Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Association of Airway Eosinophilia with Small Airway Dysfunction in Patients with Mild and at Risk for COPD
Authors Abdo M , Pedersen F, Trinkmann F , Herth FJF , Rabe KF , Kirsten AM , Watz H
Received 22 March 2022
Accepted for publication 9 June 2022
Published 17 June 2022 Volume 2022:17 Pages 1403—1408
DOI https://doi.org/10.2147/COPD.S366911
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Richard Russell
Mustafa Abdo,1 Frauke Pedersen,1,2 Frederik Trinkmann,3,4 Felix JF Herth,3 Klaus F Rabe,1 Anne-Marie Kirsten,2 Henrik Watz2
1LungenClinic Grosshansdorf, Airway Research Center North (ARCN), German Center for Lung Research (DZL), Grosshansdorf, Germany; 2Pulmonary Research Institute at LungenClinic Grosshansdorf, Airway Research Center North (ARCN), German Center for Lung Research (DZL), Grosshansdorf, Germany; 3Pneumology and Critical Care Medicine, Thoraxklinik at University Hospital Heidelberg, Translational Lung Research Center Heidelberg (TLRC), Member of German Center for Lung Research (DZL), Heidelberg, Germany; 4Department of Biomedical Informatics (DBMI) at the Center for Preventive Medicine and Digital Health Baden-Württemberg (CPD-BW), University Medical Center Mannheim, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany
Correspondence: Mustafa Abdo, LungenClinic Grosshansdorf, Airway Research Center North (ARCN), German Center for Lung Research (DZL), Wöhrendamm 80, Grosshansdorf, 22927, Germany, Email [email protected]
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by the presence of chronic airway inflammation, persistent respiratory symptoms and a progressive decline in lung function. Airway inflammation might be heterogeneous involving different cellular mechanisms. While the role of airway neutrophils in the pathogenesis of COPD is well established,1 recent data have demonstrated elevated sputum eosinophils in nearly 20% of COPD patients with different disease severities.2 So far, the role of eosinophils in COPD is almost limited to the use of blood eosinophil count as predictor for patients who are likely to benefit from inhaled corticosteroids (ICS) therapy to reduce the risk of exacerbation.3 Moreover, little is known about the prevalence of airway eosinophilia and its impact on lung function in early stages of COPD, i.e. in patients with mild stable COPD or symptomatic smokers who are at risk for COPD. It has been suggested that small airway obstruction represents the earliest stage of COPD that precedes the onset of emphysematous lung destruction.4 We have recently demonstrated that eosinophilic rather than neutrophilic airway inflammation is the main driver of small airway dysfunction in asthma.5 However, the impact of airway eosinophilic inflammation on lung function in patients with COPD remains uncertain. In this study, we sought to investigate the association between airway eosinophilic inflammation and lung function, particularly small airway dysfunction, in early stages of COPD.
