Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Efficacy of TACE Combined with Lenvatinib Plus Sintilimab for Hepatocellular Carcinoma with Tumor Thrombus in the Inferior Vena Cava and/or Right Atrium
Authors Ning S , Li X, Ma X, Liu J, Chang X
Received 21 March 2023
Accepted for publication 26 June 2023
Published 13 September 2023 Volume 2023:10 Pages 1511—1525
DOI https://doi.org/10.2147/JHC.S410967
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Jörg Trojan
Shangkun Ning,1,* Xinge Li,2,* Xiangyu Ma,1 Jibing Liu,1 Xu Chang3
1Department of Interventional Therapy I, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, Shandong, People’s Republic of China; 2Department of Oncology, Central Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, People’s Republic of China; 3Department of Interventional Therapy II, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, Shandong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xu Chang, Department of Interventional Therapy II, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, Shandong, 250117, People’s Republic of China, Tel +86-531-18769785023, Email [email protected]
Purpose: To validate the safety and effectiveness of transarterial chemoembolization (TACE) combination with lenvatinib and sintilimab in treating hepatocellular carcinoma (HCC) patients with inferior vena cava (IVC) and/or right atrium (RA) tumor thrombosis (TT).
Methods: This study retrospectively analyzed HCC patients with IVC and/or RA TT treated with TACE combined with lenvatinib plus sintilimab. Overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and disease control rate (DCR) were calculated to evaluate the anti-tumor efficacy. Treatment-related adverse events (TRAEs) were analyzed to assess the safety profiles.
Results: A total of 58 patients were screened for eligibility between March 2019 and May 2022. At the time of data collection, 48.2% of patients were still receiving treatment. The median follow-up was 23.5 months. The ORR was 48.3%, the DCR was 91.4%, the median OS was 17.3 months, and the median PFS was 13.0 months. The ORR for IVC/RA TT was 62.1%, DCR was 94.9%, and the median PFS was 14.3 months. 56.9% of patients experienced ≥ grade 3 TRAEs, such as hypertension (10.3%) and elevated liver enzymes (13.8%). No new safety signals were identified. Participants with low levels of serum PCT value had satisfactory prognoses.
Conclusion: TACE combination with lenvatinib plus sintilimab is effective in treating HCC with IVC and/or RA TT. The toxicities were manageable, with no unexpected safety signals. The baseline levels of serum PCT might be the predictive biomarkers for the triple combination therapy.
Keywords: hepatocellular carcinoma, inferior vena cava tumor thrombus, transarterial chemoembolization, lenvatinib, sintilimab
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third most common cause of cancer-related deaths worldwide.1,2 Vascular invasion is an important feature of HCC; about 12.5–62.5% of patients exhibit portal vein tumor thrombus (PVTT) at their initial visit.3 Hence, several studies4 evaluated these patients and achieved promising results. However, HCC with an extension to the inferior vena cava (IVC) and right atrium (RA) rarely occurs.5,6 Patients with tumor thrombus invading the IVC or RA, often accompanied by PVTT, have an extremely poor prognosis since the pulmonary embolization and massive involvement of the right atrial cavity can rapidly lead to death with a median survival of about 2–5 months in untreated patients.7 Surgery, transarterial chemoembolization (TACE), hepatic arterial infusion chemotherapy (HAIC), radiotherapy (RT), high-intensity focused ultrasound (HIFU), radiofrequency ablation (RFA), and microwave ablation (MWA) are the most common treatment methods for patients with IVC or RA TT.8–10 Because of the macrovascular invasion, these patients are classified as Barcelona Clinic Liver Cancer (BCLC) stage C, for which the tyrosine kinase inhibitor (TKI), sorafenib or lenvatinib, is recommended as the standard therapy. Although TKI can be used to treat these patients, its clinical effectiveness is unsatisfactory.
Immunotherapy using immune checkpoint inhibitors (ICIs) has shown promising anti-tumor efficacy in HCC. The US Food and Drug Administration (FDA) approved the combination of atezolizumab and bevacizumab for patients with unresectable or metastatic HCC who are systemic treatment-naive, based on Phase III IMbrave150 study.11 More recently, a clear superiority of the durvalumab-tremelimumab (STRIDE regimen) over sorafenib was demonstrated in the HIMALAYA phase III trial.12 In addition, studies on combination therapies, such as ICI plus TKI and ICI combined with other local treatments, presented promising results.13,14 However, only limited data on TKI, ICI, and TACE are available with respect to the treatment of HCC with IVC or RA TT. Also, there is still no standard treatment method available for these patients.
