Back to Journals » Journal of Hepatocellular Carcinoma » Volume 9
Macrotrabecular-Massive Hepatocellular Carcinoma: Light and Shadow in Current Knowledge
Authors Sessa A , Mulé S, Brustia R, Regnault H, Galletto Pregliasco A , Rhaiem R, Leroy V, Sommacale D, Luciani A, Calderaro J, Amaddeo G
Received 2 March 2022
Accepted for publication 22 June 2022
Published 27 July 2022 Volume 2022:9 Pages 661—670
DOI https://doi.org/10.2147/JHC.S364703
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Imam Waked
Anna Sessa,1– 3 Sébastien Mulé,2– 4 Raffaele Brustia,2,3,5 Hélène Regnault,1,3 Athena Galletto Pregliasco,4 Rami Rhaiem,6,7 Vincent Leroy,1– 3 Daniele Sommacale,2,3,5 Alain Luciani,2– 4 Julien Calderaro,2,3,8 Giuliana Amaddeo1– 3
1Hepatology Department, APHP, Henri Mondor University Hospital, Créteil, France; 2Université Paris-Est Créteil, Faculté de Médecine, Créteil, France; 3Inserm, U955, Team 18, Créteil, France; 4Medical Imaging Department, AP-HP, Henri Mondor University Hospital, Créteil, France; 5Department of Digestive and Hepato-Pancreato-Biliary Surgery, AP-HP, Henri Mondor University Hospital, Créteil, France; 6Department of Hepato-Biliary Pancreatic and Digestive Oncological Surgery, Robert Debré University Hospital, Reims, France; 7Reims Champagne-Ardenne University, Reims, France; 8Department of Pathology, APHP, Henri Mondor University Hospital, Créteil, France
Correspondence: Giuliana Amaddeo; Anna Sessa, Hepatology Department, APHP, Henri Mondor University Hospital, 1 rue Gustave Eiffel, Créteil, 94000, France, Tel +33 149812353, Email [email protected]; [email protected]
Abstract: The subject of this narrative review is macrotrabecular-massive hepatocellular carcinoma (MTM‐HCC). Despite their rarity, these tumours are of general interest because of their epidemiological and clinical features and for representing a distinct model of the interaction between the angiogenetic system and neoplastic cells. The MTM‐HCC subtype is associated with various adverse biological and pathological parameters (the Alfa-foetoprotein (AFP) serum level, tumour size, vascular invasion, and satellite nodules) and is a key determinant of patient prognosis, with a strong and independent predictive value for early and overall tumour recurrence. Gene expression profiling has demonstrated that angiogenesis activation is a hallmark feature of MTM-HCC, with overexpression of both angiopoietin 2 (ANGPT2) and vascular endothelial growth factor A (VEGFA).
Keywords: hepatocellular carcinoma, macrotrabecular, angiopoietin 2 inhibitors, TP53, immunotherapy
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer in the world, with 841100 new cases in 2018, and it is the second leading cause of cancer death, with a 5-year survival of 18%. In terms of sex, HCC is the fifth most common cancer in males and the ninth most common cancer in females worldwide.1 It has been reported that approximately 80% of HCC cases occur in developing countries. HCC appears frequently in patients with cirrhosis, and the most common risk factors include alcohol consumption, hepatitis B (HBV) or hepatitis C (HCV) virus infection and nonalcoholic steatohepatitis (NASH).2,3
HCC is a major health problem, and the majority of patients are diagnosed at an advanced stage, resulting in poor treatment outcomes.4
According to EASL and AASLD guidelines, current HCC diagnosis does not require histological confirmation due to excellent reported diagnostic performance of imaging procedures.4,5
In patients with cirrhosis, HCC may be diagnosed noninvasively using multiphasic computed tomography (CT) or dynamic contrast material–enhanced magnetic resonance imaging (MRI) using the Liver Imaging Reporting and Data System (LI-RADS), which standardizes HCC imaging acquisition and terminology and enables accurate stratification of the probability of HCC and overall malignancy.4,6–8
Although numerous histological tumour variants have been reported over the last decades, there has been no robust determination of the clinical impact of pathological classification to date; therefore, a liver biopsy is needed to obtain more information on the clinical implications of the HCC histological subtype.