Methods
We present a cross-sectional subgroup analysis of patients included in the cohort of Change in Airway Peripheral Tone in COPD (CAPTO-COPD), a longitudinal observational multicenter study in Germany (https://clinicaltrials.gov/ct2/show/NCT04166812). The study protocol was approved by local ethics committees (Ärztekammer Schleswig-Holstein and Medical Ethics Committee of Heidelberg University) and was performed in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. Written informed consent was obtained before enrollment. Overall, 152 subjects were recruited at baseline. Subjects included in this analysis (n = 69) were those who gave their consent to perform sputum induction. Subjects were either patients with mild COPD (post-bronchodilator FEV1/FEVC < 0.7 and FEV1 ≥ 70%) or (ex)-smokers at risk for COPD. Subjects at risk for COPD did not have fixed airflow obstruction (post-bronchodilator FEV1/FVC ≥ 0.7) but had a smoking history of ≥ 10 pack years and persistent respiratory symptoms (CAT score ≥ 10) or a regular treatment with long-acting bronchodilator. In addition to spirometry, lung function was evaluated using body plethysmography, airwave oscillometry (AOS), sulfur hexafluoride (SF6) multiple breath washout testing (MBW), according to the latest European Respiratory Society (ERS) recommendations. Sputum was induced using inhaled hypertonic saline (3%, 4%, and 5%) for seven minutes each, and processed according to standard operating procedures.6 Subjects were stratified according to sputum eosinophils count into eosinophil-high (≥ 2%) and eosinophil-low (<2%), respectively.2 For the assessment of respiratory symptoms and disease-specific health status we used St George’s Respiratory Questionnaire for COPD patients (SGRQ-c) and COPD Assessment Test (CAT). A t-test or Wilcoxon-test was used to compare clinical and lung function characteristics between both groups. In this exploratory analysis multiplicity adjustments were not done. Linear multivariate regressions were applied to investigate independent predictors of lung function. In absence of ethnic variations, the regressions were adjusted for age, sex and height. Predictors of lung function were smoking status (current vs former), smoking quantity (pack years), the percentage of sputum eosinophil and neutrophil counts. Statistical analyses were performed using R (version 3.6.2, R Foundation, Vienna, Austria). An alpha error of less than 5% was considered statistically significant.
Results
We induced sputum in 69 subjects (mean age, 65 ± 7 years; 55% male; mean body mass index, 27.5 ± 4.6 kg/m2). Thereof, 45 patients with mild COPD and 24 (ex)-smokers at risk for COPD. None of the subjects had a history of asthma and a bronchodilation test demonstrated a limited FEV1 reversibility (mean post-BD increase in FEV1: 5 ± 4%, 140 ± 110 mL). Nineteen percent of the subjects (n = 13; 8 patients with COPD and 5 (ex)-smokers at risk) were eosinophil-high and had markedly elevated sputum eosinophil and peripheral blood eosinophil counts compared with eosinophil-low subjects (Table 1). By contrast, levels of fractional exhaled nitric oxide and the sputum neutrophil count were similar in both groups (Table 1). Furthermore, no significant difference between patients with low and high eosinophils with regard to age, sex, anthropometric parameters or smoking status could be observed (Table 1). The number of subjects who had ICS therapy was similar between both groups. However, a greater proportion of eosinophil-high subjects had a therapy with long-acting bronchodilators (Table 1). In spite of this, eosinophil-high subjects had increased lung function impairment compared with eosinophil-low subjects. In addition to large airway obstruction (FEV1), eosinophil-high subjects had increased small airway dysfunction as indicated by distal airway obstruction (MEF50, MEF75), air trapping (RV, RV/TLC), lung hyperinflation (IC, FRC), and the ensuing acinar (Sacin) and global ventilation inhomogeneity (LCI), see Table 1. Measures of oscillometry (AX, R5–19) did not differ between both groups. SGRQ-c and CAT total scores were also similar in both groups. Multivariate regressions, adjusted for age, sex and height, indicated that sputum eosinophil count is an independent predictor for IC (L) (multiple R2, standard estimator, p-value for eosinophils predictor: 0.63, −0.197 and p = 0.014), IC/TLC: (0.31, −0.330, p = 0.003), RV/TLC: (0.40, 0.241, p = 0.019), Sacin: (0.29, 0.985, p = 0.018), and LCI: (0.18, 0.317, p = 0.05). A trend was found for FRC (L): (0.58, 0.149, 0.08), but neither for FEV1 (l): (0.65, −0.057, p = 0.59) nor for MEF25, 50 or 75.
