Back to Journals » Journal of Hepatocellular Carcinoma » Volume 9
Understanding the Immunoenvironment of Primary Liver Cancer: A Histopathology Perspective
Authors Chung A, Nasralla D, Quaglia A
Received 14 July 2022
Accepted for publication 1 September 2022
Published 2 November 2022 Volume 2022:9 Pages 1149—1169
DOI https://doi.org/10.2147/JHC.S382310
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Ahmed Kaseb
Annabelle Chung,1 David Nasralla,2 Alberto Quaglia1
1Department of Cellular Pathology, Royal Free Hospital, London, UK; 2Department of Hepato-Pancreato-Biliary Surgery, Royal Free Hospital, London, UK
Correspondence: Annabelle Chung, Department of Cellular Pathology, Royal Free Hospital, Pond Street, London, NW3 2QG, UK, Tel +44 20 7794 0500 ext. 35641, Email [email protected]
Abstract: One of the most common cancers worldwide, primary liver cancer remains a major cause of cancer-related mortality. Hepatocellular carcinoma and cholangiocarcinoma represent the majority of primary liver cancer cases. Despite advances in the development of novel anti-cancer therapies that exploit targets within the immune system, survival rates from liver cancer remain poor. Furthermore, responses to immunotherapies, such as immune checkpoint inhibitors, have revealed limited and variable responses amongst patients with hepatocellular carcinoma, although combination immunotherapies have shown recent breakthroughs in clinical trials. This has shifted the focus towards improving our understanding of the underlying immune and molecular characteristics of liver tumours that may influence their response to immune-modulating treatments. In this review, we outline the complex interactions that occur in the tumour microenvironment of hepatocellular carcinoma and cholangiocarcinoma, respectively, from a histopathological perspective. We explore the potential role of a classification system based on immune-specific characteristics within each cancer type, the importance of understanding inter- and intra-tumoural heterogeneity and consider the future role of histopathology and novel technologies within this field.
Keywords: hepatocellular carcinoma, cholangiocarcinoma, immunoenvironment, tertiary lymphoid structures, multiplex immunohistochemistry
Introduction
Primary malignant liver tumours include epithelial, mesenchymal, germ cell, haematolymphoid neoplasms and malignant epithelial tumours. Malignant epithelial tumours comprise hepatocellular carcinoma, cholangiocarcinoma (intrahepatic, perihilar and distal), mixed hepatocellular-cholangiocarcinoma and the paediatric neoplasm hepatoblastoma.1 Primary liver tumours represent the sixth most common malignant neoplasm and third biggest cause of cancer-related deaths worldwide. Despite these statistics, current treatment options including locoregional therapy, surgery and chemotherapy are limited and the mortality to incidence ratio remains high worldwide.2
In recent years, efforts have been made to investigate the efficacy of immunotherapies in the treatment of liver cancer by exploiting specific targets within the immunoenvironment, which have changed the landscape of first-line systemic immune-modulating therapeutic options available.3 In brief, progression of these malignant tumours requires abrogation of host effector lymphocytes and upregulation of immunosuppressive cells and tumour-associated macrophages within the tumour immunoenvironment. In addition, interaction of these immune cells with the surrounding stroma and drivers of vascular growth are key to tumourigenesis.4 Inter-tumoural and intra-tumoural heterogeneity are also significant characteristics of the liver tumour microenvironment and are recognised as a hallmark of tumour progression.5 Therefore, understanding the complex interplay between the immune system and the tumour microenvironment in the pathogenesis of cancer is imperative to find new therapeutic targets.
In this review, we focus on hepatocellular carcinoma and cholangiocarcinoma as the two most common primary epithelial liver tumours and aim to describe their respective histopathology and immunoenvironment. We also explore the therapeutic and prognostic value of understanding the immunoenvironment and the role novel techniques in histopathology may play in future research.
Hepatocellular Carcinoma
Hepatocellular carcinoma (HCC) represents around 75% to 85% of all primary liver tumours worldwide.2 A variety of risk factors can lead to the development of HCC including chronic hepatitis B (HBV) and C (HCV) viral infection, alcohol-related chronic liver disease and non-viral factors such as non-alcoholic fatty liver disease (NAFLD),4 as well as less common causes such as aflatoxin exposure and haemochromatosis. Of note, cirrhosis of the liver is the single biggest contributing factor for the development of HCC and is usually the key step in its pathogenesis.6 Furthermore, the incidence of HCC varies due to geographical and risk factor differences. Indeed, it is now increasing in Western populations including the USA and some European countries, whilst declining in high-risk regions in East Asia and Africa.7 This is likely secondary to increasing uptake of hepatitis B vaccinations and treatment for hepatitis C infection, alongside an increasing incidence of NAFLD in Western countries.6 The fifth edition of the World Health Organisation (WHO) classifies HCCs into eight distinct histological subtypes.1 This subclassification can provide insight into the distinct clinicopathological heterogeneity of the HCC immunoenvironment, as detailed later in this review.
The landscape of systemic therapeutic options for patients diagnosed with HCC is rapidly progressing. Until recently, multi-kinase inhibitors, sorafenib8 and lenvatinib,9 were first-line treatment for patients diagnosed with unresectable HCC. However, the role of immune-checkpoint inhibitors (ICIs) in the treatment of HCC has subsequently become very promising.10 Single-agent Phase III trials investigating the efficacy of programmed cell death protein 1 (PD-1) inhibitors, including nivolumab11 and pembrolizumab,12 did not produce statistically significant improvements on overall survival in pre-treated patients. Combination immunotherapies, on the other hand, have now been approved as first-line options. Atezolizumab (anti-PD-L1) plus bevacizumab (anti-vascular endothelial growth factor (VEGF)) and durvalumab (anti-PD-L1) plus tremelimumab (anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4)) were, respectively, approved as first-line therapeutic options for patients with unresectable HCC after trials showed statistically significant improvements in overall survival.13,14 Conversely, the efficacy of these immunotherapies in the adjuvant setting for HCC remains unclear.15 In a more conservative setting, other avenues of immune-modulating treatment are being explored to improve options for patients with moderate liver dysfunction (Child-Pugh B) such as metronomic chemotherapy.16 Here, chronic administration of low-dose chemotherapy aims to strike a fine balance between generating sufficient anti-cancer activity with minimising rates of toxicity in patients who would otherwise be recommended best supportive care.
Importantly, these trials have shown that only a minority of patients respond to these drugs, therefore continuing the drive to better understand the HCC immunoenvironment and its interaction with ICIs, whilst also identifying reliable biomarkers.
Immunoenvironment of HCC
The liver plays a major role in immune surveillance and receives a rich blood supply from both the hepatic artery and portal vein, allowing pathogen-derived antigens and molecules to be detected and captured by liver-resident cells.17 These cells reside in the vasculature of the liver and include Kupffer cells, specialised liver macrophages, and liver-associated lymphocytes including lymphocytes of the adaptive immune system (B and T lymphocytes) and lymphocytes of the innate immune system (natural killer and natural killer T lymphocytes). The liver develops immune tolerance to harmless molecules and antigens, such as the microflora from the gut, as a crucial part of maintaining homeostasis and preventing excessive immune activation.18 The liver immunoenvironment, in the context of tumourigenesis, is suppressed and controlled by regulatory T (Treg) cells plus tumour-associated macrophages (TAMs).19 The complex interaction of the immune cells in HCC has been widely reviewed,19–21 and key concepts are summarised in this review.
Innate Immune Cells
Natural Killer Cells
Firstly, multiple studies have shown that CD56+ natural killer (NK) cells, alongside CD8+ T cells, are one of the dominant tumour-infiltrating lymphocytes (TILs) in HCC.22,23 Furthermore, tumours with a higher density of NK cells were found to positively correlate with the presence of apoptotic tumour cells and negatively correlate with proliferating tumour cells.22 This group has also found that certain chemokine genes (CXCL10, CCL5 and CCL2) represent part of an immune signature that is a predictor of improved survival in patients diagnosed with HCC, particularly in its earlier stages.24 Expression of these chemokines within tumour sections was associated with infiltration of TILs including NK cells, T helper 1 (Th1) cells and CD8+ cells and therefore are involved in providing an immune environment collectively against HCC.
Other studies have shown NK cell populations to have reduced cytotoxicity and IFN-γ production in HCC, suggesting that functional impairment of NK cells plays a significant role in the loss of anti-tumoural response in HCC.25 Furthermore, NK cell dysfunction is an important mechanism by which tumour cells have been recognised to evade the host immune response and subsequently lead to cancer progression.23,26 Several studies have shown that NK cells in intra-tumoural tissue in advanced-stage HCC have impaired function as well as reduced population size.25,27,28 Expression by HCC tumour cells of natural killer group 2, member D (NKG2D) ligand is abundant, allowing recognition by host NK cells. However, it has been shown that as tumours progress to higher grades, loss of NKG2D expression is observed and may be linked to higher risk of early recurrence.29
Macrophages
Kupffer cells act as antigen-presenting cells and are critical in capturing pathogens and activating a cytotoxic T-cell response.30 Conversely, Kupffer cells also function to develop tolerance by inducing CD4+ T cell arrest and expanding IL-10-producing Treg cell populations.31 As such, they play a critical role in the pathogenesis of chronic liver inflammation and fibrosis progression, which is key to the development of HCC.32 Macrophages can be classified into two subtypes: classically activated “M1” macrophages and alternatively activated “M2” macrophages. M2 macrophages are representative of the phenotype taken on by TAMs19 and assist in tumour growth and metastasis.33
It is thought that macrophage polarisation is strongly influenced by tumour stage, whereby there is a dynamic switch from M1 macrophages, which induce a cytotoxic, anti-tumoural response in the setting of chronic inflammation, to a M2 phenotype in an established tumour.20,34 This switch is modulated by an array of signals released by the tumour microenvironment such as IL-10 and apoptotic cells.34 Furthermore, peri-tumoural M2 macrophages have been found to be associated with poor prognosis and increased risk of tumour spread.33,35 The role of M2 macrophages in tumour survival is highlighted by their ability to develop resistance through release of hepatocyte growth factor (HGF) against sorafenib.36 However, recent data derived from single-cell RNA sequencing of CD45+ immune cells in HCC have revealed that the M1/M2 framework may not be as simplistic.37 This group found that some macrophages expressed markers from both M1 and M2 phenotypes, suggesting that the two activation states are not mutually exclusive, and that macrophages may exist between two ends of a refined spectrum.
Adaptive Immune System
TILs, including CD3+, CD4+, CD8+ and FoxP3+ T lymphocytes, are well understood to play a pivotal role in controlling tumour progression, especially CD8+ T cells.38 Efforts into characterising the dysregulation of these TILs have been performed using single-cell RNA sequencing, which have identified 11 distinct T cell clusters, five clusters CD8+ and six CD4+, each with specific tissue distribution patterns.39 This has provided a comprehensive dataset characterising developmental relationships and activation status within the tumour microenvironment. For example, LAYN, which encodes layilin, was found to be expressed by exhausted CD8+ Treg cells, associated with negative regulation of IFN-γ and poor prognosis.