HCC is a highly heterogeneous cancer at both molecular and histological levels. The HCC phenotype appears to be closely related to particular gene mutations, tumour subgroups and/or oncogenic pathways. Several HCC subtypes characterized by histological features and growth patterns have been identified, including solid (compact), pseudoglandular/acinar, and trabecular patterns, as described in the current WHO classification.9
These subtypes correlate with clinical features and prognosis but have not been thoroughly tested as predictors of a response to local or systemic therapies, which explains their limited clinical usefulness.10
Recent studies have shown that the macrotrabecular-massive subtype of HCC (MTM-HCC) has distinct molecular features and poor prognosis among the aforementioned subtypes.11
The subject of this review is this aggressive subtype of HCC: the diagnostic, associated clinicopathological and imaging features are analysed, and treatment is discussed with particular emphasis on actual knowledge and a brief parenthetical consideration of angiopoietin 2 inhibitors, an ongoing subject of discussion.
Histological Definition: Origins to the Present
The most common growth pattern of HCC is a trabecular form that mimics normal hepatic cord plates. This growth pattern has been referred to as “macrotrabecular” (MT) when the trabeculae become >6 cells thick,12,13 and HCC in which the MT pattern constitutes >50% of the entire tumour is considered a subtype: macrotrabecular-massive hepatocellular carcinoma (MTM-HCC).11–17
MT-HCC was first recognized as a distinct growth pattern in 1983,12 but no studies supporting this observation have since been performed. There are only a few citations of MT-HCC in the literature.11,18,19
The MTM-HCC subtype was first defined in a French collaborative study conducted by Calderaro.17
In this systematic analysis of more than 340 HCCs, the cut-off for defining HCC as MTM-HCC was the presence of more than 50% of the MT pattern in a tumour, which in turn was defined as a hepatic cord thickness >6 cells.17 Using biopsy samples, cases are classified as MTM-HCC if at least one focus of the macrotrabecular pattern is identified, without taking into account the areal percentage occupied by the pattern.11
Compared to the results reported by the French group, Jean et al reported a lower cut-off of a ≥30% MTM pattern as a diagnostic criterion,15 which needs to be validated by further studies (Figure 1).
Histological Prevalence
An MTM-HCC incidence rate of approximately 10–15% has been reported in different studies.11–16
Ziol et al11 identified the macrotrabecular-massive subtype in 12% of cases in a cohort of 237 HCC surgical samples and 284 HCC liver biopsies. Rastogi performed a retrospective study on 217 HCC patients in which MT-HCC was diagnosed in 20/218 (9.2%) cases, with coexistence of macrotabecular and pseudoglandular patterns in 18% of cases.21 By comparison, Lauwers et al25 noted a macrotrabecular-predominant architecture in a higher number of cases (26.6% of 425 HCC resections).
In the study by Renne et al, MTM-HCC was identified in 7.8% of 541 resected HCCs from Italy, Korea and Japan,20 whereas Feng et al reported MTM-HCC in 38% of 170 resected HCCs in China.22
Coherently with Renne, Cannella et al found MTM-HCC in 7.8% of 295 HCCs cases.23
In a recent American study,24 MTM-HCC was reported in 7.1% of 378 HCC. However, the macrotrabecular pattern was more commonly seen in HCCs arising in non-fibrotic liver tissues compared with HCCs in cirrhosis (13.68% vs 4.95%) (Table 1).
 |
Table 1 Macrotrabecular Tumour Incidence |
Pathological Features
MTM patterns were found to be significantly correlated with the pathological features of aggressiveness, such as Edmondson grades III–IV (P = 0.001), and less pseudoglandular pattern.20
In the Calderaro study,17 the pathological features associated with the MTM-HCC subtype were satellite nodules and vascular invasion.
