175. Further analysis indicated that patients with a platelet count <175 an increased risk of death (14.9% vs 7%, P = 0.01).
The AUC of the prediction model, including history of exacerbation, EPR, hypoalbuminemia, PaO2 < 60mmHg, PaCO2 > 50mmHg and age ≥ 80, for predicting mortality was 0.854. This was significantly higher than that of eosinophil (P = 0.001), platelet (P < 0.001) and EPR (AUC = 0.685, P < 0.001) predict mortality alone respectively, presented in Figure S3.
Discussion
To our knowledge, this is the first study to reveal that EPR is a worse prognostic index for emergency department attendance in acute exacerbation of COPD. In patients with ED visit, an EPR < 0.755 was associated with a 3.2 - fold increased risk of death compared to those with an EPR ≥ 0.755 (35.6% vs 11.1%). In addition, an EPR < 0.755 and 5 other variables were independent predictors of all-cause mortality in patients with AECOPD presented at the ED.
In patients seen in ED with AECOPD, we build a simple prognostic model, incorporating clinical information and laboratory results, which can accurately predict all-cause mortality, and performed more accurately than DCEAF scores.
Blood eosinophil count has been widely investigated in COPD.19–22 And there is compelling evidence that a low eosinophil level is associated with worse outcomes of patients with an AECOPD.23–25 Recently, Bartziokas et al26 reported that in patients with AECOPD, those with eosinopenia had increased risk of noninvasive ventilation use. In the current study, the AUC of eosinophil count for predicting mortality was 0.697, with a cut-off point eosinophil count of 0.045. Further analysis indicated that patients with an eosinophil count <0.045 had higher mortality.
This finding is consistent with previous studies.6,7,9 Lv et al23 reported that patients with low eosinophils (<2%) had significantly longer length of hospital stay, and higher mortality than those with a higher eosinophil count. Wu et al24 reported that patients with a low eosinophil count often experienced poorer clinical outcomes. Prudente et al25 found that a decrease in number of peripheral eosinophils may be associated with a higher risk of death in COPD patients. In addition, a prior study reported that a low eosinophil level is associated with a higher mortality rate in patients with an AECOPD.18
Alterations of platelet count have also been reported to be related to COPD.16 We found that the AUC for platelet count to predict mortality was 0.591, with a cut-off platelet count of 175. Further analysis showed that patients with a platelet count <175 had a greater risk of death (14.9% vs 7%, P = 0.01). A number of recent studies have reported that thrombocytopenia is associated with ICU mortality.12–14 Fawzy et al16 reported that platelet count was associated with an increased risk of 3-year all-cause mortality in patients with COPD. Rahimi et al27 reported that thrombocytopenia was associated with in-hospital mortality in AECOPD. Our findings are consistent with those of the aforementioned studies.
Blood eosinophil and platelet count have become an important management tool for patients seen in the ED with an AECOPD. Our results showed that in patients seen in ED with AECOPD, a lower EPR act as a predictor of death. Our findings highlight the potential usefulness of eosinophil to platelet ratio to predict prognosis in patients with AECOPD seen in ED. For patients with ED attendance due to AECOPD, those with eosinopenia and thrombocytopenia simultaneously may have a poorer prognosis. Russell et al28 reported that in patients with an AECOPD presenting to ED, a higher blood eosinophil count is associated with a shorter length of stay and reduced mortality. Prior research has indicated that eosinophils might have an anti-infection effect, Linch et al29 reported that eosinophils might have anti-bacterial properties. In addition, in response to human rhinovirus and respiratory syncytial virus, eosinophil activation has been observed.30,31 Thrombocytopenia was also associated with mortality in patients with an AECOPD.27 A decreased platelet count might result from severe infection, which increases the risk of death. These findings may help explain why a low EPR is associated with increased mortality. Further studies are needed to better understand the role that EPR plays in AECOPD.
