Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Metabolic Score for Insulin Resistance (METS-IR) Predicts Adverse Cardiovascular Events in Patients with Type 2 Diabetes and Ischemic Cardiomyopathy
Authors Zhang X , Liu F, Li W, Zhang J, Zhang T , Yu X, Luo J, Zhao Q, Zhang J, Fang B, Yang Y , Li X
Received 26 January 2023
Accepted for publication 17 March 2023
Published 5 May 2023 Volume 2023:16 Pages 1283—1295
DOI https://doi.org/10.2147/DMSO.S404878
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Xuehe Zhang,1 Fen Liu,1 Wenling Li,1 Jixin Zhang,1 Tong Zhang,1 Xiaolin Yu,2 Junyi Luo,1 Qian Zhao,1 Jinyu Zhang,1 Binbin Fang,1 Yining Yang,2 Xiaomei Li1
1Department of Cardiology, First Affiliated Hospital of Xinjiang Medical University, Urumqi, People’s Republic of China; 2Department of Cardiology, People’s Hospital of Xinjiang Uygur Autonomous Region, Urumqi, People’s Republic of China
Correspondence: Yining Yang, Department of Cardiology, People’s Hospital of Xinjiang Uygur Autonomous Region, 91 Tianchi Road, Urumqi, 830054, People’s Republic of China, Tel +86-991-4361690, Email [email protected] Xiaomei Li, Department of Cardiology, First Affiliated Hospital of Xinjiang Medical University, 137 Liyushan South Road, Urumqi, 830054, People’s Republic of China, Tel +86-991-4362611, Email [email protected]
Purpose: This study aimed to evaluate the association between metabolic score for insulin resistance (METS-IR) and adverse cardiovascular events in patients with ischemic cardiomyopathy (ICM) and type 2 diabetes mellitus (T2DM).
Methods: METS-IR was calculated using the following formula: ln[(2 × fasting plasma glucose (mg/dL) + fasting triglyceride (mg/dL)] × body mass index (kg/m2)/(ln[high-density lipoprotein cholesterol (mg/dL)]). Major adverse cardiovascular events (MACEs) were defined as the composite outcome of nonfatal myocardial infarction, cardiac death, and rehospitalization for heart failure. Cox proportional hazards regression analysis was used to evaluate the association between METS-IR and adverse outcomes. The predictive value of METS-IR was evaluated by the area under the curve (AUC), continuous net reclassification improvement (NRI), and integrated discrimination improvement (IDI).
Results: The incidence of MACEs increased with METS-IR tertiles at a 3‑year follow‑up. Kaplan‒Meier curves showed a significant difference in event-free survival probability between METS-IR tertiles (P< 0.05). Multivariate Cox hazard regression analysis adjusting for multiple confounding factors showed that when comparing the highest and lowest METS-IR tertiles, the hazard ratio was 1.886 (95% CI:1.613– 2.204; P< 0.001). Adding METS-IR to the established risk model had an incremental effect on the predicted value of MACEs (AUC=0.637, 95% CI:0.605– 0.670, P< 0.001; NRI=0.191, P< 0.001; IDI=0.028, P< 0.001).
Conclusion: METS-IR, a simple score of insulin resistance, predicts the occurrence of MACEs in patients with ICM and T2DM, independent of known cardiovascular risk factors. These results suggest that METS-IR may be a useful marker for risk stratification and prognosis in patients with ICM and T2DM.
Keywords: metabolic score for insulin resistance, ischemic cardiomyopathy, type 2 diabetes mellitus, major adverse cardiac events, prognosis
Introduction
Ischemic cardiomyopathy (ICM) refers to left ventricular systolic dysfunction caused by obstructive coronary artery disease (CAD) and is the most common cause of heart failure (HF) with reduced ejection fraction.1 The main pathophysiological features of ischemic cardiomyopathy are left ventricular enlargement, decreased myocardial systolic and diastolic function, and further development of congestive heart failure.
Type 2 diabetes mellitus (T2DM) and HF often coexist, and 15–25% of people with heart failure have diabetes.2 The presence of diabetes is associated with reduced survival and increased hospitalization in patients with HF.3 The pathophysiological mechanisms of heart failure in patients with T2DM are complex. In addition to general heart failure risk factors such as advanced age, ethnicity, genetic predisposition, hypertension, and smoking,4 T2DM also increases the risk of ischemic heart failure by increasing the risk of CAD and directly affecting the myocardium, leading to structural and functional changes.5 However, the clustering of these classical cardiovascular risk factors is insufficient to explain the excess coronary artery disease risk in patients with diabetes. This suggests that other factors may be involved.