Rastogi et al21 performed a study series in which it showed that the macrotrabecular pattern was often associated with poor grades of differentiation, the pT3 stage, tumour sizes of 2–5 cm and >5 cm, and high AFP levels.21
In the Renne study, the MTM-HCC subtype was significantly enriched by vessels that encapsulated tumour clusters (VETC phenotype), which was defined as enrichment of ≥55% of the tumour area by CD34 immunostaining (P = 0.006).20
Molecular Biology
Hepatocellular carcinoma cells accumulate somatic DNA alterations. The most frequent genetic alterations are mutations in the TERT promoter, accounting for approximately 60% of cases, followed by mutations in TP53 in approximately 30% of cases, WNT signalling (CTNNB1 and AXIN1 in approximately 40% and 10% of cases, respectively), or chromatin remodelling (ARID1A and ARID2 in approximately 10% and 5% of cases, respectively).26
Calderaro et al17 was the first to show that a TP53 mutation and FGF19 amplification were associated with the MTM-HCC subtype. A strong association with the G3 transcriptomic subgroup, a subclass linked to cell cycle activation and chromosomal instability, was also observed.17
Interestingly, Li Li Liu et al27 reported a higher expression of tumoral programmed death-ligand 1/chemokine-like factor (CKLF)-like MARVEL transmembrane domain containing 6 (PD-L1/CMTM6) and a higher inflammatory cell density in the MTM-HCC subtype compared to other subtypes. CMTM6 belongs to the chemokine-like factor (CKLF)-like MARVEL transmembrane domain-containing (CMTM) family and is a key regulator of PD-L1; that is, CMTM6 is an important immune checkpoint inhibitor that promotes PD-L1 expression in tumour cells in the defence against T cells.28 In the Renne study, compared to other subtypes, the MTM-HCC subtype was significantly more frequent in p53+ and double-positive phenotypes (P53+ and β-catenin/GS+, with P < 0.001 and P = 0.046, respectively) and less frequent in the β-catenin/GS+ phenotype (P = 0.006).20
The Role of Biopsy
The role of tumour biopsies in the management of patients with HCC is one of the most actively debated subjects in the liver cancer community.29–31
MTM-HCC can be accurately diagnosed in biopsy samples through the identification of MTM patterns.11
Using biopsies to identify morphology and molecular alterations is critically important for elucidating the mechanisms of carcinogenesis, improving diagnosis and prognostication, and finally facilitating the development of personalized medicine by identifying tumour entities with overexpressed PDL-1 that respond to immunotherapy.
Calderaro et al32 used a molecular-driven selection of biomarkers to implement the diagnosis of HCC morphomolecular subtypes and identified ESM1 (endothelial specific molecule 1) as a reliable microenvironment immunohistochemical marker of MTM-HCC.32
The very aggressive MTM-HCC subtype may lead to a reconsideration of the role of biopsy for HCC diagnosis because the identification of this subtype during pretherapeutic work-up may have strong prognostic implications. Indeed, patients may benefit from adjuvant therapies and/or upfront registration on the liver transplant waiting list after resection or RFA. However, no data are available on the prognostic value of the MTM-HCC subtype regarding recurrence after liver transplantation.
Clinical Features
Calderaro et al showed that the MTM-HCC subtype was characterized by HBV infection, a higher level of alpha-fetoprotein, early relapse and poor survival. Subsequent work by the same group also showed that this subtype was associated with advanced tumour stages, vascular invasion, and poor prognosis.17
MTM-HCC is associated with poor survival in all studies. For example, Calderaro et al reported that the survival rate was 40% at 24 months in INSERM U1162 cohort and 60% at 24 months in validation cohort.17
Although the available data are coherent, additional study series are needed to investigate the clinical impact of MTM-HCC.
Radiological Features of Macrotrabecular-Massive Hepatocellular Carcinoma
To date, few analyses have been performed on the radiological features of histologically confirmed MTM-HCC (Table 2). Most of these have focused on MRI presentations.
 |
Table 2 Imaging Features of Macrotrabecular-Massive HCC Subtype |
Macrotrabecular-massive HCC has been reported to be associated with a peculiar microvascular pattern described as a sinusoid-like microvascular pattern or “vessels that encapsulate tumour clusters” (VETC) pattern, appear as a cobweb-like network encapsulating individual HCC clusters.33–35
HCCs with such vascular patterns exhibit a low microvascular density and frequent tumour necrosis, and low microvascular density is highly correlated with low arterial enhancement on MRI.36
In line with these results, Rhee et al14 used MRI data to show that the internal or diffuse hypovascular component (≥50% of the hypovascular component) of the arterial phase combined with two or more ancillary findings (intratumoural artery, arterial phase peritumoural enhancement, and nonsmooth tumour margin) was significantly associated with MTM-HCC and was associated with a poor prognosis.
Mulé et al16 performed a retrospective study on 189 patients, in which MTM-HCCs were classified as LIRADS-5 on multiphase contrast-enhanced MRI and presented substantial necrosis that may help to identify the MTM-HCC subtype with high specificity.