In addition, increased expression of inhibitory receptors on T lymphocytes known as immune checkpoints, such as PD-1 and CTLA-4, by TILs has been widely studied and is understood to contribute towards an immunosuppressed tumour microenvironment, making them popular therapeutic targets.40 Indeed, the statistically significant efficacy of dual immunotherapy as seen in the HIMALAYA trial is based on the synergistic effects of anti-CTLA-4 and anti-PD-1 agents, respectively. Whilst CTLA-4 inhibits the priming and activation of lymphocytes in the anti-tumour immune response,41 PD-L1 is involved in modulating the tumour immunoenvironment further downstream.42
The role of B cells in HCC pathogenesis is not as clear. On the one hand, studies have shown tumour-infiltrating T and B cells have functional interaction with each other and are associated with a better prognosis compared to T cell infiltration alone.43 In contrast, it has been suggested that a certain subset of B cells, PD-1+ B regulatory cells, are associated with more aggressive tumour growth through inhibition of specific pathways.44,45 These conflicting findings may be due to five distinct subsets of B cells that have been identified, each carrying either pro- or anti-tumoural function.46
Tertiary Lymphoid Structures
Tertiary lymphoid structures (TLSs) are aggregates of immune cells that develop in peripheral tissue, including the liver, in response to chronic inflammation in the context of autoimmune disease, infection and malignancy.47 They are characterised by an inner zone of B cells surrounded by dendritic cells and a T cell compartment largely comprised of T-follicular helper cells alongside CD4+ T helper cells, CD8+ cytotoxic T cells and T regulatory cells47 (Figure 1). They provide privileged sites where tumour antigens are presented to T cells to activate the effector function of T cells and initiate the generation of antibodies by B cells.48 In recent years, both intra- and peri-tumoural TLSs have become of great interest due to their association with improved prognoses in various cancers including breast cancer, colorectal cancer, and melanoma.49–52 However, heterogeneity within composition and location around the tumour site may have an influence on their anti-tumour effects and disease prognosis.53,54
Tertiary lymphoid structures (TLSs) are aggregates of immune cells that develop in peripheral tissue, including the liver, in response to chronic inflammation in the context of autoimmune disease, infection and malignancy.47 They are characterised by an inner zone of B cells surrounded by dendritic cells and a T cell compartment largely comprised of T-follicular helper cells alongside CD4+ T helper cells, CD8+ cytotoxic T cells and T regulatory cells47 (Figure 1). They provide privileged sites where tumour antigens are presented to T cells to activate the effector function of T cells and initiate the generation of antibodies by B cells.48 In recent years, both intra- and peri-tumoural TLSs have become of great interest due to their association with improved prognoses in various cancers including breast cancer, colorectal cancer, and melanoma.49–52 However, heterogeneity within composition and location around the tumour site may have an influence on their anti-tumour effects and disease prognosis.53,54
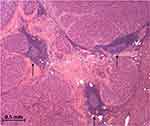 |
Figure 1 Tertiary lymphoid structures (arrows) inside a surgically resected moderately differentiated hepatocellular carcinoma (H&E 40x). |
Whilst intra-tumoural TLSs contribute towards lower risk of early recurrence in early-stage HCC,55,56 it is thought that a high density of peri-tumoural TLSs is linked to an active immunoenvironment and thus improved overall survival.57 Conversely, with regard to TLSs in non-tumoural surrounding liver tissue, Finkin et al have shown in mouse models that these TLSs may serve as niches for malignant hepatocyte progenitors, and this is correlated with an elevated risk of late HCC recurrence.58 Therefore, further work needs to be performed to understand the role of TLS in HCC and background liver and their potential use as a biomarker for prognosis and treatment.
Composition of the HCC Immune Microenvironment
Distinct Subtypes of the Immunoenvironment
Despite efforts to characterise the role of immune cells in the tumour microenvironment, understanding the heterogeneity of the HCC immunoenvironment remains a relatively new concept and may be key to predicting a patient’s response to immunotherapies. Early studies analysing gene expression profiles of HCC provided some initial insights into possible genomic-based classification systems and certain activation pathways that may be of prognostic or therapeutic importance.59,60 In recent years, several studies have also suggested different ways to classify HCC into specific immune subtypes, which have variable degrees of responsiveness to existing immunotherapies and prognostic outcomes. Indeed, underlying pathways and immune landscapes have been linked to distinct immune “hot” versus immune “cold” tumours, which each has respectively variable responses to immunotherapies.61
Kurebayashi et al62 proposed a classification of HCC into three immune subtypes: Immune-high, Immune-mid and Immune-low (in order of worsening prognosis), defined using a combination of mRNA analysis and multiplex immunohistochemistry (Figure 2A). HCC tumours belonging to the immune-high subtype, which constitutes around 25% of poorly differentiated HCCs, revealed increased B-cell, plasma cell and T-cell infiltration as well as higher expression of PD-L1 on tumour and immune cells within the tumour microenvironment. This has led to speculation that these individuals may be more susceptible to immune checkpoint inhibitors. Indeed, another study has shown that increased PD-L1 expression by tumour and immune cells is associated with tumour aggressiveness and therefore may have the potential to be better targets for PD-L1/PD-1 blockade.63 In contrast, the Immune-mid subtype revealed moderately increased T-cell infiltration, and notably lower B and plasma cell infiltration. Lastly, the Immune-low subtypes found low levels of immune cell infiltration of any kind and were associated with poor prognosis.62 mRNA sequencing analysis by Foerster et al also found three distinct immune contextures, each linked to distinctly different prognosis and survival, with infiltration by immune effector cells associated with better prognosis.64
 |
Figure 2 The immune landscape of HCC as described in three studies. (A) Three immune subtypes described as Immune-low, Immune-mid and Immune-high. Immune-low was further subcategorised into two classes, Immune-low-1 and Immune-low-2. Immune-mid was divided into Immune-mid-1 and Immune-mid-2. Immune-mid-1 was described as a more homogeneous subtype, whereas Immune-mid-2 exhibited a relative inhomogeneous subtype including areas of increase mast-cell infiltration or neutrophil infiltration or more granuloma formation.62 (B) The immune class was identified in 25% of studied HCC tumours and is subdivided into an exhausted or active immune state. Similar immune characteristics have been observed between the Immune-mid class and the exhausted subtype of the Immune class.65 (C) Zhang et al found three immune subtypes – Immunodeficient, Immunosuppressed and Immunocompetent.68 NB: this diagram has not been made to reflect the exact proportions of each subtype in relation to each other. |
The findings of the immune-high subtype appear to correlate with the “Immune class” identified by Sia et al65 (Figure 2B). Transcriptomic analysis of 956 HCC tumour samples found this “Immune class” in 25% of samples, which is rich in immune cells such as T-cells, macrophages, tertiary lymphoid structures, with high PD-1 expression. Moreover, this class was further subdivided into an “active” versus “exhausted” response based on differences in T-cell infiltration and IFN-γ expression. The active subclass was associated with lower tumour recurrence. These findings were confirmed by transcriptomic analysis of HCC cases in a Japanese cohort.66 Similarly, the immune-mid subtype appears to correlate with the exhausted immune response described by Sia et al.67
The concept of classifying HCC into three distinct subtypes has also been supported by a more recent study.68 Of note, this study evaluated the function as well as abundance of cells within the immunoenvironment (Figure 2C). On balance, this study’s classification of HCC into “immunocompetent”, “immunodeficient” and “immunosuppressed” appears to follow similar patterns to those of Kurebayashi et al. Descriptions of the “immunosuppressed” subtype appear to correlate with those of the Immune-high class from Kurebayashi et al62 and the “immune class” from Sia et al,65 where high infiltration with immunosuppressive cells including Treg cells, M2-polarised macrophages and high PD-1 expression were found. This group has also suggested that CD45+ and FoxP3+ markers can be used by immunohistochemistry to distinguish between the three subtypes with clear cut-off values. This suggests these markers could be used for prognostic evaluation and guide personalised therapeutic management. In contrast, CD3+ T cells and CD79+ B and plasma cells have been found as promising surrogate markers by immunohistochemistry by another group.62 Importantly, it should be noted that these studies are all based on very small population sizes and so further prospective investigation is warranted to understand if these observations transfer to a larger scale.
Immune Intra-Tumoural Heterogeneity
A contributing factor towards the complexity of the immunoenvironment of HCC is immune intra-tumoural heterogeneity (immune-ITH), which may partly explain why only a 20% response was observed in a recent trial of immune checkpoint inhibitors in advanced HCC.69 Knowledge of intra-tumoural heterogeneity has been established in HCC on both genomic70 and immune5 levels, with the extent of intra-tumoural heterogeneity varying considerably between patients with HCC. Regarding the immune-ITH, it has been demonstrated that it can give an insight into tumour evolution and progression.5 This study showed that the tumour microenvironment exerts a gradient of pressure whereby the immune pressure lowers as the tumour progresses and becomes an increasingly exhausted state. Indeed, they found that higher intra-tumoural heterogeneity correlates with a poorer prognosis in these patients and suggested that immune-ITH represents a hallmark of immune-tumour co-evolution parallel to tumour progression. This is supported by evidence that, even within one immune-subtype, over 50% of immunosubtype-predominant tumours contained more than one immunosubtype, although the predominant immune subtype was of prognostic value.62 Overall, immune-ITH plays a significant role in tumour progression and remains a barrier to effective therapeutic options.
Histological Subtypes of HCC
Up to 35% of HCCs can be further subclassified into eight distinct histological subtypes with varying clinicopathological outcomes: steatohepatitic (the most common subtype), fibrolamellar, scirrhous, clear cell type, macrotrabecular massive, chromophobe, neutrophil-rich and lymphocyte-rich.1 As well as displaying histologically contrasting properties, each subtype displays distinct molecular and immune interactions with the tumour microenvironment,71,72 thus contributing further to the multiple layers of heterogeneity that exist in HCC.
Lymphocyte-Rich
Lymphocyte-rich HCC is recognised by the abundance of lymphocytes out-numbering tumour cells in most fields and is a rare variant.1,73 This subtype is correlated with an immune-high microenvironment, with anti-tumoural function involving infiltration of B- and T-cells as well as better prognosis, although this evidence is limited by the rare number of cases available.1,62,74 Interestingly, PD-1 expression has been associated with lymphocyte-rich HCC. On the one hand, this suggests preference of this subtype to have better response to immunotherapies, however it also suggests a more immunosuppressive environment and thus possibly poorer outcomes.63
Steatohepatitic
Steatohepatitic HCC is the most common subtype, representing up to 20% of HCCs.1 Hallmark characteristics of this subtype include large droplet steatosis, ballooning of malignant hepatocytes, Mallory-Denk bodies and inflammation.75 Around 65% of steatohepatitic HCCs have been estimated to be associated with risk factors for NAFLD as well as NAFLD itself, and this subtype first suggested the link between NAFLD and hepatic carcinogenesis.76,77 Furthermore, this subtype has been associated with more frequent IL-6/JAK/STAT pathway activation, important in cell proliferation and differentiation, as well as a less aggressive phenotype due to lack of microvascular invasion and fewer satellite nodules.78
Macrotrabecular-Massive
This histological subtype was first described in 2018 and characterised by the presence of macrotrabeculae of more than six cells thick in more than half of the tumour.79 Individuals with this subtype have been associated with more aggressive parameters relative to other subtypes, such as size of tumour, alpha-fetoprotein (AFP) levels and vascular invasion.78,79 Furthermore, individuals with macrotrabecular-massive HCC have been found to have poor prognosis; in fact, the subtype itself is an independent predictive value for early and overall tumour recurrence.78,79 Early work into characterising biomarkers for this particularly aggressive HCC subtype has suggested a link with increased expression of pro-angiogenic factors, including VEGF.78,80 This could possibly reveal a therapeutic target for individuals with this condition.