In this study, we demonstrated that NLR and PLR were not associated with mortality in patients with AECOPD. Consistent with our study, Aksoy et al32 reported that in patients with AECOPD, the multivariate analysis showed that the NLR was not associated with mortality after adjusting for eosinophilia and other variables. In another study aimed to evaluate the association of the inflammation biomarker in patients with AECOPD with 90-day mortality, Kumar et al4 demonstrated that NLR was not significantly associated with mortality (P = 0.46). In a retrospective study, Rahimiard et al33 found that there is no significant difference in PLR values between the survival group and the non-survival group (P = 0.75), suggested that the PLR did not show significant relation to in-hospital death in AECOPD. In addition, another research34 reported that in patients with AECOPD, the PLR value of non-survival patients was not significantly higher than that in survival participants (P > 0.05), and the diagnostic value of PLR in the prognosis of in-hospital mortality was found to be insignificant (P = 0.18), indicated that PLR was not associated with mortality. Therefore, the relationship between NLR, PLR and prognosis of AECOPD needed to be further clarified.
Chen et al35 found that in patients presenting to the ED with an AECOPD and hypercapnic respiratory failure, respiratory rate, lactic acid, PaCO2, blood urea nitrogen, haemoglobin, and platelet count were predictors of in-hospital mortality. Other study has reported that patients with an AECOPD and hypercapnia have an increased mortality rate.36 Matkovic et al37 found that hypercapnia in patients admitted for an AECOPD was an independent predictor of death. Our results are in line with prior study.
We also found that hypoalbuminemia was an independent risk factor for mortality. Chen et al38 demonstrated that severe hypoalbuminemia was a strong risk factor for acute respiratory failure in patients with COPD. Additionally, a meta-analysis by Lomholt et al39 found that hypoalbuminemia was associated with increased mortality in patients with COPD. A low albumin level may be a sign of worsening nutrition status or increased inflammation during an AECOPD.
There are some limitations of this study that should be considered. As a retrospective study, our study sample was relatively small and this can have an impact on the results. Second, multi-centre clinical trials with a larger sample size are still needed.
Conclusion
In conclusion, our data demonstrated that in patients with ED attendance due to AECOPD, an EPR <0.755 was associated with an increased risk of death.
Abbreviations
AECOPD, chronic obstructive pulmonary disease; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; ICU, intensive care unit; RR, relatively risk; ARF, acute respiratory failure; EPR, eosinophil to platelet ratio; LRTI, lower respiratory tract infection; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; forced expiratory rate of the 1st second (FEV1%); LOH, length of hospitalization; ED, emergency department; IQR, interquartile range; ROC, receiver operating characteristic curve; AUC, area under the curves; LASSO, least absolute shrinkage selection operator; OCS, oral corticosteroids; OR, odds ratio.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Dalian Friendship Municipal Hospital (protocol code YY-LL-2021-050, date of approval: 2021.12.31). Due to no specific patient identifiable information was used, patient consent was officially waived by the Ethics Committees of Dalian Municipal Friendship Hospital.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was funded by the National Natural Science Foundation of China (82370029, 82070030), and the Basic and Applied Basic Research Foundation of Guangdong province (2022A1515012064, 2023A1515010406).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Christenson SA, Smith BM, Bafadhel M, et al. Chronic obstructive pulmonary disease. Lancet. 2022;399(10342):2227–2242. doi:10.1016/s0140-6736(22)00470-6
2. Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi:10.1136/thx.2005.040527
3. Xiong W, Xu M, Zhao Y, et al. Can we predict the prognosis of COPD with a routine blood test? Int J Chron Obstruct Pulmon Dis. 2017;12:615–625. doi:10.2147/COPD.S124041
4. Kumar P, Law S, Sriram KB. Evaluation of platelet lymphocyte ratio and 90-day mortality in patients with acute exacerbation of chronic obstructive pulmonary disease. J Thorac Dis. 2017;9(6):1509–1516. doi:10.21037/jtd.2017.05.77