Feng et al22 confirmed previously reported results on MRI and were the first to report that substantial necrosis on CT was related to the presence of VETC.22
Recently, Zhu et al37 reported that for 88 patients with histologically confirmed HCC, intratumour fat was determined to be an independent predictor of MT-HCC, whereas necrosis was not.37
A macrotrabecular area has also been more frequently observed in HCCs with arterial phase rim-like enhancement on gadoxetic acid-enhanced MRI.15
Hence, the presence of substantial necrosis on imaging may lead to consideration for lesion biopsy with therapeutic and prognostic intent, especially as the MTM-HCC subtype can be accurately diagnosed in biopsy samples.11
In a recent Italo-French study,23 the larger size, the presence of tumor in vein (TIV), at least 1 LR-M feature (LI-RADS major features, ie non-rim APHE, non-peripheral “washout”, and enhancing “capsule”), infiltrative appearance and necrosis or severe ischaemia were significantly more frequent in MTM-HCCs than in other subtypes on contrast-enhanced CT).
In addition, TIV and larger size tended to be more frequent in MTM-HCCs on MRI too.23
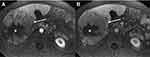
Imaging findings of MTM-HCC could help select HCC patients who show poor outcomes after curative treatment as potential candidates for clinical trials of neoadjuvant strategies (Figure 2).
Treatment
According to the Barcelona Clinic Liver Cancer (BCLC), the only curative treatments for HCC to date – whatever the histological subtype (including MTM) – are liver transplantation, resection and in some cases thermal ablation. Compared to the standard surgical approach in HCC, there are no dedicated technical strategies allowing to improve survival outcomes. Systemic treatment is the only option for advanced HCC.4 Sorafenib was the first agent shown to improve the survival of patients with advanced HCC or in cases of intermediate-stage disease despite progression with transarterial therapies.38 Treatment practices have changed in recent years with the advent of Lenvatinib39 as another first-line treatment choice and Regorafenib,40 Ramucirumab,41 and Cabozantinib42 as second-line treatment options. Checkpoint-blockade immunotherapy targeting programmed cell death protein 1 (PD-1) has recently shown promising efficacy for HCC. In May 2020, the FDA approved a combination treatment of Atezolizumab and Bevacizumab for patients with unresectable or metastatic HCC who have not received prior systemic therapy based on the primary analysis results of the IMbrave150 study.43
Li Li Lu et al27 reported that the MTM-HCC subtype was characterized by a high expression of CMTM6/PD-L1, suggesting that MTM-HCC patients are suitable candidates for tumour immunotherapy.
Targeting particular subsets of tumours remains a promising approach, as shown by the recent success of the REACH-2 trial41 that assessed the efficacy of Ramucirumab, an anti-angiogenic drug, in patients with elevated alpha-fetoprotein serum levels. Interestingly, MTM-HCC is characterized by increased levels of alpha-fetoprotein and activation of angiogenesis; the antitumour effect of Ramucirumab or other anti-angiogenic drugs in patients with this subtype of HCC should be assessed.41
During trans-arterial chemoembolization (TACE), the ischemic damage caused by the arterial occlusion induces the production of the hypoxia-inducible factor (HIF)-1α, a known modulator of PD-L1 expression, decreased the percentage of T-regs and increased the CD4+/CD8+ ratio.44
In particular, Montasser et al in their series with 11 cases of MTM-HCC demonstrated an increase of PD-1 and PD-L1 expression in HCC following TACE that supported the use of TACE in combination with immunotherapy.45
This rationale guided the design of trials testing the combination of TACE and anti-PD-1 mAb (like, in NCT03397654 trial – pembrolizumab after TACE- or NCT03143270 trial – nivolumab with drug-eluting bead-TACE). More data are needed to evaluate the benefits of treatment combining TACE and checkpoint inhibitors in MTT-HCC.
Prognosis
HCC is a highly malignant tumour associated with a poor prognosis, and only a subset of patients are eligible for curative treatment options. Moreover, the recurrence rate is high.5 The tumour size, vascular invasion, grading and staging are important variables for predicting HCC prognosis.46
Although the MTM-HCC subtype is more prevalent in intermediate or advanced HCC, MTM-HCC represented a significant fraction (10%) of tumours classified as very early or early HCC (BCLC 0/A) in a cohort studied by Ziol et al.11
Macrotrabecular-massive HCC (MTM-HCC) appears to be associated with early relapse after resection or percutaneous ablation, independent of classical clinical and radiological prognostic factors.34
The MTM-HCC pattern was found to promote the metastatic potential of a mouse HCC cell line in vivo through the release of tumour clusters into the bloodstream in an epithelial-to-mesenchymal transition-independent manner.34
In the same line, in a recent American study,47 the authors identified the MTM subtype as the most common histo-morphologic features in primary tumors associated with metastasis amongst 39 HCC cases studied.