The main pathophysiological abnormalities of type 2 diabetes are insulin resistance (IR) and impaired insulin secretion. IR refers to the state in which insulin secretion is normal, but the sensitivity of tissues, organs and cells to insulin is reduced, and the role of insulin in promoting glucose uptake and utilization in target organs is reduced.6 Insulin resistance plays an important role in the development of early vascular diseases in patients with diabetes. T2DM patients experience a long period of insulin resistance before the clinical onset of the disease, while asymptomatic vascular injury develops long before the diagnosis of diabetes.7,8 There is increasing evidence that cardiometabolic disorders and insulin resistance are among the earliest alterations in the myocardium caused by diabetes, preceding functional and pathological changes.9 However, HF itself is thought to lead to insulin resistance.10,11 IR and T2DM combined with HF are mutually causal, forming a vicious circle and worsening the disease. These pathological injuries can damage the heart, resulting in impaired myocardial calcium handling and contractility, reduced cardiac energy efficiency, myocardial cell apoptosis and cardiac fibrosis.12 With the development of T2DM combined with HF, IR is also aggravated. In particular, patients with end-stage HF are accompanied by severe IR. Therefore, it is necessary to detect insulin resistance in the early stage of the disease. The hyperinsulinemic-euglycemic clamp technique is the gold standard for evaluating insulin resistance, but it is not practical in clinical practice. Therefore, alternative markers such as homeostasis model assessment of IR (HOMA-IR) have been developed to assess IR.13 However, HOMA-IR also requires additional blood samples and analysis costs. At the same time, because the actual state of IR cannot be reflected, it may not be effective in patients receiving subcutaneous treatment with basic insulin. Recently published studies have evaluated several new surrogate markers of insulin resistance. For example, triglyceride-glucose (TyG),14 TyG-body mass index (TyG-BMI),15 triglyceride to high-density lipoprotein cholesterol (TG/HDL-C),16 etc. However, to date, there is conflicting evidence regarding the role of these indicators in the screening, diagnosis, and prognosis of CAD, especially in patients with coexisting different metabolic diseases. These biomarkers may be useful for different cardiovascular disease subgroups.
The metabolic score for IR (METS-IR) is a new method to evaluate insulin resistance, and it has a higher consistency with the hyperinsulinemic-euglycemic clamp technique.17,18 METS‐IR, as a valid surrogate for insulin sensitivity that incorporates both laboratory and anthropometric measures, was considered to identify subjects more susceptible to early development of type 2 diabetes and metabolic syndrome. Apart from metabolic syndrome, METS‐IR has also been used for early identification of subjects at high risk of CAD, especially type 2 diabetes.17 Compared with other indicators, it is easier to identify high-risk groups of cardiovascular diseases.19 To our knowledge, no study has evaluated the association of METS-IR with adverse outcomes in ICM. Therefore, this study aims to evaluate the relationship between METS-IR and adverse cardiovascular events in ICM patients with diabetes.
Methods
Study Population
This study is a single-center, retrospective cohort study that included patients with type 2 diabetes mellitus and ICM who were admitted to the First Affiliated Hospital of Xinjiang Medical University from July 2013 to November 2019. In this study, patients with T2DM history, who are currently using insulin or hypoglycemic drugs, who have fasting plasma glucose≥7.0 mmol/L or 2 h blood glucose level≥11.1 mmol/L in the oral glucose tolerance test (OGTT), and who were diagnosed with diabetes by the OGTT during hospitalization were included. ICM was diagnosed as follows: 1. patients with a previous vascular reconstruction history; 2. main or proximal stenosis of the left anterior descending branch >75% and/or two main epicardial coronary artery stenoses >75% confirmed by coronary angiography; 3. echocardiography showed that the left ventricular function was lower than 40%. The exclusion criteria of this study included severe valvular disease or congenital heart disease, malignant tumor, blood system disease, familial hypertriglyceridemia, and hormone shock therapy. The study was conducted in accordance with the principles of the Declaration of Helsinki. Approved by the Medical Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University (20141201-03-1701A).