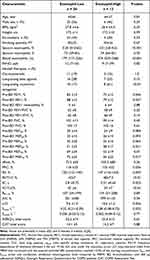 |
Table 1 Clinical and Lung Physiology Characteristics in Patients Stratified According to Sputum Eosinophils Count |
Sputum neutrophil count was also an independent predictor for FRC (L): (0.58, 0.207, p = 0.022) and RV (L): (0.43, 0.226, p = 0.02). Smoking status, yet not quantity, was an independent predictor for FEV1, FRC and RV/TLC, (all p-values for smoking status predictor <0.05). The regression analysis for measures of oscillometry (AX, R5–19) yielded poor fitting models (multiple R2<0.10) and did not demonstrate significant predictor of both measures.
Among predictors of lung function, sputum eosinophil count was a stronger predictor for small airway dysfunction (Sacin, LCI, IC/TLC and RV/TLC) than for central airway obstruction (FEV1, sRtot) or the inhomogeneity in the conductive airway (Scond), Figure 1. In addition, the sputum eosinophil count was a stronger predictor than the sputum neutrophil count for Sacin, LCI, IC/TLC and RV/TLC, Figure 1. Smoking status (former vs current) was the strongest predictor of lung function and no association between the smoking status and the classification of patients to eosinophil-high and eosinophil-low could be observed (Fisher’s exact test p = 0.54). Likewise, sputum neutrophil count was similar between current and former smokers (75 [64–82] vs 72 [58–81], %), p = 0.44).
Discussion
This study demonstrates that sputum eosinophil count is an independent predictor for small airway dysfunction, rather for central airflow obstruction, after adjustment for potential predictors of lung function in COPD. Despite using a long-acting bronchodilator more frequently, eosinophil-high patients had worse lung function than those with low eosinophils. This study also demonstrates that airway eosinophilia is common in early COPD and can be found in nearly 20% of these patients even in absence of concurrent asthma features. The reported prevalence of airway eosinophilia in our study is comparable to previous findings of Hastie et al who reported a prevalence between 14% and 20% based on a sputum eosinophil cutoff of 1.25% and 2% in a population of COPD patients with different disease severities and a treatment with ICS in nearly one third of the patients.2 In our study, the early impairment in lung function was best measured through markers of small airway dysfunction, despite the fact that no significant differences in the FEV1/FVC ratio between patients with low and high eosinophils were found. This finding is in line with the previously reported results from surgically resected lung tissue which indicated that small airway dysfunction occurs earlier and independently from central airflow obstruction,7 which is routinely used for the diagnosis of COPD. It is also noteworthy that sputum eosinophil count was an independent predictor for small airway dysfunction rather than for the FEV1. The relationship between lung function and airways eosinophils in COPD is still not fully understood, particularly with regard to the FEV1, as the role of airway eosinophilia in large airflow obstruction remains uncertain.7,8 However, the reported relationship between lung function measure of small airway dysfunction and airway eosinophilia in our study confirms the previous findings from quantitative computed tomography, indicating that increased indices of air trapping and emphysema in COPD is associated with high sputum eosinophils.2 However, the long-term impact of airway eosinophils on the progressive decline of lung function needs to be investigated in longitudinal studies.
Identifying airway eosinophilia and its ensuing impact on small airway dysfunction might have therapeutic potential in selected patients with COPD. Currently, a therapy with ICS in COPD is only recommended in patients with frequent exacerbation and high blood eosinophil count,3 as ICS has demonstrated a limited impact on lung function of COPD patients. However, trials on this matter have almost only used the measure of FEV1 to assess the changes in lung function in non-selected populations of COPD patients.9 Therefore, the pursuance of targeting airway eosinophils in a subgroup of eosinophilic COPD patients and its impact on small airway dysfunction and patients’ symptoms seems to be reasonable.
The cross-sectional design and small cohort size were the main limitations of this study. However, to the best of our knowledge, this is the first study demonstrating an association between airway eosinophilia and lung function markers of small airway dysfunction in patients with mild or at risk for COPD. Therefore, larger interventional trials investigating the longitudinal nature of this association and its therapeutic potentials are warranted.