Calderaro et al17 observed an association between MTM-HCC and poor prognosis (early relapse and poor survival). Major aggressiveness of this HCC subtype (major incidence of vascular invasion and satellite nodules) was also reported.
These features were confirmed in a report by Ziol et al11 of a significantly higher recurrence rate and worse overall survival in patients with this HCC subtype compared to other subtypes. Similar results were found by Jeon et al15 for patients who underwent liver resection/transplantation. Li Li Liu et al27 showed that at the molecular level, CMTM6/PD-L1 expression was an independent prognostic factor for patient survival in the MTM-HCC subtype population, with increased risk of HCC progression and death.
The above-mentioned reported data are in line with those of Zhu et al,48 showing that elevated expression of CMTM6 in a cohort of HCC cases was frequently accompanied by worsening of malignant phenomena, including high AFP levels, large tumour sizes, advanced TNM stages, and vascular invasion.
This association between macrotrabecular-massive growth pattern and higher AFP expression was recently confirmed by the recent studies of Cannella et al23 Ridder et al49 (Supplementary Material Figure S1).
New Perspectives
Angiopoietin 2 Inhibitors, An Ongoing Subject of Discussion
In MTM-HCC, angiopoietin 2 (ANGPT2) mRNA levels are overexpressed, along with a significant almost increasing trend in the vascular endothelial growth factor (VEGF) level.17
To date, ANGPT2, in cooperation with VEGFA, is known to promote neoangiogenesis and endothelial sprouting in the tumoral environment.50
It has also been shown that secretion of ANGPT2 by various primary solid tumours can induce metastasis via the extravasation of circulating tumour cells51 by loosening endothelial cell junctions and increasing vascular permeability of distant organs.34,52
In HCC, ANGPT2 produced by neoplastic cells may disseminate directly in adjacent liver parenchyma and contribute to the increased frequency of satellite nodules observed in MTM-HCC.
It is reasonable to expect that silencing the expression of ANGPT2 growth factor by neoplastic cells could disrupt the formation of this particular pattern and reduce both intrahepatic neoplasia and extrahepatic metastases.34
Hashizume et al53 have suggested that Ang2 inhibitors and VEGF inhibitors may have complementary antiangiogenic actions and effects on reducing tumour growth.
Ang2 inhibition prevents the growth of new vessels by endothelial sprout formation, whereas VEGF inhibition causes tumour vessel regression, and the formation of empty basement membrane sleeves reduces tumour growth. The combined blockade of Ang2 and VEGF has additive effects on sprouting and vessel regression and decelerates tumour growth.53
Schmittnaegel et al54 also demonstrated that blocking angiogenesis using these types of antibodies enhanced antitumour immunity (by increasing extravasation and perivascular accumulation of antitumour CD8+ cells and interferon-γ-producing cytotoxic T lymphocytes).54
The synergistic use of ANGPT2 inhibitors and Bevacizumab (anti-VEGF) combined with immunotherapy could open novel therapeutic avenues for this highly aggressive HCC subtype and may represent a new tool for HCC treatment, bringing HCC molecular classification into clinical practice.
Artificial Intelligence: Which Role in the Future for MTT-HCC
The artificial intelligence (AI), computer-based algorithms for data analysis and by the construction of predictive models based on the usage of various imaging techniques, especially in combination with HCC molecular biomarkers, recently used for improving image recognition and representation in HCC diagnosis and prognosis.55 For example, in their most recent article that represents a proof-of-principle study, Zeng et al56 propose a novel approach using AI to predict activation of inflammatory gene signatures associated with increased sensitivity to immunotherapy, for improving personalized allocation of therapy. In this trend, the combination of artificial intelligence and automated computerized image analysis is likely to provide a new tool for MTM-HCC diagnosis and for characterisation of biomarkers to help in therapy work-up in the near future.
Conclusion
This article is a narrative review of the most recent data in the field of MTM-HCC, and provides insights on future directions and challenges.
In conclusion, MTM-HCC is an aggressive subtype of HCC characterized by vascular invasion, a higher level of alpha-fetoprotein, early relapse and poor survival.