This study investigated the safety and efficacy of TKI, ICI, and TACE in the treatment of patients with IVC and/or RA TT.
Materials and Methods
Case Selection and General Information
A total of 58 unresectable HCC patients with IVC and/or RA TT treated with TACE combined with lenvatinib plus sintilimab between March 2019 and May 2022 were recruited in this study (Figure 1A). All patients were diagnosed with HCC based on the non-invasive criteria or biopsy. The non-invasive diagnostic criteria for HCC in patients with cirrhosis were as follows: liver cirrhosis, tumor diameter > 1 cm based on four-phase multidetector computed tomography (MDCT) or dynamic magnetic resonance imaging (MRI), and arterial hypervascularization with venous or delayed phase washout.15,16
 |
Figure 1 (A): Flowchart of the patient cohort. (B): The schema of the combination therapy. |
The inclusion criteria were as follows: (1) patient aged between 18 and 80 years; (2) liver function classification (Child-Pugh) of grade A or B; (3) Eastern Cooperative Oncology Group (ECOG) score 0–2; (4) IVC and/or RATT diagnosed before treatment; (5) conformed to the indications for lenvatinib plus sintilimab; (6) stable thrombus in patients with pulmonary embolism. The exclusion criteria were: (1) surgery, liver tumor radiofrequency ablation, particle implantation, or liver transplantation before or after admission; (2) received radiotherapy and/or systemic chemotherapy before admission; (3) patients taking other targeted drugs in addition to lenvatinib; (4) with severe dysfunction of the heart, lung, liver, or kidneys; (5) Child-Pugh class C or ECOG score > 2; (6) serious medical complications.
This single-center retrospective study was approved by the ethics committee of the hospital. Written informed consent was acquired from all patients before the operation in accordance with the 1955 Declaration of Helsinki.
Study Design
Patients received oral lenvatinib (EISAI Merck & Co. Inc., Japan, USA) at 12mg/day (for body weight > 60kg) or 8mg/day (for body weight < 60 kg) 3–7 days for initial confirmation of tolerability. Dose interruptions followed by reductions for lenvatinib-related toxicities were allowed. Sintilimab (Innovent, Soochow, China), as an ICI, was administered intravenously at a dose of 200 mg on day 1 of a 21-day therapy cycle after TACE (Figure 1B). The TACE was repeated at intervals of 4–6 weeks until the complete disappearance of the viable intrahepatic tumor or when the hepatic function was not preserved. Adverse events were assessed using the National Cancer Institute patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE).17
TACE Procedure
The entire treatment was conducted using digital subtraction angiography (DSA) monitoring (Allura Xper FD20, Philips N. V, Netherlands) with the patient under local anesthesia. The 5F vascular sheath was inserted into the right femoral artery via the Seldinger technique. All patients underwent angiography of the celiac and superior mesenteric artery to identify the intrahepatic tumor-feeding branches. Arteries supplying to the IVC and RATT were identified as follows: (1) in patients with lesions in the right lobe of the liver, followed by angiography of the right inferior phrenic artery; (2) for patients with lesions in the left lobe, left gastric artery, and left inferior phrenic artery, angiography was carried out; (3) internal mammary artery angiography was performed in patients with no definite tumor thrombus-feeding branches. If the blood supply vessel of the tumor, IVC, or RA TT was identified, it was maximally superselected using a microcatheter (Terumo, Japan). Then, a mixture of iodized oil (Jiangsu Hengrui Medicine, Lianyungang, China) and chemotherapeutic agents, such as lobaplatin (Guizhouyibai, Guizhou, China) and epirubicin (Pfizer, New York, USA), were administered into the target artery. Lipiodol was injected until it stopped flowing in the artery, directly feeding the tumor, and a gelatin sponge (Ailikang, Hangzhou, China) or coil (COOK, Bloomington, USA) was injected as a supplement when necessary.
Classification of IVC/RA TT
Cheng et al18 summarized the correlation between the anatomical location of the proximal end of the tumor thrombus in the inferior vena cava and prognosis, dividing the hepatic vein (HV)/IVC TT into three categories: (1) hepatic vein type (type I): tumor thrombus confined within the hepatic vein; (2) subdiaphragmatic type (type II): tumor thrombus located in the posterior inferior vena cava of the liver but below the plane of the diaphragm; (3) supradiaphragmatic type (type III): tumor thrombus crossed the plane of the diaphragm (type IIIa), or tumor thrombus entered the RA (type IIIb).