Data Collection and Definitions
Clinical data were collected from all medical records by trained clinicians who were blinded to the study objectives. The data included age, sex, smoking history, drinking history, history of hypertension, previous stroke, height, weight, systolic blood pressure (SBP) and diastolic blood pressure (DBP). Left ventricular ejection fraction(LVEF), early-to-late diastolic flow velocity ratio (E/A), left ventricular end diastolic dimension (LVEDD), and left atrial diameter (LAD) were recorded. Peripheral venous blood samples were collected in the morning after overnight fasting on admission. Concentrations of serum creatinine, fasting plasma glucose (FPG), triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), hemoglobin A1c (HbA1c), and B-type natriuretic peptide (BNP). Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). Renal function was assessed using the baseline estimated glomerular filtration rate (eGFR). EGFR was calculated as [(140-age) ×weight (kg)] ×0.85 (if female)/ [72×serum creatinine (mg/dL)]20. METS-IR was calculated as ln [(2 × FPG (mg/dL) + fasting TG (mg/dL)] × BMI (kg/m2))/(ln[HDL-C (mg/dL]).17
Follow-Up and Endpoint Events
Regular follow-up was performed with the patients, mainly through clinical visits or telephone contact. Clinical events were recorded, and dates were determined from patients enrolled in the study. The primary endpoint was major adverse cardiovascular events (MACEs), defined as a combination of cardiac death, nonfatal myocardial infarction (MI), or rehospitalization for heart failure. Secondary endpoints included nonfatal MI and cardiac death. Cardiac death was defined as death caused by MI, HF, sudden cardiac death, or cardiac procedures. If more than one event occurred during follow-up, the most severe endpoint event was selected for analysis.
Statistical Analysis
Continuous variables are presented as the mean ± standard deviation or median (interquartile range) according to the results of normality tests, and ANOVA or the Kruskal‒Wallis test was used to compare continuous variables in tertiles. Categorical variables were compared using the chi-square test. Spearman correlation coefficient was used to analyze the correlation between variables. Kaplan‒Meier curves were used to assess event-free survival associated with METS-IR tertiles, and the Log rank test was used for comparison. A multivariate Cox proportional hazards regression model was constructed to identify independent predictors of clinical endpoints. Model 1 was adjusted for age and sex, and Model 2 was adjusted for smoking, drinking, hypertension, previous stroke, and New York Heart Association (NYHA) class on the basis of Model 1. Model 3 was additionally adjusted for eGFR, LDL-C, HbA1c, BNP, LVEF, and E/A. Restricted cubic spline analysis was applied using the R package “rms” to explore the relationship between METS-IR and clinical endpoint events. A receiver operating characteristic curve (ROC) was used to compare the predictive ability of different indicators for the risk of clinical endpoints. The area under the curve (AUC), net reclassification improvement (NRI), and integrated discrimination improvement (IDI) were used to compare the incremental value of FPG, TGs, BMI, HDL-C, and METS-IR in predicting clinical endpoints based on established risk factors. IDI and NRI were performed using the R package “PredictABEL”. The R package “survminer” was used to visualize the survival curve, and the “survival package” was used to analyze the survival data. SPSS 25.0 (IBM Corp, Armonk, NY, USA) and R version 4.0.2 software (Vienna, Austria) were used for all analyses. P value <0.05 was considered statistically significant.
Results
Baseline Characteristics of Patients
A total of 1610 patients were included for follow-up (Figure 1), of whom 174 (10.8%) were lost to follow-up. A total of 1436 patients (89.2%) completed the 3-year clinical follow-up. The mean age of these patients was 65±10 years, including 1069 males (74.4%) and 367 females (25.6%). The distribution of admission METS-IR scores is shown in Figure 2. According to the METS-IR score at admission, the patients were divided into tertiles: T1 (n=479, METS-IR<40.9), T2 (n=473, 40.9≤METS-IR<48.0), and T3 (n=484, METS-IR≥48.0). The clinical baseline characteristics of the patients are shown in Table 1. There were statistically significant differences in age, sex, smoking, drinking, BMI, eGFR, FPG, TGs, HDL-C, HbA1c, BNP, LVEF, LVEDD, and LAD among the three groups. There were no significant differences in any other variables. Spearman rank correlation analysis showed that METS-IR was significantly correlated with DBP, eGFR, HbA1c, LVEF, LVEDD, and LAD (Table 2).