Although the available data are coherent, additional study series are needed to investigate the clinical impact of MTM-HCC on treatment response. The development of particular therapeutic strategies and/or trials focusing on the particularly aggressive subtype of MTM-HCC should be considered. One limitation is the subjective nature of pathology and nonoptimal interobserver agreement among pathologists; however, artificial intelligence and automated computerized image analysis are likely to provide a unique opportunity to achieve consensus in MTM-HCC diagnosis in the near future.
Abbreviations
HCC, hepatocellular carcinoma; MTM-HCC, macrotrabecular hepatocarcinoma; LT, liver transplantation; PD-1, checkpoint-blockade immunotherapy targeting programmed cell death protein 1; CMTM6, chemokine-like factor-like (CKLF) MARVEL transmembrane domain-containing 6; ANGPT2, angiopoietin; VEGF, vascular endothelial growth factor; AFP, alpha-fetoprotein.
Funding
There is no funding to report.
Disclosure
Hélène Regnault reports personal fees from Boston Scientific, outside the submitted work. Vincent Leroy reports personal fees from AbbVie, Intercept, and Mayoly, and grants and personal fees from Gilead, outside the submitted work. Julien Calderaro reports shares in Crosscope, and personal fees from Keen Eye, outside the submitted work. The authors report no other potential conflicts of interest in relation to this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264–1273.e1. doi:10.1053/j.gastro.2011.12.061
3. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi:10.1002/hep.28431
4. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
5. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–750. doi:10.1002/hep.29913
6. Tang A, Bashir MR, Corwin MT, et al.; LI-RADS Evidence Working Group. Evidence supporting LI-RADS major features for CT- and MR imaging-based diagnosis of hepatocellular carcinoma: a systematic review. Radiology. 2018;286(1):29–48. doi:10.1148/radiol.2017170554
7. Chernyak V, Tang A, Flusberg M, et al. LI-RADS® ancillary features on CT and MRI. Abdom Radiol. 2018;43(1):82–100. doi:10.1007/s00261-017-1220-6
8. Elsayes KM, Kielar AZ, Elmohr MM, et al. White paper of the Society of Abdominal Radiology hepatocellular carcinoma diagnosis disease-focused panel on LI-RADS v2018 for CT and MRI. Abdom Radiol. 2018;43(10):2625–2642. doi:10.1007/s00261-018-1744-4
9. Nagtegaal ID, Odze RD, Klimstra D, et al.; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76(2):182–188. doi:10.1111/his.13975
10. Zucman-Rossi J, Villanueva A, Nault JC, Llovet JM. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology. 2015;149(5):1226–1239.e4. doi:10.1053/j.gastro.2015.05.061
11. Ziol M, Poté N, Amaddeo G, et al. Macrotrabecular-massive hepatocellular carcinoma: a distinctive histological subtype with clinical relevance. Hepatology. 2018;68(1):103–112. doi:10.1002/hep.29762
12. Kishi K, Shikata T, Hirohashi S, Hasegawa H, Yamazaki S, Makuuchi M. Hepatocellular carcinoma. A clinical and pathologic analysis of 57 hepatectomy cases. Cancer. 1983;51(3):542–548. doi:10.1002/1097-0142(19830201)51:3<542::AID-CNCR2820510330>3.0.CO;2-2
13. Kondo F, Wada K, Kondo Y. Morphometric analysis of hepatocellular carcinoma. Virchows Arch A Pathol Anat Histopathol. 1988;413(5):425–430. doi:10.1007/BF00716991
14. Rhee H, Cho ES, Nahm JH, et al. Gadoxetic acid-enhanced MRI of macrotrabecular-massive hepatocellular carcinoma and its prognostic implications. J Hepatol. 2021;74(1):109–121. doi:10.1016/j.jhep.2020.08.013
15. Jeon Y, Benedict M, Taddei T, Jain D, Zhang X. Macrotrabecular hepatocellular carcinoma: an aggressive subtype of hepatocellular carcinoma. Am J Surg Pathol. 2019;43(7):943–948. doi:10.1097/PAS.0000000000001289
16. Mulé S, Galletto Pregliasco A, Tenenhaus A, et al. Multiphase liver MRI for identifying the macrotrabecular-massive subtype of hepatocellular carcinoma. Radiology. 2020;295(3):562–571. doi:10.1148/radiol.2020192230