5. Yao C, Liu X, Tang Z. Prognostic role of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio for hospital mortality in patients with AECOPD. Int J Chron Obstruct Pulmon Dis. 2017;12:2285–2290. doi:10.2147/COPD.S141760
6. Abidi K, Khoudri I, Belayachi J, et al. Eosinopenia is a reliable marker of sepsis on admission to medical intensive care units. Crit Care. 2008;12(2):R59. doi:10.1186/cc6883
7. Holland M, Alkhalil M, Chandromouli S, et al. Eosinopenia as a marker of mortality and length of stay in patients admitted with exacerbations of chronic obstructive pulmonary disease. Respirology. 2010;15(1):165–167. doi:10.1111/j.1440-1843.2009.01651.x
8. Yu S, Zhang J, Fang Q, et al. Blood eosinophil levels and prognosis of hospitalized patients with acute exacerbation of chronic obstructive pulmonary disease. Am J Med Sci. 2021;362(1):56–62. doi:10.1016/j.amjms.2021.02.013
9. MacDonald MI, Osadnik CR, Bulfin L, et al. Low and high blood eosinophil counts as biomarkers in hospitalized acute exacerbations of COPD. Chest. 2019;156(1):92–100. doi:10.1016/j.chest.2019.02.406
10. Chan MC, Yeung YC, Elm Y, et al. Blood eosinophil and risk of exacerbation in chronic obstructive pulmonary disease patients: a retrospective cohort analysis. Int J Chron Obstruct Pulmon Dis. 2020;15:2869–2877. doi:10.2147/COPD.S268018
11. Prins HJ, Duijkers R, Lutter R, et al. Blood eosinophilia as a marker of early and late treatment failure in severe acute exacerbations of COPD. Respir Med. 2017;131:118–124. doi:10.1016/j.rmed.2017.07.064
12. Vanderschueren S, De Weerdt A, Malbrain M, et al. Thrombocytopenia and prognosis in intensive care. Crit Care Med. 2000;28(6):1871–1876.
13. Strauss R, Wehler M, Mehler K, et al. Thrombocytopenia in patients in the medical intensive care unit: bleeding prevalence, transfusion requirements, and outcome. Crit Care Med. 2002;30(8):1765–1771.
14. Stéphan F, Hollande J, Richard O, et al. Thrombocytopenia in a surgical ICU. Chest. 1999;115(5):1363–1370.
15. Lopez-Delgado JC, Rovira A, Esteve F, et al. Thrombocytopenia as a mortality risk factor in acute respiratory failure in H1N1 influenza. Swiss Med Wkly. 2013:143:w13788. doi:10.4414/smw.2013.13788
16. Fawzy A, Anderson JA, Cowans NJ, et al. Association of platelet count with all-cause mortality and risk of cardiovascular and respiratory morbidity in stable COPD. Respir Res. 2019;20(1):86. doi:10.1186/s12931-019-1059-1
17. Harrison MT, Short P, Williamson PA, et al. Thrombocytosis is associated with increased short and long term mortality after exacerbation of chronic obstructive pulmonary disease: a role for antiplatelet therapy? Thorax. 2014;69(7):609–615. doi:10.1136/thoraxjnl-2013-203996
18. Steer J, Gibson J, Bourke SC. The DECAF Score: predicting hospital mortality in exacerbations of chronic obstructive pulmonary disease. Thorax. 2012;67(11):970–976. doi:10.1136/thoraxjnl-2012-202103
19. Pascoe S, Locantore N, Dransfield MT, et al. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. 2015;3(6):435–442. doi:10.1016/S2213-2600(15)00106-X
20. Pavord ID, Chanez P, Criner GJ, et al. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N Engl J Med. 2017;377(17):1613–1629. doi:10.1056/NEJMoa1708208
21. Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–659. doi:10.1016/S0140-6736(12)60988-X
22. Vedel-Krogh S, Nielsen SF, Lange P, et al. Blood eosinophils and exacerbations in chronic obstructive pulmonary disease. the Copenhagen general population study. Am J Respir Crit Care Med. 2016;193(9):965–974. doi:10.1164/rccm.201509-1869OC
23. Lv MY, Qiang LX, Li ZH, et al. The lower the eosinophils, the stronger the inflammatory response? The relationship of different levels of eosinophils with the degree of inflammation in acute exacerbation chronic obstructive pulmonary disease (AECOPD). J Thorac Dis. 2021;13(1):232–243. doi:10.21037/jtd-20-2178
24. Wu H-X, Zhuo K-Q, Cheng D-Y. Peripheral blood eosinophil as a biomarker in outcomes of acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2019;14:3003–3015. doi:10.2147/COPD.S226783
25. Prudente R, Ferrari R, Mesquita CB, et al. Peripheral blood eosinophils and Nine years mortality in COPD patients. Int J Chron Obstruct Pulmon Dis. 2021;16:979–985. doi:10.2147/COPD.S265275
26. Bartziokas K, Papathanasiou E, Papaioannou AI, et al. Eosinopenia as a prognostic biomarker for noninvasive ventilation use in COPD exacerbations. J Pers Med. 2023;13(4):686.