Neutrophil-Rich
Neutrophil-rich subtype is an extremely rare form of HCC (less than 1% of all HCC tumours) and is characterised by diffuse neutrophilic infiltration within the tumour.1 These cases are often associated with leucocytosis and a raised C-reactive protein, and granulocyte colony-stimulating factor (G-CSF) is a key molecular finding. G-CSF is a glycoprotein, which stimulates cell differentiation and enhances neutrophil function. Based on case report findings, G-CSF-producing HCC is associated with a poorly differentiated appearance, rapid progression and poor prognosis.81 Furthermore, this subtype confers a poor prognosis despite early and aggressive surgical management of this subtype, suggesting other treatments such as immunotherapies may be of interest to investigate.82
Fibrolamellar
Fibrolamellar HCC, commonly occurring in adolescents and young adults without primary liver disease, is composed of large polygonal cells with eosinophilic granular cytoplasm, prominent nucleoli and abundant intratumoural lamellar fibrosis.83 The immune profile of this subtype is distinct from other HCC subtypes as it expresses CD68, a histiocytic marker as well as CK7, a biliary marker, which can be stained to support the diagnosis by immunohistochemistry.84,85 On a molecular level, fusion of genes DNAJB1 and PRKACA on chromosome 19 have been found to have 100% specificity to fibrolamellar HCC and has since become a diagnostic molecular marker, therefore raising interest as a specific therapeutic target.86 Interestingly, this oncogenic fusion protein has more recently been identified in certain pancreato-biliary neoplasms and thus conflicts with previous findings.87 More recently, a small study of 32 cases of fibrolamellar HCC has highlighted a CD8+ T cell inflamed tumour microenvironment, especially around the tumour interface, with around two-thirds of cases exhibiting membranous PD-L1 expression on tumour cells as well as PD-L1+ TILs and TAMs.88 These findings have not only highlighted the possible role of PD-L1+ TILs as a prognostic biomarker for response to immunotherapy in fibrolamellar HCC but also demonstrate that expression of multiple immune-checkpoint molecules including PD-1, PD-L1, B7-H3 and IDO plays an important role in adaptive immune resistance.
Scirrhous
Scirrhous HCC is described to have abundant and diffuse fibrosis involving at least 50% of the tumour.83 For this reason, it is often misdiagnosed as fibrolamellar HCC – however, distinct differences exist on both clinical and molecular levels between the two subtypes. Current studies have shown that scirrhous HCC tends to arise in individuals with a background of chronic hepatitis or cirrhosis.89 It is linked to mutations in tuberous sclerosis complex gene (TSC1/TSC2) leading to activation of the PI3K/AKT/mTOR pathway, which is important in cell proliferation and survival making it a popular therapeutic target.78 Of note, fibrous tissue in this subtype is more enriched with CAFs and tumour cells expressing pSTAT3, which is associated with higher macrophage infiltration, larger tumour size and poorer prognosis compared to fibrolamellar HCC.90 Furthermore, prominent intratumoural CD8+ T lymphocyte infiltration has been observed in scirrhous HCC and has been suggested as an important factor in improving prognosis due to their anti-tumoural effects.91
Chromophobe
A relatively new subtype of HCC, chromophobe HCC, is characterised by tumour cells showing striking nuclear pleomorphism scattered throughout a background of cells containing bland nuclear changes and the appearance of eosinophilic cytoplasm.92 Although there is lack of substantial literature to our knowledge on the tumour immunoenvironment of this subtype, it is strongly associated with activation of alternative lengthening of telomeres (ALT), a telomerase-independent mechanism of telomere maintenance.93 In some cases of other ALT-enriched cancers, such as neuroblastoma, ALT has been shown to be an indicator of poor prognosis.94 This has highlighted interest in exploring mechanisms behind ALT activation as a target for therapeutic purposes.95
Clear Cell
Clear-cell type HCC is recognised by the abundance of clear cytoplasm, filled with glycogen and fat droplets, that does not stain with haematoxylin and eosin. It is usually defined by a minimum of 50% of clear cells present to meet diagnostic requirements.83 In general, this subtype tends to be well to moderately differentiated and smaller in size with lower rates of vascular invasion than conventional HCC;96 however, there is a lack of robust literature, which further characterises the immunoenvironemnt of clear cell HCC. On a molecular level, mutations in isocitrate dehydrogenase 1 (IDH1), a regulator of lipid metabolism, have been suggested to be linked to the morphological features of the tumour and carry a comparatively worse prognosis compared to clear-cell HCC with wild-type IDH1.97
How HCC Aetiology Can Contribute Towards Immune Heterogeneity
It is well established that HBV and HCV are key players in the pathogenesis of hepatocellular carcinoma.98,99 Chronic infection with HBV or HCV is closely linked with the degree of inflammation and, in most cases, leads to progressive liver fibrosis and cirrhosis.100 Importantly, the development of chronic infection is associated with acquiring an immunosuppressive environment and T cell exhaustion through various mechanisms. For example, intratumoural regulatory T cells expressed more PD-1 in HBV-related HCC compared to non-HBV-related HCC, thus creating a more immunosuppressive environment, whilst CD8+PD-1+ T cells were found to be more functionally exhausted than in non-HBV-related HCC.101 This suggests a mechanism behind how this virus can induce an immunosuppressive microenvironment.101
Similarly, the immune landscape of non-alcoholic steatohepatitis (NASH)-associated HCC differs vastly. Mouse and human models have demonstrated that different immune cells can control NASH development and thus limit HCC development. For example, depletion of CD4+ T cells can lead to progression of NASH and increased tumour growth, suggesting critical importance of this cell type in HCC progression.102 CD8+ T cells have been found to play a key role in activating the NF-kB pathway, an important pathway in the transition from NASH to HCC.103 Infiltration of neutrophils in NASH, through increased activity via release of neutrophil extracellular traps (NETs), has been found to contribute towards release of inflammatory cytokines, action of macrophages and progression of a chronic inflammatory microenvironment that can transform into HCC.104
Of note, the importance of these underlying aetiological immune mechanisms in generating a specific immune environment is unclear, as no significant aetiological differences were found between HCCs of different immune subtypes in studies exploring the distinct subtypes of the HCC immunoenvironment.62,64,65
Spontaneous Regression of HCC
Spontaneous regression of HCC is an extremely rare phenomenon, with its true incidence in HCC difficult to discern due to its rarity. One systematic review of 1640 patients estimated its incidence to be 0.4%.105 This phenomenon is defined as partial or complete disappearance of a malignant tumour in the absence of any treatment.106 Underlying mechanisms are unclear, however significant hypoxic events, systemic inflammatory response, or both, are suggested to be the main responsible culprits in documented case reports, with up to one-third of cases caused by each aetiology respectively.107
Severe hypoxic injury to the tumour can be secondary to either (i) thrombosis of the hepatic artery or portal veins or (ii) severe systemic hypotension compromising perfusion of the liver.107,108 This mechanism is in keeping with the strategies used by current therapeutic options which induce hypoxic conditions, such as transarterial embolization (TAE). Mechanisms causing a systemic inflammatory response are less clear. Specific aetiologies including abstinence from alcohol, tobacco and exogenous androgens have been suggested in the literature to stimulate an inflammatory response in some cases.108,109 On the other hand, the role of the immunoenvironment has been implicated with certain cytokines and inflammatory markers, such as IL-18,110 TNF-α111 and IL-2, IL-6 and IFN-γ112 identified in some case reports of spontaneous regression of HCC lesions. Furthermore, one case study demonstrated a higher proportion of activated NK cells and a highly pro-inflammatory and cytotoxic T cell response in ex vivo stimulation measured by expression of TNF-α, IFN-γ and granzyme B.113 Although spontaneous regression of HCC is profoundly rare and the mechanisms underpinning the condition lack robust evidence, these cases highlight that the host immune response has the potential to effectively target HCC tumour cells and illustrates the value of studying individual immune profiles in these rare instances. It has also been suggested that it is likely a combination of different mechanisms that leads to significant regression of the tumour. In fact, several studies have found secondary regression of pulmonary metastases following treatment of the primary liver tumour with TAE or induction of hypoxia by thrombi. This implies that initial necrosis from medical intervention to the primary tumour triggers some form of immunological response causing regression of metastatic lesions.114,115 Further work into the exact mechanisms of the interplay between the immune system and its anti-tumoural response in this condition may reveal potential therapeutic targets that could be exploited.
Cholangiocarcinoma
Cholangiocarcinoma (CCA) is a neoplasm showing features of biliary differentiation and is the second most common primary liver tumour, following hepatocellular carcinoma.116 CCA can be subdivided into three classes based on anatomical location: intrahepatic, perihilar and distal CCA,1 with intrahepatic CCA (iCCA) representing 10% to 15% of primary liver neoplasms worldwide.2 Intrahepatic CCA can be further subclassified based on histological findings into large bile duct and small bile duct types.116
Incidence varies widely depending on geographical location – its highest incidence in Thailand and areas of East Asia and lowest in Western countries such as the USA, although this appears to be on the rise.117 This large geographic discrepancy is thought to be due to complex interactions between genetic predisposition and certain geographical risk factors. For example, the majority of CCA cases in endemic areas of East Asia are secondary to liver fluke infection.118 In contrast, primary sclerosing cholangitis is the most common underlying condition in Western countries.119 Furthermore, it has been suggested that aetiological differences exist between intrahepatic CCA (iCCA) versus perihilar and distal CCAs due to the two distinct stem cell niches present along the biliary tree and the varying injuries they are exposed to.120 Other established aetiologies include toxins such as Thorotrast, biliary-duct cysts, and hepatolithiasis – the latter two showing a stronger association with development of intrahepatic CCA over periductal or ductal CCA.121
The Immunoenvironment in CCA
The composition of the tumour microenvironment of cholangiocarcinoma is characterised by dense desmoplastic stroma, in which intrahepatic and periductal CCA are rich in cancer-associated fibroblasts (CAFs) that surround malignant ducts and glands.119 Although technically not a constituent of the immune system, CAFs express α-smooth muscle actin (α-SMA) and contribute towards progression and metastasis of CCA tumours through multiple pathways and have been suggested by early studies to be of potential prognostic value.122 For example, as demonstrated in prostate cancer, CAFs recruit TAMs through the release of various interleukins, chemokines and proteinases.123 They also aid in inducing M2 polarisation of these macrophages to contribute towards an immunosuppressive microenvironment.124 Synergistically, TAMs release their own growth factors that in turn stimulate CAFs to promote the growth and spread of the tumour cells. Furthermore, presence of TAMs infiltrated within tumours is associated with poorer prognosis.125 TAMs and CAFs also play an important role in immune escape mechanisms. Their release of the chemokine, CCL2, stimulates TILs to express CD25 and transform into regulatory T cells and shift to an immunosuppressive environment.126 The importance of CAFs has also been demonstrated by single-cell RNA sequencing from which six distinct fibroblast subsets were identified in iCCA tumour samples, the most prevalent type vascular-CAFs (vCAFs). The IL-6/IL-6 receptor axis was found to be enriched between vCAFs and iCCA tumour cells, involved in upregulating epigenetic factors further downstream and promoting tumour progression.127 Notably, further studies revealed that IL-6 receptor neutralising antibody, tocilizumab, significantly abrogated these pro-tumoural effects, thus highlighting potential new therapeutic targets in iCCA.