17. Calderaro J, Couchy G, Imbeaud S, et al. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification. J Hepatol. 2017;67(4):727–738. doi:10.1016/j.jhep.2017.05.014
18. Torbenson MS. Morphologic subtypes of hepatocellular carcinoma. Gastroenterol Clin North Am. 2017;46(2):365–391. doi:10.1016/j.gtc.2017.01.009
19. Tan PS, Nakagawa S, Goossens N, et al. Clinicopathological indices to predict hepatocellular carcinoma molecular classification. Liver Int. 2016;36(1):108–118. doi:10.1111/liv.12889
20. Renne SL, Woo HY, Allegra S, et al. Vessels Encapsulating Tumor Clusters (VETC) is a powerful predictor of aggressive hepatocellular carcinoma. Hepatology. 2020;71(1):183–195. doi:10.1002/hep.30814
21. Rastogi A, Maiwall R, Ramakrishna G, et al. Hepatocellular carcinoma: clinicopathologic associations amidst marked phenotypic heterogeneity. Pathol Res Pract. 2021;217:153290. doi:10.1016/j.prp.2020.153290
22. Feng Z, Li H, Zhao H, et al. Preoperative CT for characterization of aggressive macrotrabecular-massive subtype and vessels that encapsulate tumor clusters pattern in hepatocellular carcinoma. Radiology. 2021;300(1):219–229. doi:10.1148/radiol.2021203614
23. Cannella R, Dioguardi Burgio M, Beaufrère A, et al. Imaging features of histological subtypes of hepatocellular carcinoma: implication for LI-RADS. JHEP Rep. 2021;3(6):100380. doi:10.1016/j.jhepr.2021.100380
24. Jain A, Mazer B, Deng Y, et al. Hepatocellular carcinoma: does the background liver with or without cirrhosis matter? Am J Clin Pathol. 2021;2021:aqab125.
25. Lauwers GY, Terris B, Balis UJ, et al.; International Cooperative Study Group on Hepatocellular Carcinoma. Prognostic histologic indicators of curatively resected hepatocellular carcinomas: a multi-institutional analysis of 425 patients with definition of a histologic prognostic index. Am J Surg Pathol. 2002;26(1):25–34. doi:10.1097/00000478-200201000-00003
26. Schulze K, Nault JC, Villanueva A. Genetic profiling of hepatocellular carcinoma using next-generation sequencing. J Hepatol. 2016;65(5):1031–1042. doi:10.1016/j.jhep.2016.05.035
27. Liu LL, Zhang SW, Chao X, et al. Coexpression of CMTM6 and PD-L1 as a predictor of poor prognosis in macrotrabecular-massive hepatocellular carcinoma. Cancer Immunol Immunother. 2021;70(2):417–429. doi:10.1007/s00262-020-02691-9
28. Burr ML, Sparbier CE, Chan YC, et al. CMTM6 maintains the expression of PD-L1 and regulates anti-tumour immunity. Nature. 2017;549(7670):101–105. doi:10.1038/nature23643
29. Torbenson M, Schirmacher P. Liver cancer biopsy–back to the future?! Hepatology. 2015;61(2):431–433. doi:10.1002/hep.27545
30. Mueller C, Waldburger N, Stampfl U, et al. Non-invasive diagnosis of hepatocellular carcinoma revisited. Gut. 2018;67(5):991–993. doi:10.1136/gutjnl-2017-314981
31. Poté N, Cauchy F, Albuquerque M, et al. Contribution of virtual biopsy to the screening of microvascular invasion in hepatocellular carcinoma: a pilot study. Liver Int. 2018;38(4):687–694. doi:10.1111/liv.13585
32. Calderaro J, Meunier L, Nguyen CT, et al. ESM1 as a marker of macrotrabecular-massive hepatocellular carcinoma. Clin Cancer Res. 2019;25(19):5859–5865. doi:10.1158/1078-0432.CCR-19-0859
33. Tanigawa N, Lu C, Mitsui T, Miura S. Quantitation of sinusoid-like vessels in hepatocellular carcinoma: its clinical and prognostic significance. Hepatology. 1997;26(5):1216–1223. doi:10.1053/jhep.1997.v26.pm0009362365
34. Fang JH, Zhou HC, Zhang C, et al. A novel vascular pattern promotes metastasis of hepatocellular carcinoma in an epithelial-mesenchymal transition-independent manner. Hepatology. 2015;62(2):452–465. doi:10.1002/hep.27760
35. Yoon JH, Kim H. CT characterization of aggressive macrotrabecular-massive hepatocellular carcinoma: a step forward to personalized medicine. Radiology. 2021;300(1):230–232. doi:10.1148/radiol.2021210379