Abbreviations
AX, area under the reactance curve; CAT, COPD Assessment Test; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in first second; FRC, functional residual capacity; FVC, forced vital capacity; IC, inspiratory capacity; LCI, lung clearance index; MBW, multiple breath washout; MEF, maximal expiratory flow; R5–19, frequency dependence of resistance between 5 Hz and 19 Hz; RV, residual volume; Sacin, acinar ventilation inhomogeneity; SAD, small airway dysfunction; SBW, single breath washout; Scond, conductive ventilation inhomogeneity; SGRQ-c, St George’s Respiratory Questionnaire for COPD patients; sRtot, total specific airway resistance; TLC, total lung capacity.
Data Sharing Statement
The datasets used during the current study are available from the corresponding author on reasonable request.
Ethics Approval
The study is a part of the cohort of Change in Airway Peripheral Tone in COPD (CAPTO-COPD), (https://clinicaltrials.gov/ct2/show/NCT04166812). The study protocol was approved by local ethics committees (Ärztekammer Schleswig-Holstein and Medical Ethics Committee of Heidelberg University) and was performed in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines.
Funding
This study is supported by an unrestricted research grant from Chiesi GmbH, Hamburg, Germany.
Disclosure
Mustafa Abdo and Frauke Pedersen report no relevant conflicts of interest. Frederik Trinkmann reports grants from Chiesi for the conduct of the study. He also received travel support from Actelion, Berlin Chemie, Boehringer Ingelheim, Chiesi, Novartis, Mundipharma and TEVA as well as speaker or consultation fees from AstraZeneca, Berlin Chemie, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, GlaxoSmithKline, Novartis and Roche, Sanofi Aventis, all outside the submitted work. Felix Herth and Klaus F. Rabe report no relevant conflict of interest. Anne-Marie Kirsten reports grants from Chiesi for the conduct of the study. Henrik Watz reports grants from Chiesi for the conduct of the study. Grants, personal fees and non-financial support from AZ, grants, personal fees and non-financial support from GSK, grants, personal fees and non-financial support from Chiesi, grants, personal fees and non-financial support from Novartis, grants, personal fees and non-financial support from Bayer, grants, personal fees and non-financial support from BI, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Hoenderdos K, Condliffe A. The neutrophil in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2013;48(5):531–539. doi:10.1165/rcmb.2012-0492TR
2. Hastie AT, Martinez FJ, Curtis JL, et al. Sputum or blood eosinophil association with clinical measures of COPD severity in the SPIROMICS cohort. Lancet Respir Med. 2017;5(12):956–967. doi:10.1016/S2213-2600(17)30432-0
3. Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5):1900164. doi:10.1183/13993003.00164-2019
4. McDonough JE, Yuan R, Suzuki M, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365(17):1567–1575. doi:10.1056/NEJMoa1106955
5. Abdo M, Pedersen F, Kirsten A-M, et al. Longitudinal impact of sputum inflammatory phenotypes on small airway dysfunction and disease outcomes in asthma. J Allergy Clin Immunol. 2022;10:1545–1553.e2. doi:10.1016/j.jaip.2022.02.020
6. Pedersen F, Trinkmann F, Abdo M, et al. Influence of cell quality on inflammatory biomarkers in COPD sputum supernatant. Int J Chron Obstruct Pulmon Dis. 2021;16:487–493. doi:10.2147/COPD.S284938
7. Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645–2653. doi:10.1056/NEJMoa032158
8. Lams BE, Sousa AR, Rees PJ, Lee TH. Subepithelial immunopathology of the large airways in smokers with and without chronic obstructive pulmonary disease. Eur Respir J. 2000;15(3):512–516. doi:10.1034/j.1399-3003.2000.15.14.x
9. Pauwels RA, Löfdahl CG, Laitinen LA, et al. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European respiratory society study on chronic obstructive pulmonary disease. N Engl J Med. 1999;340(25):1948–1953. doi:10.1056/NEJM199906243402503
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