 |
Table 1 Baseline Characteristics of the Patients Stratified by the METS-IR Tertiles |
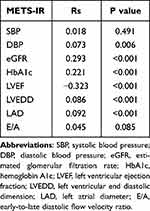 |
Table 2 Correlation Between the METS-IR and Clinical Variables |
 |
Figure 1 Flow diagram for inclusion of study subjects. |
 |
Figure 2 Histogram showing the population distribution of METS-IR. |
METS-IR and Cardiovascular Events
During the follow-up period of 3-year, 1091 (75.9%) MACEs were recorded, including 213 (14.8%) nonfatal MI, 728 (50.7%) cardiac death and 150 (10.4%) rehospitalization for heart failure. Kaplan‒Meier curves showed that event-free survival rates for nonfatal MI, cardiac death, and MACEs decreased with increasing METS-IR tertile (Figure 3). Using T1 as a reference, univariate Cox proportional hazards regression showed that METS-IR in T2 and T3 groups increased the hazard ratio (HR) of endpoint events, including nonfatal MI (HR: 3.298, 95% CI: 2.287–4.756, P<0.001), cardiac death (HR: 2.077, 95% CI: 1.731–2.492, P<0.001), and MACEs (HR: 1.863, 95% CI: 1.610–2.156, P<0.001). Multivariate adjustment models were established, and Model 1 was adjusted for age and sex. Model 2 was adjusted for variables related to general information, smoking, drinking, hypertension, previous stroke, and NYHA. Model 3 was adjusted for risk factors for heart failure on the basis of Model 2, including eGFR, LDL-C, HbA1c, BNP, LVEF, and E/A. METS-IR is independently associated with adverse outcomes in patients with ischemic cardiomyopathy and T2DM (Table 3). The multivariate HR increased with METS-IR levels at different cardiovascular endpoints(P<0.05). The relationship between METS-IR and adverse outcomes is shown in Figure 4. The results showed that METS-IR was an independent risk factor for poor prognosis in patients with ICM.
 |
Table 3 Univariate and Multivariate Cox Regression Analysis for Predicting MACE |
 |
Figure 3 Kaplan‒Meier survival curve for clinical outcomes across METS-IR tertiles. (A), Nonfatal MI (B), Cardiovascular death (C), MACEs. |
 |
Figure 4 Restricted cubic splines for the relationship between METS-IR and the hazard ratio for clinical endpoints. (A), Nonfatal MI (B), Cardiovascular death (C), MACEs. |
Predictive Ability of the METS-IR for the Risk of MACEs
ROC curve was used to assess the predictive ability of METS-IR for different clinical endpoints. For the prediction of nonfatal MI, cardiac death, and MACEs, the AUC of METS-IR was significantly higher than that of any individual variable involved in the calculation (Table 4). Established risk factors had significant predictive value for adverse cardiovascular events. Using FPG, TGs, BMI or HDL-C alone to predict the incidence of adverse outcomes on the basis of established risk factors has little incremental value. Adding METS-IR to the established risk factors improved the AUC for the prediction of nonfatal MI, cardiac death, and MACEs. Moreover, in terms of NRI and IDI, the addition of METS-IR had incremental prognostic value for predicting MACEs, especially when compared with established risk factors (Table 5). The prognostic value of METS-IR in MACEs in each subgroup is shown in Table 6. After adjusting for age, sex, smoking, drinking, hypertension, previous stroke, NYHA, eGFR, LDL-C, HbA1c, BNP, LVEF and E/A, METS-IR can independently predict the occurrence of MACEs in T2DM patients with ICM, and it is not related to advanced age (age ≥ 60), sex, history of hypertension, or renal insufficiency (eGFR<60).
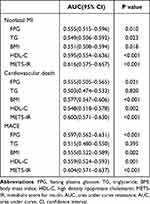 |
Table 4 Predictive Ability of Different Variables for Clinical Endpoints |
 |
Table 5 Evaluation of Predictive Models for Clinical Outcomes |
 |
Table 6 Prognostic Value of METS-IR for MACE in Various Subgroups |
Discussion
The current study demonstrates that METS-IR is a novel marker for assessing the risk of insulin resistance, which has been reported to have a strong relationship with CVD risk.19 In this study, for the first time, we identified METS-IR as an important risk factor for adverse outcomes in ICM patients with diabetes. The risk of nonfatal myocardial infarction, cardiac death and MACEs events increased with the increase in METS-IR, even after adjusting for general information and risk factors for heart failure.