36. Wang B, Gao ZQ, Yan X. Correlative study of angiogenesis and dynamic contrast-enhanced magnetic resonance imaging features of hepatocellular carcinoma. Acta Radiol. 2005;46(4):353–358. doi:10.1080/02841850510021247
37. Zhu Y, Weng S, Li Y, et al. A radiomics nomogram based on contrast-enhanced MRI for preoperative prediction of macrotrabecular-massive hepatocellular carcinoma. Abdom Radiol. 2021;46(7):3139–3148. doi:10.1007/s00261-021-02989-x
38. Llovet JM, Ricci S, Mazzaferro V, et al.; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi:10.1056/NEJMoa0708857
39. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi:10.1016/S0140-6736(18)30207-1
40. Bruix J, Qin S, Merle P, et al.; RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. doi:10.1016/S0140-6736(16)32453-9
41. Zhu AX, Kang YK, Yen CJ, et al.; REACH-2 study investigators. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282–296. doi:10.1016/S1470-2045(18)30937-9
42. Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63. doi:10.1056/NEJMoa1717002
43. Finn RS, Qin S, Ikeda M, et al.; IMbrave150 Investigators. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
44. Liao J, Xiao J, Zhou Y, et al. Effect of transcatheter arterial chemoembolization on cellular immune function and regulatory T cells in patients with hepatocellular carcinoma. Mol Med Rep. 2015;12(4):6065–6071. doi:10.3892/mmr.2015.4171
45. Montasser A, Beaufrère A, Cauchy F, et al. Transarterial chemoembolisation enhances programmed death‐1 and programmed death‐ligand 1 expression in hepatocellular carcinoma. Histopathology. 2021;79(1):36–46. doi:10.1111/his.14317
46. Zhang Y, Chen SW, Liu LL, Yang X, Cai SH, Yun JP. A model combining TNM stage and tumor size shows utility in predicting recurrence among patients with hepatocellular carcinoma after resection. Cancer Manag Res. 2018;10:3707–3715. doi:10.2147/CMAR.S175303
47. Kumar D, Hafez O, Jain D, Zhang X. Can primary hepatocellular carcinoma histomorphology predict extrahepatic metastasis? Hum Pathol. 2021;113:39e46. doi:10.1016/j.humpath.2021.04.008
48. Zhu X, Qi G, Li C, et al. Expression and clinical significance of CMTM6 in hepatocellular carcinoma. DNA Cell Biol. 2019;38(2):193–197. doi:10.1089/dna.2018.4513
49. Ridder DA, Weinmann A, Schindeldecker M, et al. Comprehensive clinicopathologic study of alpha fetoprotein-expression in a large cohort of patients with hepatocellular carcinoma. Int J Cancer. 2022;150(6):1053–1066. doi:10.1002/ijc.33898
50. Gerald D, Chintharlapalli S, Augustin HG, Benjamin LE. Angiopoietin-2: an attractive target for improved antiangiogenic tumor therapy. Cancer Res. 2013;73(6):1649–1657. doi:10.1158/0008-5472.CAN-12-4697
51. Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147(2):275–292. doi:10.1016/j.cell.2011.09.024
52. Huang Y, Song N, Ding Y, et al. Pulmonary vascular destabilization in the premetastatic phase facilitates lung metastasis. Cancer Res. 2009;69(19):7529–7537. doi:10.1158/0008-5472.CAN-08-4382
53. Hashizume H, Falcón BL, Kuroda T, et al. Complementary actions of inhibitors of angiopoietin-2 and VEGF on tumor angiogenesis and growth. Cancer Res. 2010;70(6):2213–2223. doi:10.1158/0008-5472.CAN-09-1977
54. Schmittnaegel M, Rigamonti N, Kadioglu E, et al. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci Transl Med. 2017;9(385):eaak9670. doi:10.1126/scitranslmed.aak9670
55. Moldogazieva NT, Mokhosoev IM, Zavadskiy SP, et al. Proteomic profiling and artificial intelligence for hepatocellular carcinoma translational medicine. Biomedicines. 2021;9:159. doi:10.3390/biomedicines9020159
56. Zeng Q, Klein C, Caruso S, et al. Artificial intelligence predicts immune and inflammatory gene signatures directly from hepatocellular carcinoma histology. J Hepatol. 2022;77:116–127. doi:10.1016/j.jhep.2022.01.018
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.