With regard to the adaptive immune response, infiltration of T and B lymphocytes appears to decrease as the cancer progresses.128 A recent systematic review of TILs in CCA revealed that presence of both cytotoxic CD8+ T lymphocytes and FoxP3–CD4+ T lymphocytes, which were largely found at the tumoural interface, both confer better long-term survival. In contrast, the role of FoxP3+ Treg cells in CCA is more disputed and requires further investigation.129 Furthermore, a recent study has shown that high infiltration of CD8+ T cells, low FoxP3+ cell infiltration and low PD-1 expression was associated with high microvessel density of intrahepatic CCA and better overall survival.130 This suggests an interplay between tumour microvessels and regulation of cytotoxic and regulatory TILs.
Intrahepatic CCA
The WHO classifies iCCA into two distinct and contrasting histological subtypes: large duct type and small duct type.1 Large duct type arises from the larger bile ducts near the hilum and proximal to the left and right hepatic ducts. It forms lesions which are either mass-forming or have a periductal infiltrating pattern. Large duct type often features mucin-secreting glands and involves perineural or lymphovascular invasion, overall resembling perihilar CCA. On the other hand, small duct type arises from the periphery of the liver and form mass lesions. It features small ductal components sparing mucin production and can mimic HCC.
The contrasting characteristics between these subtypes are a result of several proposed factors, including high molecular heterogeneity between CCA subtypes and different cells of origin,131 and this remains a significant barrier to effective therapeutic options. Within intrahepatic CCA, histological differences, as described above, are reflected on a molecular level. Small duct type is characterised by IDH1/IDH2 mutations or translocations in fibroblast growth factor receptor (FGFR2),132,133 whilst large duct type (as well as pCCA and dCCA) displays frequent KRAS and TP53 gene mutations.134 Secondly, distinct groups of cells of origin have been identified responsible for small duct type iCCA compared to large duct type iCCA, pCCA and dCCA. It has been suggested that hepatic progenitor or stem cells (HpSCs), mature cholangiocytes or even hepatocytes may be the original cells of these malignancies, based on experimental models; however, this requires further research to conclude on a definitive answer.135–137 Another concept further contributing to the heterogeneity and complexity within CCA is the presence of epithelial–mesenchymal transition (EMT) markers found in iCCA138 with increasing evidence showing these EMT markers expressed by cancer stem cells (CSCs) in CCA. This phenomenon can aid epithelial cancer cells to acquire more aggressive, metastatic qualities and contributes towards CCA heterogeneity depending on their microenvironment.139
The immune landscape in iCCA is less well defined than in HCC. iCCA has previously been described as either immune “hot” or immune “cold” tumours; however, it is becoming clear that it may not be as simplistic.140 Evidence from Phase II clinical trials shows response rates of 6% to 13% to pembrolizumab, an immune checkpoint inhibitor, in advanced CCA.141 This only reinforces the importance of understanding the immunoenvironment of this malignancy. The WHO has recognised the molecular classification of iCCA into two different classes in its latest edition1 – proliferation (62%) and inflammation (38%). The proliferation class has been associated with oncogenic signalling pathways, increased tumour aggressiveness and disease recurrence, whilst the inflammatory class is characterised by inflammation-related pathways and associated with better prognosis and recurrence rates.142
Meanwhile, Job et al proposed the existence of four discrete immune subtypes within iCCA based on gene expression profiles of the tumour microenvironment, which were correlated with immunohistochemistry143 (Figure 3A). They suggested the following subtypes: immune desert (I1), immunogenic (I2), myeloid (I3) and mesenchymal (I4). The immunogenic subtype, which represents 10% of the studied cohort, revealed an inflammatory phenotype enriched with an abundance of immune cells and overexpression of immune checkpoints, thus suggesting potential responsiveness to targeted immunotherapies. This concept has been reinforced by other studies where subsets of tumours displayed PD-L1 expression that correlated with increased densities of CD3+ tumour infiltrating cells.144 In contrast, the immune desert phenotype (48% of iCCA) is characterised by very weak expression of any functional immune signalling. The myeloid subtype (14%) features prominent density of myeloid and monocyte-derived cells, particularly M2 macrophages, whilst infiltrating peritumoural T cell populations fail to form an efficient anti-tumoural response. The mesenchymal subtype (28%) is represented by a high abundance of haematopoietic stem cells and primary fibroblasts as well as tumorigenic factors involved in extracellular matrix remodelling and angiogenesis. Tumours with high stromal density showed a poor prognosis compared to those with immune subtypes associated with higher immune tumour cell infiltration. This study shows that a clearer understanding of the immunoenvironment immune subtype could help inform individualised therapeutic protocols. For example, it has been suggested that those with immune “cold” tumours, including the immune desert subtype in the above study, could potentially be targeted with priming agents to induce a more inflammatory phenotype that would subsequently become more responsive to immunotherapies.145 Unfortunately, this classification system does not correlate to key molecular subtypes that have previously been described in iCCA138,142,146 and therefore its relevance in influencing therapeutic strategies is limited.
 |
Figure 3 The immune landscape of iCCA as described in three studies. (A) Job et al discovered four classes - Immune desert, Mesenchymal, Myeloid and Immunogenic.143 (B) Ding et al subdivided the immunoenvironment of iCCA according to distribution and density of tertiary lymphoid structures from Class I to Class IV.147 (C) Martin-Serrano et al has presented 5 STIM classes broadly categorised by inflamed and non-inflamed classes, further subdivided according to genotype-immuno-phenotype properties.148 NB: this diagram has not been made to reflect the exact proportions of each subtype in relation to each other. Abbreviations: HSC, hematopoietic stem cell; Tfh, T follicular helper cells. |
Furthermore, another study subdivided iCCAs into four distinct classes based on a scoring system measuring the distribution and densities of intra- and peri-tumoural tertiary lymphoid structures.147 Class I represents cases of iCCA with TLSs predominantly in the peri-tumoural regions and Class IV consists of those mainly in the intra-tumoural region (Figure 3B). As such, Class I was classified as an immune-excluded class and was found to correlate with weaker immune responses and poorer overall survival, whereas the latter indicated strong immune action and better overall survival. The abundance of Treg cells in intra-tumoural TLSs alongside high frequency of peri-tumoural TLSs suggests a potential interplay between presence of peri-tumoural TLSs with an immunosuppressive intra-tumoural environment. Classes II and III are thought to be heterogeneous with regard to TLS distribution and reflected an “immune-altered” and intermediate anti-tumoural response.
Martin-Serrano et al from the group who previously proposed the molecular classification of “inflammation” and “proliferation” in iCCA142 have recently deepened their understanding of the tumour microenvironment by using a classification system that combines characteristics of the stroma, tumour and immune microenvironment (“STIM”).148 As a result, five STIM classes have been introduced (Figure 3C), which split broadly into inflamed (35%) and non-inflamed (65%) profiles. The inflamed subtypes, immune classical and inflammatory stroma, are characterised by high immune infiltrate and contrast in oncogenic signalling pathways, with the latter much richer in desmoplastic stroma. Conversely, the non-inflamed classes, which correlate with the “cold” tumours typically known to exist in iCCA, include desert-like, hepatic-stem like and tumour classical and represent the majority of iCCAs. Tumours belonging to these non-inflamed classes are identified by low immune infiltrate, desert-like class scoring the lowest, whilst hepatic stem-like displays stem-like features and tumour classical shows high expression of cholangiocyte markers. Importantly, this group identified that inflamed classes have an abundance of CD8+ cells and CAFs whilst non-inflamed display immunosuppressive properties. Of note, this study revealed overlap between the inflamed classes of iCCA with the immune exhausted HCC subset,65 sharing properties of T cell exhaustion and stromal deposition. Similarly, the immune classical iCCA subtype shares characteristics with the immune active HCC cohort; however, no overlap was observed between the immune classes of pCCA, dCCA and iCCA or HCC.
Extrahepatic CCA – Perihilar and Distal
Perihilar and distal CCA are cholangiocarcinomas of the right, left or common bile ducts (perihilar) or distal to the insertion of the cystic duct.149 One retrospective study on a cohort of extrahepatic CCA proposed a high-risk signature of tumour-associated neutrophils (TANs) and Tregs with low infiltration of CD8+ T cells was associated with worse overall survival.150 Higher rates of lymph node spread, post-operative distant recurrence as well as resistance to mainstay chemotherapy option, gemcitabine, in post-operative recurrence were also observed. This is consistent with evidence from immunohistochemistry that a subset of extrahepatic HCC contains a higher ratio of PD-1+/CD8+ TILs. This was associated with poorer overall survival in patients who had undergone surgery and adjuvant chemotherapy, with PD-1+ cells correlating with tumour location.151,152 Therefore, these subsets may be candidates for a more intensive systemic treatment and may be used to stratify patients according to their tumour microenvironment to guide further therapy.
Another study which performed transcriptomic analysis of perihilar and distal CCA tumour samples has revealed four discrete subclasses based on biological features and its tumour microenvironment: Metabolic, Proliferation, Mesenchymal (the most frequent) and Immune.153 The Immune class, which represented 11.5% of the cohort, was characterised by upregulation of the adaptive immune genes, infiltration with dysfunctional lymphocytes and high expression of PD-1, thereby potentially presenting a target for immune checkpoint inhibitors. It has also been suggested by the authors that HER2 inhibitors may be suitable treatment for the proliferation class based on the involvement of well-known oncogenic pathways such as AKT/mTOR. Therefore, although the above studies are on a relatively small scale, they highlight the complexities and diversity of the immunoenvironment of intrahepatic and extrahepatic CCA and provide evidence towards the importance of personalised targeted therapies. They also raise the clinical potential of using a classification system of the tumour microenvironment of each patient to guide further management.
Combined HCC-CCA
Combined hepatocellular-cholangiocarcinoma (cHCC-CCA) is a rare but aggressive form of primary liver cancer characterised by unequivocal components of both hepatocellular carcinoma and cholangiocarcinoma.1,154 Estimates suggest that cHCC-CCA represents between 0.4% and 14.2% of primary liver tumours with varying incidence across different regions.155 It has been hypothesised that it derives from bipotent hepatic progenitor cells, capable of differentiating into either hepatocellular cells or bile duct epithelial cells.156 Accurate diagnosis of this cancer is difficult, often due to sampling error in biopsy specimens.157 Where surgical options are deemed appropriate, either major or minor hepatic resection can be limited by the extent of underlying liver disease and often leads to liver transplantation as the only remaining option.158 Indeed, hepatic resection and liver transplantation have been found by systematic review of cHCC-CCA in the US to have the best 5-year overall survival, although still relatively dismal at 27–28% and 40%, respectively.159
Little is known about the molecular and immune landscape of cHCC-CCA and so this may also be a contributing factor to the limited effective treatment options available currently. However, a new study has proposed two immune subtypes within cHCC-CCAs, immune-high and immune-low.160 This immune-high subset, through gene profiling and immunohistochemistry, has identified a subgroup that may be amenable to immunomodulatory therapies.