As a common secondary cardiomyopathy, ICM is caused by coronary atherosclerotic heart disease, but it can also be caused by recurrent coronary artery spasm, coronary artery inflammation, and connective tissue disease. Patients develop diffuse fibrosis of the myocardium due to myocardial ischemia, manifested as dilated cardiomyopathy with systolic or diastolic dysfunction or both. When diabetes and heart failure coexist, morbidity and mortality risks are significantly increased, and adverse patient outcomes are synergistically amplified.21,22 Insulin resistance and hyperglycemia are key central metabolic disorders in T2DM, leading to specific metabolic abnormalities that adversely affect myocardial function.3 Since both glucose and fatty acid metabolism in the heart are tightly controlled by insulin signaling, insulin resistance plays a key role in the pathogenesis of heart failure. Indeed, impairment of insulin signaling and development of insulin resistance are major determinants of heart failure progression.23 Although insulin is considered a survival hormone, the presence of insulin resistance in patients with diabetes may compromise the protective effects of this hormone. Insulin may lose its cardioprotective effect because of impaired MAPK phosphatase-1 expression or because hyperglycemia directly impairs insulin signaling.24 In T2DM, insulin resistance is present for several years before hyperglycemia develops. Thus, the effect of hyperglycemia alone on cardiovascular disease and its risk factors are difficult to assess because virtually all patients have insulin resistance.25 Studies have shown that diabetes mellitus (DM) and IR are not the only pathogenic factors of HF, but HF patients with DM or IR have more severe left ventricular dysfunction and higher mortality than patients without HF and diabetes or IR. IR is not simply a problem of inadequate glucose uptake induced by insensitivity to insulin but rather a multifaceted syndrome that significantly increases the risk of cardiovascular disease.
Studies on alternative markers of insulin resistance suggested that METS‐IR could be a useful surrogate measure of insulin sensitivity for the earlier identification of patients with T2DM.17,26 The risk of cardiovascular disease persists in dyslipidemia, which is characteristic of atherosclerosis. The dyslipidemia caused by insulin resistance and type 2 diabetes (dyslipidemia in diabetes) is characterized by (1) hypertriglyceridemia, (2) low HDL cholesterol levels, (3) the presence of small-density low-density lipoprotein, and postprandial hyperlipemia.27 One of the main mechanisms behind dyslipidemia in the state of insulin resistance is the increased flow of free fatty acids from adipose tissue to the liver. Accumulation of intracellular lipid metabolites in the liver appears to contribute to hepatic insulin resistance. The liver accounts for 30% of insulin-stimulated glucose metabolism, and insulin resistance leads to impaired glucose output and fatty acid metabolism. Free fatty acids promote increased hepatic triglyceride synthesis, which leads to the secretion of very low-density lipoprotein. METS-IR, as a combined indicator of FPG, TG, BMI and HDL-C, can better reflect the metabolic risk of diabetic patients, and as a more favorable predictor of cardiovascular disease in patients with diabetes.
Previous studies have shown that the impact of diabetes on the prognosis of patients with heart failure is significantly influenced by the underlying etiology, and is particularly detrimental in ischemic heart disease.22,28,29 Left ventricular dysfunction and adverse remodeling in ICM indicate a poor prognosis for recurrent MI, HF, and arrhythmia. On this basis, diabetes can further worsen the long-term outcomes of ischemic injury, including increased incidence of heart failure and all-cause mortality. Patients with T2DM have an accelerated atherosclerotic process with more lipid-rich and unstable atherosclerotic plaques, resulting in a 2-4-fold increase in cardiovascular disease morbidity and mortality compared to patients with atherosclerosis without diabetes.30–32 Fibrin clots formed in patients with diabetes were denser and had less porous structures than those in healthy individuals. Patients with hyperglycemia due to insulin resistance are susceptible to concurrent insulin-driven fibrinolytic injury and glucose-driven clot-activated thrombotic events.33 Expression of Plasminogen activator inhibitor 1 (PAI-1) was also associated with insulin resistance.34,35 IR patients are more likely to develop fibrinolytic disorders. In addition, insulin resistance may lead to the accumulation of macrophages in the vessel wall through a variety of mechanisms, thereby increasing the instability of atherosclerosis and vulnerable plaques.36 Finally, many human and animal studies have shown that insulin resistance increases the degree of myocardial damage during myocardial ischemia, possibly leading to an increased risk of heart failure in affected individuals.37 Our study showed that patients in the METS-IR high group had a significantly higher incidence of nonfatal MI, cardiac death, and MACEs after discharge than patients in the METS-IR low group. At the same time, compared with FPG, TGs, BMI and HDL-C, whether independently predicted or added to the established risk factors for nonfatal MI, cardiac death, and MACEs, the predictive value of METS-IR was higher and significantly improved the established risk prediction.