27. Rahimi-Rad MH, Soltani S, Rabieepour M, et al. Thrombocytopenia as a marker of outcome in patients with acute exacerbation of chronic obstructive pulmonary disease. Pneumonol Alergol Pol. 2015;83(5):348–351. doi:10.5603/PiAP.2015.0056
28. Russell R, Beer S, Pavord ID, et al. The acute wheezy adult with airways disease in the emergency department: a retrospective case-note review of exacerbations of COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:971–977. doi:10.2147/COPD.S190085
29. Linch SN, Kelly AM, Danielson ET, et al. Mouse eosinophils possess potent antibacterial properties in vivo. Infect Immun. 2009;77(11):4976–4982. doi:10.1128/IAI.00306-09
30. Rosenberg HF, Dyer KD, Domachowske JB. Respiratory viruses and eosinophils: exploring the connections. Antiviral Res. 2009;83(1):1–9. doi:10.1016/j.antiviral.2009.04.005
31. Handzel ZT, Busse WW, Sedgwick JB, et al. Eosinophils bind rhinovirus and activate virus-specific T cells. J Immunol. 1998;160(3):1279–1284.
32. Aksoy E, Güngör S, Ağca MÇ, et al. A revised treatment approach for hospitalized patients with eosinophilic and neutrophilic exacerbations of chronic obstructive pulmonary disease. Turk Thorac J. 2018;19(4):193–200. doi:10.5152/TurkThoracJ.2018.18004
33. Rahimirad S, Ghaffary MR, Rahimirad MH, et al. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute exacerbation of chronic obstructive pulmonary disease. Tuberk Toraks. 2017;65(1):25–31.
34. Emami Ardestani M, Alavi-Naeini N. Evaluation of the relationship of neutrophil-to lymphocyte ratio and platelet-to-lymphocyte ratio with in-hospital mortality in patients with acute exacerbation of chronic obstructive pulmonary disease. Clin Respir J. 2021;15(4):382–388. doi:10.1111/crj.13312
35. Chen L, Chen L, Zheng H, et al. Emergency admission parameters for predicting in-hospital mortality in patients with acute exacerbations of chronic obstructive pulmonary disease with hypercapnic respiratory failure. BMC Pulm Med. 2021;21:1.
36. Yang H, Xiang P, Zhang E, et al. Is hypercapnia associated with poor prognosis in chronic obstructive pulmonary disease? A long-term follow-up cohort study. BMJ Open. 2015;5(12):e008909. doi:10.1136/bmjopen-2015-008909
37. Matkovic Z, Huerta A, Soler N, et al. Predictors of adverse outcome in patients hospitalised for exacerbation of chronic obstructive pulmonary disease. Respir Int Rev Thoracic Dis. 2012;84(1):17–26. doi:10.1159/000335467
38. Chen C-W, Chen -Y-Y, C-l L, et al. Severe hypoalbuminemia is a strong independent risk factor for acute respiratory failure in COPD: a nationwide cohort study. Int J Chron Obstruct Pulmon Dis. 2015;10:1147–1154. doi:10.2147/COPD.S85831
39. Lomholt FK, Laulund AS, Bjarnason NH, et al. Meta-analysis of routine blood tests as predictors of mortality in COPD. Eur Clin Respir J. 2014;1. doi:10.3402/ecrj.v1.24110
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