The Future of Histopathology
New Techniques in Multiplex Immunohistochemistry
The use of multiplex immunohistochemistry (mIHC), which allows detection of multiple markers on a single tissue section, has become an important method of characterising the tumour immunoenvironment. Indeed, a meta-analysis of 8135 patients revealed that multiplex immunohistochemistry/immunofluorescence (mIHC/IF) was associated with better performance in predicting response to PD-L1/PD-1 immunotherapy compared to PD-L1 IHC alone in ten different tumour types, thus showing the value of this technique in clinical and translational research.161 Multiple approaches can be taken to perform mIHC involving various staining techniques and methods for imaging and detection, which have previously been reviewed.162 Each of these techniques has their own respective limitations; however, a general drawback of using mIHC at present is that the images are 2-dimensional and may only represent a specific plane of the immunoenvironment in one tissue section.
As such, there have been efforts to develop new techniques to overcome the limitations of 2D immunohistochemistry. For example, a new method named “Histo-cytometry” combines the accurate phenotypic characterisation and quantitative function of flow-cytometry with the ability of 3-dimensional digital microscopy to visualise and understand the spatial arrangement of these cell populations within tissue sections by multiple fluorescent markers.163 Clearing-enhanced 3D (Ce3D) multiple volumetric imaging allows for large volume tissue samples to undergo direct antibody-based immunolabeling and produces high-quality images of a diverse range of tissues and cell types in any plane of interest.164 Current limitations of this technique include variable antibody penetration in thick tissue specimens, however it remains an exciting field of interest. Furthermore, integrating the use of spatial transcriptomics with mIHC has been suggested to aid in gaining a better understanding of the functional interactions between cells in the tumour microenvironment.165 New advances in automated sectioning techniques combined with AI and advances in 3D reconstruction software could also help in reducing the limiting labour-intensive steps of 3D histology reconstruction and make it more readily available for the study of the tumour immunoenvironment. There is hope that these forward-thinking imaging techniques can be adapted to clinical practice providing a more efficient way for pathologists to review, with more representative sections of tissue.166
Digital Pathology
The concept of digital pathology has been around since the 1980s167 and has remained an important component in efforts to modernise the traditional methods of histopathology. Digital pathology involves the integration of information technology to aid in generating and sharing data and images and it forms the foundation for recent advances in quantitative pathologic assessments such as whole slide imaging (WSI) and artificial intelligence (AI).168 WSI involves scanning and digitising prepared tissue and cytological samples from glass slides and can even provide spatial dimensions of a sample when several layers of the specimen are imaged. Comparison of WSI and conventional light microscopy for primary diagnostic purposes showed high concordance when tested on certain subspecialties of histopathology and thus suggests reassurance that WSI could be used as an alternative to conventional methods on a clinical level.169 As such, WSI presents several potential advantages that could revolutionise modern pathology, such as remote diagnosis and teleconsultation, collaborative opportunities with subspecialist colleagues, improved time efficiency and opportunities to involve AI and clinical research. However, WSI is not yet in widespread use and limitations to integrating this concept into clinical practice need to be addressed, including high-cost expenditure, lack of storage and network resources as well as legal and safety issues.
Artificial Intelligence and Computational Pathology
AI represents an exciting concept in histopathology, with its potential appealing and vast especially for tissue section analysis. This technology is also known as computational pathology (CPATH)170 and there has been a significant drive to apply CPATH algorithms to aid in certain clinical aspects of the histopathologist’s role such as tumour detection and classification, cell detection and cell counting, and tumour grading, which have been extensively reviewed.171–173 Some realistic expectations involving the use of machine learning in the possible near future may include its role in automating repetitive and time-consuming tasks including analysis of lymph nodes174 or providing objective analysis of multiplex IHC on single sections of slides facilitated by digital pathology.162 This may provide support to an increasing workload that pathologists are having to manage and allow time for the pathologist to focus on higher-level tasks that are required to perform more accurate and extensive diagnostic assessments.
Nevertheless, the use of AI on a clinical scale still presents a myriad of challenges.175 Among these limitations, there is a lack of robust randomised controlled trials within both pathology and the wider medical field to support integration of AI technologies into standards of care at present. There is also a concern that data sets used to generate CPATH algorithms are not reflective of those in the patient population in clinical practice, and that variation in slide preparation, staining techniques and scanning technologies between centres is also not mirrored in these trials.176 Despite these challenges, AI continues to represent the exciting potential to revolutionise the specialty in both a clinical and research setting.
Conclusion
The immunoenvironment of primary liver cancer is diverse and complex. Research has proposed several ways to categorise the immune and molecular landscape of hepatocellular carcinoma and cholangiocarcinoma, which may help guide targeted therapies. This suggests the potential value of using an immunological classification system alongside existing molecular and histopathological frameworks in the diagnostic phase of cancer management. Furthermore, in the era of transcriptomics, this research emphasises that histopathology and its transition on to the digital platform will remain a vital component in navigating our understanding in this field of work. It is likely that the integration of digital pathology and artificial intelligence will transform the field of histopathology and provide a supportive role to the way these specialists operate in order to produce more efficient and accurate outcomes.
Abbreviations
HCC, hepatocellular carcinoma; HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD, non-alcoholic fatty liver disease; ICI, immune checkpoint inhibitor; VEGF, vascular endothelial growth factor; PD-1, programmed cell death protein 1; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; Treg, regulatory T cells; TAM, tumour-associated macrophage; NK cells, natural killer cells; TILs, tumour-infiltrating lymphocytes; Th1, T helper cell 1; NKG2D, natural killer group 2 member D; HGF, hepatocyte growth factor; TLS, tertiary lymphoid structure; immune-ITH, immune intratumoural heterogeneity; AFP, alpha-fetoprotein; G-CSF, granulocyte colony-stimulating factor; IDH1, isocitrate dehydrogenase 1; HpSCs, hepatic progenitor or stem cells; EMT, epithelial-mesenchymal-tumour; CSC, cancer stem cell; STIM, stroma, tumour and immune microenvironment; NASH, non-alcoholic steatohepatitis; TAE, transarterial embolization; CCA, cholangiocarcinoma; iCCA, intrahepatic cholangiocarcinoma; CAFs, cancer-associated fibroblasts; α-SMA, α-smooth muscle actin; TANs, tumour associated neutrophils; cHCC-CCA, combined hepatocellular carcinoma and cholangiocarcinoma; mIHC, multiplex immunohistochemistry; WSI, whole slide imaging; AI, artificial intelligence; CPATH, computational pathology.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lokuhetty D, White VA, Watanabe R, Cree IA; World Health Organisation, International Agency for Research on Cancer. The 2019 WHO Classification of Tumours of the Digestive System.
2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
3. Sangro B, Sarobe P, Hervás-Stubbs S, Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18(8):525–543. doi:10.1038/s41575-021-00438-0
4. Jeng K, Chang C, Jeng W, Sheen I, Jeng C. Heterogeneity of hepatocellular carcinoma contributes to cancer progression. Crit Rev Oncol Hematol. 2015;94(3):337–347. doi:10.1016/j.critrevonc.2015.01.009
5. Nguyen PHD, Ma S, Phua CZJ, et al. Intratumoural immune heterogeneity as a hallmark of tumour evolution and progression in hepatocellular carcinoma. Nat Commun. 2021;12(1):1–13.
6. Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: new trends. J Hepatol. 2020;72(2):250–261. doi:10.1016/j.jhep.2019.08.025
7. Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019;156(2):477–491.e1. doi:10.1053/j.gastro.2018.08.065
8. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi:10.1056/NEJMoa0708857
9. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi:10.1016/S0140-6736(18)30207-1
10. Rizzo A, Ricci AD, Gadaleta-Caldarola G, Brandi G. First-line immune checkpoint inhibitor-based combinations in unresectable hepatocellular carcinoma: current management and future challenges. Expert Rev Gastroenterol Hepatol. 2021;15(11):1245–1251. doi:10.1080/17474124.2021.1973431
11. Yau T, Park J, Finn RS, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23(1):77–90. doi:10.1016/S1470-2045(21)00604-5
12. Finn RS, Ryoo B, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. JCO. 2020;38(3):193–202. doi:10.1200/JCO.19.01307
13. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
14. Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022;1(8):EVIDoa2100070. doi:10.1056/EVIDoa2100070
15. Rizzo A, Ricci AD, Brandi G. Systemic adjuvant treatment in hepatocellular carcinoma: tempted to do something rather than nothing. Future Oncol. 2020;16(32):2587–2589. doi:10.2217/fon-2020-0669
16. De Lorenzo S, Tovoli F, Barbera MA, et al. Metronomic capecitabine vs. best supportive care in Child-Pugh B hepatocellular carcinoma: a proof of concept. Sci Rep. 2018;8(1):1–7. doi:10.1038/s41598-018-28337-6
17. Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol. 2013;14(10):996–1006. doi:10.1038/ni.2691
18. Robinson MW, Harmon C, O’Farrelly C. Liver immunology and its role in inflammation and homeostasis. Cell Mol Immunol. 2016;13(3):267–276. doi:10.1038/cmi.2016.3
19. Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12(12):681–700. doi:10.1038/nrgastro.2015.173
20. Capece D, Fischietti M, Verzella D, et al. The inflammatory microenvironment in hepatocellular carcinoma: a pivotal role for tumor-associated macrophages. Biomed Res Int. 2012;2013:e187204.