In 1995, Stern proposed the “Common Soil Hypothesis”, which posits insulin resistance as a common risk factor for hypertension, diabetes, heart disease, and stroke.38 Insulin resistance stimulates sympathetic activity, which leads to increased blood pressure.39 Clinical studies have shown that approximately 50% of hypertensive subjects have comorbid hyperinsulinemia or glucose intolerance, while at least 80% of patients with type 2 diabetes have comorbid hypertension.40 Meanwhile, in patients with diabetes, heart failure and renal insufficiency often coexist.41 Due to chronic inflammation, uremic toxins, and vitamin D deficiency, chronic kidney disease patients have a higher IR than the general population.42 Subgroup analysis showed that after adjusting for important variables, METS-IR was an independent predictor of adverse cardiovascular outcomes regardless of age ≥ 60 years, sex, hypertension and renal insufficiency.
In fact, the hearts of people with diabetes face significant stress from hyperlipidemia, hyperglycemia, and inflammation. There is sufficient evidence that cardiovascular disease risk begins with insulin resistance as an asymptomatic state that develops long before overt diabetes. Insulin resistance may be the major intracellular event leading to the ultimate fate of the heart in patients with diabetes.13,43 Therefore, proactive clinical management of patients at high risk of insulin resistance should be initiated early to prevent the development of adverse cardiovascular events. Our study demonstrates the significant predictive value of METS-IR for adverse outcomes in patients with ICM and T2DM. In practice, METS-IR can be used to identify high-risk patients with diabetes for early intervention, reduce the incidence of MACEs, and improve the prognosis of patients.
Study Limitations
This study is a single-center retrospective study, and regardless of the analysis adjustment, it is still difficult to exclude the influence of some potential confounders. These findings need to be further verified by a multicenter prospective cohort study. Second, the baseline level of METS-IR was derived from the admission assessment, and FPG or TG may be affected by antidiabetic and lipid-lowering drugs during follow-up. It is unclear whether changes in METS-IR affect its clinical predictive value. Third, the comparison of predictive value between METS-IR and HOMA-IR and the hyperinsulinemic euglycemic clamp technique requires further study. Despite these limitations, this study has important clinical implications because it is the first study of the association between METS-IR and adverse cardiovascular events in patients with diabetes and ICM.
Conclusion
METS-IR is a score that is easy to obtain and calculate in the clinic. In ICM patients with T2DM, METS-IR was significantly associated with the occurrence of MACEs, and even after adjusting for other confounding factors, this relationship was still significant. Adding METS-IR to established risk prediction models has incremental value for MACEs prediction.
Data sharing Statement
The datasets used and/or analyzed in the study are available from the corresponding author upon reasonable request.
Ethics Approval
The study was conducted in accordance with the principles of the Declaration of Helsinki. Informed consent for participation was obtained in this study. Approved by the Medical Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University (20141201-03-1701A).
Acknowledgments
We thank all the investigators and subjects who participated in this project.
Funding
This work was supported by Xinjiang Uygur Autonomous Region University Scientific Research Program (No: XJEDU2020I013); Xinjiang Uygur Autonomous Region Postgraduate Scientific Research Innovation Project (XJ2022G158).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Perera D, Clayton T, Petrie MC, et al. Percutaneous revascularization for ischemic ventricular dysfunction: rationale and design of the REVIVED-BCIS2 trial: percutaneous coronary intervention for ischemic cardiomyopathy. JACC Heart Fail. 2018;6(6):517–526. doi:10.1016/j.jchf.2018.01.024
2. Teupe C, Rosak C. Diabetic cardiomyopathy and diastolic heart failure - difficulties with relaxation. Diabetes Res Clin Pract. 2012;97(2):185–194. doi:10.1016/j.diabres.2012.03.008
3. Dei Cas A, Khan SS, Butler J, et al. Impact of diabetes on epidemiology, treatment, and outcomes of patients with heart failure. JACC Heart Fail. 2015;3(2):136–145. doi:10.1016/j.jchf.2014.08.004
4. Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev. 2004;25(4):543–567. doi:10.1210/er.2003-0012
5. Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30(6):595–602. doi:10.1016/0002-9149(72)90595-4
6. Semenkovich CF. Insulin resistance and a long, strange trip. N Engl J Med. 2016;25:160217112012002.
7. Rhee EJ, Kim JH, Park HJ, et al. Increased risk for development of coronary artery calcification in insulin-resistant subjects who developed diabetes: 4-year longitudinal study. Atherosclerosis. 2016;245:132–138. doi:10.1016/j.atherosclerosis.2015.12.010
8. Henry RM, Kostense PJ, Spijkerman AM, et al. Arterial stiffness increases with deteriorating glucose tolerance status: the Hoorn Study. Circulation. 2003;107(16):2089–2095. doi:10.1161/01.CIR.0000065222.34933.FC
9. Stanley WC, Lopaschuk GD, McCormack JG. Regulation of energy substrate metabolism in the diabetic heart. Cardiovasc Res. 1997;34(1):25–33. doi:10.1016/S0008-6363(97)00047-3
10. Swan JW, Anker SD, Walton C, et al. Insulin resistance in chronic heart failure: relation to severity and etiology of heart failure. J Am Coll Cardiol. 1997;30(2):527–532. doi:10.1016/S0735-1097(97)00185-X
11. Paternostro G, Pagano D, Gnecchi-Ruscone T, Bonser RS, Camici PG. Insulin resistance in patients with cardiac hypertrophy. Cardiovasc Res. 1999;42:246–253.
12. Fujii S. Insulin resistance and heart failure. J Cardiovasc Pharmacol. 2013;62(4):379–380. doi:10.1097/FJC.0b013e3182a18c56
13. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412. doi:10.1007/BF00280883
14. Sang BL, Min KK, Kang S, et al. Triglyceride glucose index is superior to the homeostasis model assessment of insulin resistance for predicting nonalcoholic fatty liver disease in Korean adults. Endocrinol Metabol. 2019;34(2). doi:10.3803/EnM.2019.34.2.179
15. Raimi TH, Dele-Ojo BF, Dada SA, Fadare JO, Ajayi OA. Triglyceride-glucose index and related parameters predicted metabolic syndrome in Nigerians. Metab Syndr Relat Disord. 2020;19(2):76–82. doi:10.1089/met.2020.0092
16. Zhang Y, Qin P, Lou Y, et al.Association of TG/HDLC ratio trajectory and risk of type 2 diabetes: a retrospective cohort study in China. J Diabetes. 2020. doi:10.1111/1753-0407.13123
17. Bello-Chavolla OY, Almeda-Valdes P, Gomez-Velasco D, et al. METS-IR, a novel score to evaluate insulin sensitivity, is predictive of visceral adiposity and incident type 2 diabetes. Eur J Endocrinol. 2018;178(5):533–544. doi:10.1530/EJE-17-0883
18. Liu XZ, Fan J, Pan SJ. METS-IR, a novel simple insulin resistance indexes, is associated with hypertension in normal-weight Chinese adults. J Clin Hypertens. 2019;21(8):1075–1081. doi:10.1111/jch.13591
19. Yoon J, Jung D, Lee Y, Park B. The Metabolic Score for Insulin Resistance (METS-IR) as a predictor of incident ischemic heart disease: a longitudinal study among Korean without diabetes. J Pers Med. 2021;11(8):742. doi:10.3390/jpm11080742
20. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi:10.1159/000180580
21. Kenny HC, Abel ED. Heart failure in type 2 diabetes mellitus. Circ Res. 2019;124(1):121–141. doi:10.1161/CIRCRESAHA.118.311371
22. Johansson I, Dahlström U, Edner M, Näsman P, Rydén L, Norhammar A. Prognostic implications of type 2 diabetes mellitus in ischemic and nonischemic heart failure. J Am Coll Cardiol. 2016;68(13):1404–1416. doi:10.1016/j.jacc.2016.06.061
23. Ingelsson E, Sundstrom J, Arnlov J, Zethelius B, Lind L. Insulin resistance and congestive heart failure—reply. J Am Med Assoc. 2005;15:294.