21. Ringelhan M, Pfister D, O’Connor T, Pikarsky E, Heikenwalder M. The immunology of hepatocellular carcinoma. Nat Immunol. 2018;19(3):222–232. doi:10.1038/s41590-018-0044-z
22. Chew V, Tow C, Teo M, et al. Inflammatory tumour microenvironment is associated with superior survival in hepatocellular carcinoma patients. J Hepatol. 2010;52(3):370–379. doi:10.1016/j.jhep.2009.07.013
23. Sun C, Sun H, Xiao W, Zhang C, Tian Z. Natural killer cell dysfunction in hepatocellular carcinoma and NK cell-based immunotherapy. Acta Pharmacol Sin. 2015;36(10):1191–1199. doi:10.1038/aps.2015.41
24. Chew V, Chen J, Lee D, et al. Chemokine-driven lymphocyte infiltration: an early intratumoural event determining long-term survival in resectable hepatocellular carcinoma. Gut. 2012;61(3):427–438. doi:10.1136/gutjnl-2011-300509
25. Cai L, Zhang Z, Zhou L, et al. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin Immunol. 2008;129(3):428–437. doi:10.1016/j.clim.2008.08.012
26. Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29(1):235–271. doi:10.1146/annurev-immunol-031210-101324
27. Wu Y, Kuang D, Pan W, et al. Monocyte/macrophage-elicited natural killer cell dysfunction in hepatocellular carcinoma is mediated by CD48/2B4 interactions. Hepatology. 2013;57(3):1107–1116. doi:10.1002/hep.26192
28. Pang Y, Zhang H, Peng J, et al. The immunosuppressive tumor microenvironment in hepatocellular carcinoma. Cancer Immunol Immunother. 2009;58(6):877–886. doi:10.1007/s00262-008-0603-5
29. Kamimura H, Yamagiwa S, Tsuchiya A, et al. Reduced NKG2D ligand expression in hepatocellular carcinoma correlates with early recurrence. J Hepatol. 2012;56(2):381–388. doi:10.1016/j.jhep.2011.06.017
30. Hou J, Zhang H, Sun B, Karin M. The immunobiology of hepatocellular carcinoma in humans and mice: basic concepts and therapeutic implications. J Hepatol. 2020;72(1):167–182. doi:10.1016/j.jhep.2019.08.014
31. Heymann F, Peusquens J, Ludwig-Portugall I, et al. Liver inflammation abrogates immunological tolerance induced by Kupffer cells. Hepatology. 2015;62(1):279–291. doi:10.1002/hep.27793
32. Tian Z, Hou X, Liu W, Han Z, Wei L. Macrophages and hepatocellular carcinoma. Cell Biosci. 2019;9. doi:10.1186/s13578-019-0342-7
33. Yeung OWH, Lo C, Ling C, et al. Alternatively activated (M2) macrophages promote tumour growth and invasiveness in hepatocellular carcinoma. J Hepatol. 2015;62(3):607–616. doi:10.1016/j.jhep.2014.10.029
34. Yu L, Ling Y, Wang H. Role of nonresolving inflammation in hepatocellular carcinoma development and progression. NPJ Precis Oncol. 2018;2(1):6. doi:10.1038/s41698-018-0048-z
35. Zhu X, Zhang J, Zhuang P, et al. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. JCO. 2008;26(16):2707–2716. doi:10.1200/JCO.2007.15.6521
36. Dong N, Shi X, Wang S, et al. M2 macrophages mediate sorafenib resistance by secreting HGF in a feed-forward manner in hepatocellular carcinoma. Br J Cancer. 2019;121(1):22–33. doi:10.1038/s41416-019-0482-x
37. Zhang Q, He Y, Luo N, et al. Landscape and dynamics of single immune cells in hepatocellular carcinoma. Cell. 2019;179(4):829–845.e20. doi:10.1016/j.cell.2019.10.003
38. Pagès F, Galon J, Dieu-Nosjean M, Tartour E, Sautès-Fridman C, Fridman W. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29(8):1093–1102. doi:10.1038/onc.2009.416
39. Zheng C, Zheng L, Yoo J, et al. Landscape of infiltrating T cells in liver cancer revealed by single-cell sequencing. Cell. 2017;169(7):1342–1356.e16. doi:10.1016/j.cell.2017.05.035
40. Zhou G, Sprengers D, Boor PPC, et al. Antibodies against immune checkpoint molecules restore functions of tumor-infiltrating T cells in hepatocellular carcinomas. Gastroenterology. 2017;153(4):1107–1119.e10. doi:10.1053/j.gastro.2017.06.017
41. Rowshanravan B, Halliday N, Sansom DM. CTLA-4: a moving target in immunotherapy. Blood. 2018;131(1):58–67. doi:10.1182/blood-2017-06-741033
42. Chen D, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi:10.1016/j.immuni.2013.07.012
43. Garnelo M, Tan A, Her Z, et al. Interaction between tumour-infiltrating B cells and T cells controls the progression of hepatocellular carcinoma. Gut. 2017;66(2):342–351. doi:10.1136/gutjnl-2015-310814
44. Shao Y, Lo CM, Ling CC, et al. Regulatory B cells accelerate hepatocellular carcinoma progression via CD40/CD154 signaling pathway. Cancer Lett. 2014;355(2):264–272. doi:10.1016/j.canlet.2014.09.026
45. Kessel A, Haj T, Peri R, et al. Human CD19+CD25high B regulatory cells suppress proliferation of CD4+ T cells and enhance Foxp3 and CTLA-4 expression in T-regulatory cells. Autoimmun Rev. 2012;11(9):670–677. doi:10.1016/j.autrev.2011.11.018
46. Zhang Z, Ma L, Goswami S, et al. Landscape of infiltrating B cells and their clinical significance in human hepatocellular carcinoma. OncoImmunology. 2019;8(4):e1571388. doi:10.1080/2162402X.2019.1571388
47. Schumacher TN, Thommen DS. Tertiary lymphoid structures in cancer. Science. 2022;375. doi:10.1126/science.abf9419
48. Sautès-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer. 2019;19(6):307–325. doi:10.1038/s41568-019-0144-6
49. Fridman WH, Zitvogel L, Sautès–Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14(12):717–734. doi:10.1038/nrclinonc.2017.101
50. Cabrita R, Lauss M, Sanna A, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020;577(7791):561–565. doi:10.1038/s41586-019-1914-8
51. Di Caro G, Bergomas F, Grizzi F, et al. Occurrence of tertiary lymphoid tissue is associated with T-cell infiltration and predicts better prognosis in early-stage colorectal cancers. Clin Cancer Res. 2014;20(8):2147–2158. doi:10.1158/1078-0432.CCR-13-2590
52. Liu X, Tsang JYS, Hlaing T, et al. Distinct tertiary lymphoid structure associations and their prognostic relevance in HER2 positive and negative breast cancers. Oncologist. 2017;22(11):1316–1324. doi:10.1634/theoncologist.2017-0029
53. Dieu-Nosjean M, Goc J, Giraldo NA, Sautès-Fridman C, Fridman WH. Tertiary lymphoid structures in cancer and beyond. Trends Immunol. 2014;35(11):571–580. doi:10.1016/j.it.2014.09.006
54. Colbeck EJ, Ager A, Gallimore A, Jones GW. Tertiary lymphoid structures in cancer: drivers of antitumor immunity, immunosuppression, or bystander sentinels in disease? Front Immunol. 2017;8. doi:10.3389/fimmu.2017.01830
55. Calderaro J, Petitprez F, Becht E, et al. Intra-tumoral tertiary lymphoid structures are associated with a low risk of early recurrence of hepatocellular carcinoma. J Hepatol. 2019;70(1):58–65. doi:10.1016/j.jhep.2018.09.003
56. Li H, Wang J, Liu H, et al. Existence of intratumoral tertiary lymphoid structures is associated with immune cells infiltration and predicts better prognosis in early-stage hepatocellular carcinoma. Aging. 2020;12(4):3451–3472. doi:10.18632/aging.102821
57. Li H, Liu H, Fu H, et al. Peritumoral tertiary lymphoid structures correlate with protective immunity and improved prognosis in patients with hepatocellular carcinoma. Front Immunol. 2021;12:648812.
58. Finkin S, Yuan D, Stein I, et al. Ectopic lymphoid structures function as microniches for tumor progenitor cells in hepatocellular carcinoma. Nat Immunol. 2015;16(12):1235–1244. doi:10.1038/ni.3290
59. Hoshida Y, Nijman SMB, Kobayashi M, et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 2009;69(18):7385–7392. doi:10.1158/0008-5472.CAN-09-1089
60. Boyault S, Rickman DS, de Reyniès A, et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology. 2007;45(1):42–52. doi:10.1002/hep.21467
61. Kudo M. Limited impact of anti-PD-1/PD-L1 monotherapy for hepatocellular carcinoma. LIC. 2020;9(6):629–639.
62. Kurebayashi Y, Ojima H, Tsujikawa H, et al. Landscape of immune microenvironment in hepatocellular carcinoma and its additional impact on histological and molecular classification. Hepatology. 2018;68(3):1025–1041. doi:10.1002/hep.29904
63. Calderaro J, Rousseau B, Amaddeo G, et al. Programmed death ligand 1 expression in hepatocellular carcinoma: relationship with clinical and pathological features. Hepatology. 2016;64(6):2038–2046. doi:10.1002/hep.28710
64. Foerster F, Hess M, Gerhold-Ay A, et al. The immune contexture of hepatocellular carcinoma predicts clinical outcome. Sci Rep. 2018;8(1):1–11. doi:10.1038/s41598-018-21937-2
65. Sia D, Jiao Y, Martinez-Quetglas I, et al. Identification of an immune-specific class of hepatocellular carcinoma, based on molecular features. Gastroenterology. 2017;153(3):812–826. doi:10.1053/j.gastro.2017.06.007
66. Shimada S, Mogushi K, Akiyama Y, et al. Comprehensive molecular and immunological characterization of hepatocellular carcinoma. EBioMedicine. 2019;40:457–470. doi:10.1016/j.ebiom.2018.12.058
67. Kurebayashi Y, Kubota N, Sakamoto M. Immune microenvironment of hepatocellular carcinoma, intrahepatic cholangiocarcinoma and liver metastasis of colorectal adenocarcinoma: relationship with histopathological and molecular classifications. Hepatol Res. 2021;51(1):5–18. doi:10.1111/hepr.13539
68. Zhang Q, Lou Y, Yang J, et al. Integrated multiomic analysis reveals comprehensive tumour heterogeneity and novel immunophenotypic classification in hepatocellular carcinomas. Gut. 2019;68(11):2019–2031. doi:10.1136/gutjnl-2019-318912
69. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label Phase 2 trial. Lancet Oncol. 2018;19(7):940–952. doi:10.1016/S1470-2045(18)30351-6