24. Morisco C, Marrone C, Trimarco V, et al. Insulin resistance affects the cytoprotective effect of insulin in cardiomyocytes through an impairment of MAPK phosphatase-1 expression. Cardiovasc Res. 2007;76(3):453–464. doi:10.1016/j.cardiores.2007.07.012
25. Stancáková A, Javorský M, Kuulasmaa T, Haffner SM, Kuusisto J, Laakso M. Changes in insulin sensitivity and insulin release in relation to glycemia and glucose tolerance in 6414 Finnish men. Diabetes. 2009;58(5):1212–1221. doi:10.2337/db08-1607
26. Yu X, Wang L, Zhang W, et al. Fasting triglycerides and glucose index is more suitable for the identification of metabolically unhealthy individuals in the Chinese adult population: a nationwide study. J Diabetes Investig. 2019;10(4):1050–1058. doi:10.1111/jdi.12975
27. Goldberg IJ. Clinical review 124: diabetic dyslipidemia: causes and consequences. J Clin Endocrinol Metab. 2001;86(3):965–971. doi:10.1210/jcem.86.3.7304
28. De Groote P, Lamblin N, Mouquet F, et al. Impact of diabetes mellitus on long-term survival in patients with congestive heart failure. Eur Heart J. 2004;25(8):656–662. doi:10.1016/j.ehj.2004.01.010
29. Dries DL, Sweitzer NK, Drazner MH, Stevenson LW, Gersh BJ. Prognostic impact of diabetes mellitus in patients with heart failure according to the etiology of left ventricular systolic dysfunction. J Am Coll Cardiol. 2001;38(2):421–428. doi:10.1016/S0735-1097(01)01408-5
30. Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339(4):229–234. doi:10.1056/NEJM199807233390404
31. Rao Kondapally Seshasai S, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829–841.
32. Schramm TK, Gislason GH, Køber L, et al. Diabetes patients requiring glucose-lowering therapy and nondiabetics with a prior myocardial infarction carry the same cardiovascular risk: a population study of 3.3 million people. Circulation. 2008;117(15):1945–1954. doi:10.1161/CIRCULATIONAHA.107.720847
33. Stegenga ME, van der Crabben SN, Levi M, et al. Hyperglycemia stimulates coagulation, whereas hyperinsulinemia impairs fibrinolysis in healthy humans. Diabetes. 2006;55(6):1807–1812. doi:10.2337/db05-1543
34. Juhan-Vague I, Alessi MC, Vague P. Increased plasma plasminogen activator inhibitor 1 levels. A possible link between insulin resistance and atherothrombosis. DIABETOLOGIA. 1991;34(7):457–462. doi:10.1007/BF00403280
35. Schneider,David J. Abnormalities of coagulation, platelet function, and fibrinolysis associated with syndromes of insulin resistance. Coron Artery Dis. 2005;16(8):473–476. doi:10.1097/00019501-200512000-00003
36. Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011;14(5):575–585. doi:10.1016/j.cmet.2011.07.015
37. Aurigemma GP, de Simone G, Fitzgibbons TP. Cardiac remodeling in obesity. Circ Cardiovasc Imaging. 2013;6(1):142–152. doi:10.1161/CIRCIMAGING.111.964627
38. Stern MP. Diabetes and cardiovascular disease. The “common soil” hypothesis. Diabetes. 1995;44(4):369–374. doi:10.2337/diab.44.4.369
39. Huggett RJ, Scott EM, Gilbey SG, Stoker JB, Mackintosh AF, Mary DA. Impact of type 2 diabetes mellitus on sympathetic neural mechanisms in hypertension. Circulation. 2003;108(25):3097–3101. doi:10.1161/01.CIR.0000103123.66264.FE
40. Zhou MS, Schulman IH, Zeng Q. Link between the renin-angiotensin system and insulin resistance: implications for cardiovascular disease. Vasc Med. 2012;17(5):330–341. doi:10.1177/1358863X12450094
41. Banerjee S, Panas R. Diabetes and cardiorenal syndrome: understanding the “Triple Threat”. Hellenic J Cardiol. 2017;58(5):342–347. doi:10.1016/j.hjc.2017.01.003
42. Becker B, Kronenberg F, Kielstein JT, et al. Renal insulin resistance syndrome, adiponectin and cardiovascular events in patients with kidney disease: the mild and moderate kidney disease study. J Am Soc Nephrol. 2005;16(4):1091–1098. doi:10.1681/ASN.2004090742
43. Nakamura K, Sakurai M, Miura K, et al. Homeostasis model assessment of insulin resistance and the risk of cardiovascular events in middle-aged non-diabetic Japanese men. Diabetologia. 2010;53(9):1894. doi:10.1007/s00125-010-1803-z
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