70. Xue R, Li R, Guo H, et al. Variable intra-tumor genomic heterogeneity of multiple lesions in patients with hepatocellular carcinoma. Gastroenterology. 2016;150(4):998–1008. doi:10.1053/j.gastro.2015.12.033
71. Vij M, Calderaro J. Pathologic and molecular features of hepatocellular carcinoma: an update. World J Hepatol. 2021;13(4):393–410. doi:10.4254/wjh.v13.i4.393
72. Calderaro J, Ziol M, Paradis V, Zucman-Rossi J. Molecular and histological correlations in liver cancer. J Hepatol. 2019;71(3):616–630. doi:10.1016/j.jhep.2019.06.001
73. Wada Y, Nakashima O, Kutami R, Yamamoto O, Kojiro M. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology. 1998;27(2):407–414. doi:10.1002/hep.510270214
74. Chan AWH, Tong JHM, Pan Y, et al. Lymphoepithelioma-like hepatocellular carcinoma: an uncommon variant of hepatocellular carcinoma with favorable outcome. Am J Surg Pathol. 2015;39(3):304–312. doi:10.1097/PAS.0000000000000376
75. Salomao M, Remotti H, Vaughan R, Siegel AB, Lefkowitch JH, Moreira RK. The steatohepatitic variant of hepatocellular carcinoma and its association with underlying steatohepatitis. Hum Pathol. 2012;43(5):737–746. doi:10.1016/j.humpath.2011.07.005
76. Salomao M, Yu WM, Brown RS, Emond JC, Lefkowitch JH. Steatohepatitic hepatocellular carcinoma (SH-HCC): a distinctive histological variant of HCC in hepatitis C virus-related cirrhosis with associated NAFLD/NASH. Am J Surg Pathol. 2010;34(11):1630–1636. doi:10.1097/PAS.0b013e3181f31caa
77. Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51(5):1820–1832. doi:10.1002/hep.23594
78. Calderaro J, Couchy G, Imbeaud S, et al. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification. J Hepatol. 2017;67(4):727–738. doi:10.1016/j.jhep.2017.05.014
79. Ziol M, Poté N, Amaddeo G, et al. Macrotrabecular-massive hepatocellular carcinoma: a distinctive histological subtype with clinical relevance. Hepatology. 2018;68(1):103–112. doi:10.1002/hep.29762
80. Calderaro J, Meunier L, Nguyen CT, et al. ESM1 as a marker of macrotrabecular-massive hepatocellular carcinoma. Clin Cancer Res. 2019;25(19):5859–5865. doi:10.1158/1078-0432.CCR-19-0859
81. Nagata H, Komatsu S, Takaki W, et al. Granulocyte colony-stimulating factor-producing hepatocellular carcinoma with abrupt changes. World J Clin Oncol. 2016;7(5):380–386. doi:10.5306/wjco.v7.i5.380
82. Araki K, Kishihara F, Takahashi K, et al. Hepatocellular carcinoma producing a granulocyte colony-stimulating factor: report of a resected case with a literature review. Liver Int. 2007;27(5):716–721. doi:10.1111/j.1478-3231.2007.01468.x
83. Torbenson MS. Morphologic subtypes of hepatocellular carcinoma. Gastroenterol Clin North Am. 2017;46(2):365–391. doi:10.1016/j.gtc.2017.01.009
84. Ross HM, Daniel HD, Vivekanandan P, et al. Fibrolamellar carcinomas are positive for CD68. Mod Pathol. 2011;24(3):390–395. doi:10.1038/modpathol.2010.207
85. Ward SC, Huang J, Tickoo SK, Thung SN, Ladanyi M, Klimstra DS. Fibrolamellar carcinoma of the liver exhibits immunohistochemical evidence of both hepatocyte and bile duct differentiation. Mod Pathol. 2010;23(9):1180–1190. doi:10.1038/modpathol.2010.105
86. Honeyman JN, Simon EP, Robine N, et al. Detection of a recurrent DNAJB1-PRKACA chimeric transcript in fibrolamellar hepatocellular carcinoma. Science. 2014;343:1010–1014. doi:10.1126/science.1249484
87. Vyas M, Hechtman JF, Zhang Y, et al. DNAJB1-PRKACA fusions occur in oncocytic pancreatic and biliary neoplasms and are not specific for fibrolamellar hepatocellular carcinoma. Mod Pathol. 2020;33(4):648–656. doi:10.1038/s41379-019-0398-2
88. Kim AK, Gani F, Layman AJ, et al. Multiple immune-suppressive mechanisms in fibrolamellar carcinoma. Cancer Immunol Res. 2019;7(5):805–812. doi:10.1158/2326-6066.CIR-18-0499
89. Murtha-Lemekhova A, Fuchs J, Schulz E, et al. Scirrhous hepatocellular carcinoma: systematic review and pooled data analysis of clinical, radiological, and histopathological features. J Hepatocell Carcinoma. 2021;8:1269–1279. doi:10.2147/JHC.S328198
90. Kim Y, Rhee H, Yoo JE, et al. Tumour epithelial and stromal characteristics of hepatocellular carcinomas with abundant fibrous stroma: fibrolamellar versus scirrhous hepatocellular carcinoma. Histopathology. 2017;71(2):217–226. doi:10.1111/his.13219
91. Kurogi M, Nakashima O, Miyaaki H, Fujimoto M, Kojiro M. Clinicopathological study of scirrhous hepatocellular carcinoma. J Gastroenterol Hepatol. 2006;21(9):1470–1477. doi:10.1111/j.1440-1746.2006.04372.x
92. Wood LD, Heaphy CM, Daniel HD, et al. Chromophobe hepatocellular carcinoma with abrupt anaplasia: a proposal for a new subtype of hepatocellular carcinoma with unique morphological and molecular features. Mod Pathol. 2013;26(12):1586–1593. doi:10.1038/modpathol.2013.68
93. Kang HJ, Oh J, Kim YW, et al. Clinicopathological and molecular characterization of chromophobe hepatocellular carcinoma. Liver Int. 2021;41(10):2499–2510. doi:10.1111/liv.14975
94. Dilley RL, Greenberg RA. ALTernative telomere maintenance and cancer. Trends Cancer. 2015;1(2):145–156. doi:10.1016/j.trecan.2015.07.007
95. Zhang J, Zou L. Alternative lengthening of telomeres: from molecular mechanisms to therapeutic outlooks. Cell Biosci. 2020;10. doi:10.1186/s13578-020-00391-6
96. Li T, Fan J, Qin L, et al. Risk factors, prognosis, and management of early and late intrahepatic recurrence after resection of primary clear cell carcinoma of the liver. Ann Surg Oncol. 2011;18(7):1955–1963. doi:10.1245/s10434-010-1540-z
97. Lee JH, Shin DH, Park WY, et al. IDH1 R132C mutation is detected in clear cell hepatocellular carcinoma by pyrosequencing. World J Surg Oncol. 2017;15. doi:10.1186/s12957-017-1144-1
98. Yang H, Lu S, Liaw Y, et al. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002;347(3):168–174. doi:10.1056/NEJMoa013215
99. Saito I, Miyamura T, Ohbayashi A, et al. Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc Natl Acad Sci U S A. 1990;87(17):6547–6549. doi:10.1073/pnas.87.17.6547
100. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264–1273.e1. doi:10.1053/j.gastro.2011.12.061
101. Lim CJ, Lee YH, Pan L, et al. Multidimensional analyses reveal distinct immune microenvironment in hepatitis B virus-related hepatocellular carcinoma. Gut. 2019;68(5):916–927. doi:10.1136/gutjnl-2018-316510
102. Ma C, Kesarwala AH, Eggert T, et al. NAFLD causes selective CD4+ T lymphocyte loss and promotes hepatocarcinogenesis. Nature. 2016;531(7593):253–257. doi:10.1038/nature16969
103. Wolf MJ, Adili A, Piotrowitz K, et al. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell. 2014;26(4):549–564. doi:10.1016/j.ccell.2014.09.003
104. van der Windt DJ, Sud V, Zhang H, et al. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology. 2018;68(4):1347–1360. doi:10.1002/hep.29914
105. Oquiñena S, Guillen-Grima F, Iñarrairaegui M, Zozaya JM, Sangro B. Spontaneous regression of hepatocellular carcinoma: a systematic review. Eur J Gastroenterol Hepatol. 2009;21(3):254–257. doi:10.1097/MEG.0b013e328324b6a2
106. Cole WH, Everson TC. Spontaneous regression of cancer: preliminary report. Ann Surg. 1956;144(3):366–383. doi:10.1097/00000658-195609000-00007
107. Sakamaki A, Kamimura K, Abe S, et al. Spontaneous regression of hepatocellular carcinoma: a mini-review. World J Gastroenterol. 2017;23(21):3797–3804. doi:10.3748/wjg.v23.i21.3797
108. Huz JI, Melis M, Sarpel U. Spontaneous regression of hepatocellular carcinoma is most often associated with tumour hypoxia or a systemic inflammatory response. HPB. 2012;14(8):500–505. doi:10.1111/j.1477-2574.2012.00478.x
109. McCaughan GW, Bilous MJ, Gallagher ND. Long-term survival with tumor regression in androgen-induced liver tumors. Cancer. 1985;56(11):2622–2626. doi:10.1002/1097-0142(19851201)56:11<2622::AID-CNCR2820561115>3.0.CO;2-0
110. Abiru S, Kato Y, Hamasaki K, Nakao K, Nakata K, Eguchi K. Spontaneous regression of hepatocellular carcinoma associated with elevated levels of interleukin 18. Am J Gastroenterol. 2002;97(3):774–775. doi:10.1111/j.1572-0241.2002.05580.x
111. Ohba K, Omagari K, Nakamura T, et al. Abscopal regression of hepatocellular carcinoma after radiotherapy for bone metastasis. Gut. 1998;43(4):575–577. doi:10.1136/gut.43.4.575
112. Jozuka H, Jozuka E, Suzuki M, Takeuchi S, Takatsu Y. Psycho-neuro-immunological treatment of hepatocellular carcinoma with major depression--A single case report. Curr Med Res Opin. 2003;19(1):59–63. doi:10.1185/030079902125001362
113. Arjunan V, Hansen A, Deutzmann A, Sze DY, Dhanasekaran R. Spontaneous regression of hepatocellular carcinoma: when the immune system stands up to cancer. Hepatology. 2021;73(4):1611–1614. doi:10.1002/hep.31489
114. Pectasides E, Miksad R, Pyatibrat S, Srivastava A, Bullock A. Spontaneous regression of hepatocellular carcinoma with multiple lung metastases: a case report and review of the literature. Dig Dis Sci. 2016;61(9):2749–2754. doi:10.1007/s10620-016-4141-2
115. Heianna J, Miyauchi T, Suzuki T, Ishida H, Hashimoto M, Watarai J. Spontaneous regression of multiple lung metastases following regression of hepatocellular carcinoma after transcatheter arterial embolization. A case report. Hepatogastroenterology. 2007;54(77):1560–1562.
116. Lendvai G, Szekerczés T, Illyés I, et al. Cholangiocarcinoma: classification, histopathology and molecular carcinogenesis. Pathol Oncol Res. 2020;26(1):3–15. doi:10.1007/s12253-018-0491-8
117. Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: epidemiology and risk factors. Liver Int. 2019;39(S1):19–31. doi:10.1111/liv.14095
118. Sripa B, Kaewkes S, Sithithaworn P, et al. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007;4(7):e201. doi:10.1371/journal.pmed.0040201
119. Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145(6):1215–1229. doi:10.1053/j.gastro.2013.10.013
120. Bragazzi MC, Ridola L, Safarikia S, et al. New insights into cholangiocarcinoma: multiple stems and related cell lineages of origin. Ann Gastroenterol. 2018;31(1):42–55. doi:10.20524/aog.2017.0209
121. Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54(1):173–184. doi:10.1002/hep.24351
122. Guedj N, Blaise L, Cauchy F, Albuquerque M, Soubrane O, Paradis V. Prognostic value of desmoplastic stroma in intrahepatic cholangiocarcinoma. Mod Pathol. 2021;34(2):408–416. doi:10.1038/s41379-020-00656-y
123. Comito G, Giannoni E, Segura CP, et al. Cancer-associated fibroblasts and M2-polarized macrophages synergize during prostate carcinoma progression. Oncogene. 2014;33(19):2423–2431. doi:10.1038/onc.2013.191
124. Gentilini A, Pastore M, Marra F, Raggi C. The role of stroma in cholangiocarcinoma: the intriguing interplay between fibroblastic component, immune cell subsets and tumor epithelium. Int J Mol Sci. 2018;19(10):2885. doi:10.3390/ijms19102885
125. Hasita H, Komohara Y, Okabe H, et al. Significance of alternatively activated macrophages in patients with intrahepatic cholangiocarcinoma. Cancer Sci. 2010;101(8):1913–1919. doi:10.1111/j.1349-7006.2010.01614.x
126. Whiteside TL. What are regulatory T cells (treg) regulating in cancer and why? Semin Cancer Biol. 2012;22(4):327–334. doi:10.1016/j.semcancer.2012.03.004
127. Zhang M, Yang H, Wan L, et al. Single-cell transcriptomic architecture and intercellular crosstalk of human intrahepatic cholangiocarcinoma. J Hepatol. 2020;73(5):1118–1130. doi:10.1016/j.jhep.2020.05.039
128. Goeppert B, Frauenschuh L, Zucknick M, et al. Prognostic impact of tumour-infiltrating immune cells on biliary tract cancer. Br J Cancer. 2013;109(10):2665–2674. doi:10.1038/bjc.2013.610
129. Liu D, Heij LR, Czigany Z, et al. The role of tumor-infiltrating lymphocytes in cholangiocarcinoma. J Exp Clin Cancer Res. 2022;41(1):127. doi:10.1186/s13046-022-02340-2
130. Yugawa K, Itoh S, Yoshizumi T, et al. Prognostic impact of tumor microvessels in intrahepatic cholangiocarcinoma: association with tumor-infiltrating lymphocytes. Mod Pathol. 2021;34(4):798–807. doi:10.1038/s41379-020-00702-9
131. Banales JM, Marin JJG, Lamarca A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17(9):557–588. doi:10.1038/s41575-020-0310-z
132. Graham RP, Barr Fritcher EG, Pestova E, et al. Fibroblast growth factor receptor 2 translocations in intrahepatic cholangiocarcinoma. Hum Pathol. 2014;45(8):1630–1638. doi:10.1016/j.humpath.2014.03.014
133. Borger DR, Tanabe KK, Fan KC, et al. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist. 2012;17(1):72–79. doi:10.1634/theoncologist.2011-0386
134. Yoon JG, Kim MH, Jang M, et al. Molecular characterization of biliary tract cancer predicts chemotherapy and programmed death 1/programmed death-ligand 1 blockade responses. Hepatology. 2021;74(4):1914–1931. doi:10.1002/hep.31862
135. Wang J, Dong M, Xu Z, et al. Notch2 controls hepatocyte-derived cholangiocarcinoma formation in mice. Oncogene. 2018;37(24):3229–3242. doi:10.1038/s41388-018-0188-1
136. Guest RV, Boulter L, Kendall TJ, et al. Cell lineage tracing reveals a biliary origin of intrahepatic cholangiocarcinoma. Cancer Res. 2014;74(4):1005–1010. doi:10.1158/0008-5472.CAN-13-1911
137. Komuta M, Spee B, Vander Borght S, et al. Clinicopathological study on cholangiolocellular carcinoma suggesting hepatic progenitor cell origin. Hepatology. 2008;47(5):1544–1556. doi:10.1002/hep.22238
138. Andersen JB, Spee B, Blechacz BR, et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology. 2012;142(4):1021–1031.e15. doi:10.1053/j.gastro.2011.12.005
139. Cardinale V, Renzi A, Carpino G, et al. Profiles of cancer stem cell subpopulations in cholangiocarcinomas. Am J Pathol. 2015;185(6):1724–1739. doi:10.1016/j.ajpath.2015.02.010
140. Carapeto F, Bozorgui B, Shroff RT, et al. The immunogenomic landscape of resected intrahepatic cholangiocarcinoma. Hepatology. 2022;75(2):297–308. doi:10.1002/hep.32150
141. Piha-Paul SA, Oh D, Ueno M, et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: results from the KEYNOTE-158 and KEYNOTE-028 studies. Int J Cancer. 2020;147(8):2190–2198. doi:10.1002/ijc.33013
142. Sia D, Hoshida Y, Villanueva A, et al. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology. 2013;144(4):829–840. doi:10.1053/j.gastro.2013.01.001
143. Job S, Rapoud D, Santos AD, et al. Identification of four immune subtypes characterized by distinct composition and functions of tumor microenvironment in intrahepatic cholangiocarcinoma. Hepatology. 2020;72(3):965–981. doi:10.1002/hep.31092
144. Fontugne J, Augustin J, Pujals A, et al. PD-L1 expression in perihilar and intrahepatic cholangiocarcinoma. Oncotarget. 2017;8(15):24644–24651. doi:10.18632/oncotarget.15602
145. Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18(3):197–218. doi:10.1038/s41573-018-0007-y
146. Oishi N, Kumar MR, Roessler S, et al. Transcriptomic profiling reveals hepatic stem-like gene signatures and interplay of miR-200c and epithelial-mesenchymal transition in intrahepatic cholangiocarcinoma. Hepatology. 2012;56(5):1792–1803. doi:10.1002/hep.25890
147. Ding G, Ma J, Yun J, et al. Distribution and density of tertiary lymphoid structures predict clinical outcome in intrahepatic cholangiocarcinoma. J Hepatol. 2022;76(3):608–618. doi:10.1016/j.jhep.2021.10.030
148. Martin-Serrano MA, Kepecs B, Torres-Martin M, et al. Novel microenvironment-based classification of intrahepatic cholangiocarcinoma with therapeutic implications. Gut. 2022;gutjnl-2021–326514. doi:10.1136/gutjnl-2021-326514
149. Banales JM, Cardinale V, Carpino G, et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European network for the study of cholangiocarcinoma (ENS-CCA). Nat Rev. 2016;13(5):261–280. doi:10.1038/nrgastro.2016.51
150. Kitano Y, Okabe H, Yamashita Y, et al. Tumour-infiltrating inflammatory and immune cells in patients with extrahepatic cholangiocarcinoma. Br J Cancer. 2018;118(2):171–180. doi:10.1038/bjc.2017.401
151. Lim YJ, Koh J, Kim K, et al. High ratio of programmed cell death protein 1 (PD-1)+/CD8+ tumor-infiltrating lymphocytes identifies a poor prognostic subset of extrahepatic bile duct cancer undergoing surgery plus adjuvant chemoradiotherapy. Radiother Oncol. 2015;117(1):165–170. doi:10.1016/j.radonc.2015.07.003
152. Walter D, Herrmann E, Schnitzbauer AA, et al. PD-L1 expression in extrahepatic cholangiocarcinoma. Histopathology. 2017;71(3):383–392. doi:10.1111/his.13238
153. Montal R, Sia D, Montironi C, et al. Molecular classification and therapeutic targets in extrahepatic cholangiocarcinoma. J Hepatol. 2020;73(2):315–327. doi:10.1016/j.jhep.2020.03.008
154. Goodman ZD, Ishak KG, Langloss JM, Sesterhenn IA, Rabin L. Combined hepatocellular-cholangiocarcinoma. A histologic and immunohistochemical study. Cancer. 1985;55(1):124–135. doi:10.1002/1097-0142(19850101)55:1<124::AID-CNCR2820550120>3.0.CO;2-Z
155. Kassahun WT, Hauss J. Management of combined hepatocellular and cholangiocarcinoma. Int J Clin Pract. 2008;62(8):1271–1278. doi:10.1111/j.1742-1241.2007.01694.x
156. Yeh MM. Pathology of combined hepatocellular-cholangiocarcinoma. J Gastroenterol Hepatol. 2010;25(9):1485–1492. doi:10.1111/j.1440-1746.2010.06430.x
157. Sapisochin G, Fidelman N, Roberts JP, Yao FY. Mixed hepatocellular cholangiocarcinoma and intrahepatic cholangiocarcinoma in patients undergoing transplantation for hepatocellular carcinoma. Liver Transplant. 2011;17(8):934–942. doi:10.1002/lt.22307
158. Lunsford KE, Court C, Lee YS, et al. Propensity-matched analysis of patients with mixed hepatocellular-cholangiocarcinoma and hepatocellular carcinoma undergoing liver transplantation. Liver Transplant. 2018;24(10):1384–1397. doi:10.1002/lt.25058
159. Garancini M, Goffredo P, Pagni F, et al. Combined hepatocellular-cholangiocarcinoma: a population-level analysis of an uncommon primary liver tumor. Liver Transplant. 2014;20(8):952–959. doi:10.1002/lt.23897
160. Nguyen CT, Caruso S, Maille P, et al. Immune profiling of combined hepatocellular- cholangiocarcinoma reveals distinct subtypes and activation of gene signatures predictive of response to immunotherapy. Clin Cancer Res. 2022;28(3):540–551. doi:10.1158/1078-0432.CCR-21-1219
161. Lu S, Stein JE, Rimm DL, et al. Comparison of biomarker modalities for predicting response to PD-1/PD-L1 checkpoint blockade: a systematic review and meta-analysis. JAMA Oncol. 2019;5(8):1195–1204. doi:10.1001/jamaoncol.2019.1549
162. Stack EC, Wang C, Roman KA, Hoyt CC. Multiplexed immunohistochemistry, imaging, and quantitation: a review, with an assessment of tyramide signal amplification, multispectral imaging and multiplex analysis. Methods. 2014;70(1):46–58. doi:10.1016/j.ymeth.2014.08.016
163. Gerner M, Kastenmuller W, Ifrim I, Kabat J, Germain R. Histo-cytometry: a method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes. Immunity. 2012;37(2):364–376. doi:10.1016/j.immuni.2012.07.011
164. Li W, Germain RN, Gerner MY. Multiplex, quantitative cellular analysis in large tissue volumes with clearing-enhanced 3D microscopy (Ce3D). Proc Natl Acad Sci U S A. 2017;114(35):E7321–E7330. doi:10.1073/pnas.1708981114
165. Widodo SS, Hutchinson RA, Fang Y, et al. Toward precision immunotherapy using multiplex immunohistochemistry and in silico methods to define the tumor immune microenvironment. Cancer Immunol Immunother. 2021;70(7):1811–1820. doi:10.1007/s00262-020-02801-7
166. Sabdyusheva Litschauer I, Becker K, Saghafi S, et al. 3D histopathology of human tumours by fast clearing and ultramicroscopy. Sci Rep. 2020;10(1):1–16. doi:10.1038/s41598-020-71737-w
167. Weinstein RS. Prospects for telepathology. Hum Pathol. 1986;17(5):433–434. doi:10.1016/S0046-8177(86)80028-4
168. Pallua JD, Brunner A, Zelger B, Schirmer M, Haybaeck J. The future of pathology is digital. Pathology. 2020;216(9):153040. doi:10.1016/j.prp.2020.153040
169. Araújo ALD, Arboleda LPA, Palmier NR, et al. The performance of digital microscopy for primary diagnosis in human pathology: a systematic review. Virchows Arch. 2019;474(3):269–287. doi:10.1007/s00428-018-02519-z
170. Fuchs TJ, Buhmann JM. Computational pathology: challenges and promises for tissue analysis. Comput Med Imaging Graph. 2011;35(7):515–530. doi:10.1016/j.compmedimag.2011.02.006
171. van der Laak J, Litjens G, Ciompi F. Deep learning in histopathology: the path to the clinic. Nat Med. 2021;27(5):775–784. doi:10.1038/s41591-021-01343-4
172. Bera K, Schalper KA, Rimm DL, Velcheti V, Madabhushi A. Artificial intelligence in digital pathology — new tools for diagnosis and precision oncology. Nat Rev Clin Oncol. 2019;16(11):703–715. doi:10.1038/s41571-019-0252-y
173. Niazi MKK, Parwani AV, Gurcan MN. Digital pathology and artificial intelligence. Lancet Oncol. 2019;20(5):e253–e261. doi:10.1016/S1470-2045(19)30154-8
174. Ehteshami Bejnordi B, Veta M, Johannes van Diest P, et al. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA. 2017;318(22):2199–2210. doi:10.1001/jama.2017.14585
175. Kelly CJ, Karthikesalingam A, Suleyman M, Corrado G, King D. Key challenges for delivering clinical impact with artificial intelligence. BMC Med. 2019;17(1):195. doi:10.1186/s12916-019-1426-2
176. Abels E, Pantanowitz L, Aeffner F, et al. Computational pathology definitions, best practices, and recommendations for regulatory guidance: a white paper from the digital pathology association. J Pathol. 2019;249(3):286–294. doi:10.1002/path.5331
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