Response Evaluation
The response was evaluated by two investigators and reviewed by an independent radiologist at the time of study completion. Modified Response Evaluation Criteria In Solid Tumors (mRECIST)19 was used to evaluate the primary lesions. The overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and disease control rate (DCR) were calculated to evaluate the anti-tumor efficacy. OS was defined as the time from the first TACE treatment to death or the last follow-up. PFS was defined as the time from the first TACE treatment to disease progression or the last follow-up. The objective response rate (ORR) was the percentage of patients with complete or partial response (CR and PR), and the DCR was the percentage of patients with complete or partial response (CR and PR) or stable disease (SD).
Sequential CT-enhanced scans were used to assess the IVC or RA TT response by calculating the diameter of the tumor thrombus and comparing it to the initial value. Based on the changes in IVC or RA TT diameter measurements after treatment, we evaluated the effects in this study based on four categories as follows: (1) CR: the tumor thrombus disappeared completely or was completely covered by iodinated oil; (2) PR: the diameter of the tumor thrombus was reduced ≥ 30%; (3) SD: the diameter of tumor thrombus did not change or did not meet the standard for improvement and progress; (4) progressive disease (PD): tumor thrombus diameter increased by ≥ 20%, or new lesions appeared.
The side effects of TACE, including fever, gastrointestinal reactions, liver function, and pain, were assessed. In addition, the liver function tests (serum albumin, bilirubin, and prothrombin time) were conducted four weeks after the first TACE treatment to assess the toxicity of the treatment on the liver.
Blood Assessment for Procalcitonin and Determination of the Cutoff Value
Measuring procalcitonin (PCT) concentration before the first cycle of the combined treatment, blood samples were collected into a blood collection tube containing a serum-separating agent or EDTA and centrifuged at 3000 rpm for 20 minutes. The concentration of PCT was measured using a chemiluminescent enzyme immunoassay. The cutoff value was defined using the software X tile. A blood culture was performed for the patients with PCT value >0.5 ng/mL to confirm that there were no active infectious.
Follow-Up
Follow-up visits were performed 4–6 weeks after the TACE procedure. Contrast-enhanced CT or dynamic MRI was conducted after each follow-up to assess the efficacy of the procedure. In addition, echocardiography was used every three months to assess thrombus stability for patients with pulmonary embolisms. Laboratory tests included hematological and biochemical analyses, such as complete blood count, prothrombin time, methemoglobin, aspartate aminotransferase, alanine aminotransferase, total bilirubin, and serum albumin.
Telephone follow-up and outpatient interviews were continued and ended on November 1st, 2022. For patients out of contact during the follow-up, the OS was calculated as the time from the diagnosis to the last follow-up.
Statistical Analysis
R Programming Language and SPSS 26.0 were used for all statistical analyses. OS and PFS were evaluated using the Kaplan-Meier method and Log rank test, with p < 0.05 as a statistically significant difference. Cox proportional risk regression models were used for univariate and multivariate analyses. Univariate analysis was used, and variables with p < 0.1 were selected for multivariate analysis, with p < 0.05 considered statistically significant.
Results
Patients and Tumor Characteristics
The characteristics of 58 patients are listed in Table 1. All patients were treated with TACE plus lenvatinib and sintilimab. The cohort comprised 9 females and 49 males with a mean age of 56.71 ± 9.54 years. The sum of the maximum diameters of all intrahepatic lesions was ≤ 5 cm in 6.9% (4/58), 5–10 cm in 39.7% (23/58), and ≥ 10 cm in 53.4% (31/58) of cases. In addition, 84.5% (49/58) were Child-Pugh A and the remaining were B. Also, 89.7% (52/58) of the patients had an underlying chronic liver disease caused by hepatitis B virus infection; 55.2% (32/58) patients had PVTT with 9, 12, and 11 cases of type II, III, and IV, respectively, according to the Japanese VP classification; 53.4% (31/58) of the patients had distant metastases, and some had multiple metastases, including 25 lymph nodes and 18 lung, 2 adrenal gland, 2 abdominal wall, and 1 bone metastases. Pulmonary embolism was observed in 8 cases, but none had any obvious symptoms, and 5 cases had lower limb edema.
 |
Table 1 Characteristics of the Enrolled 58 Patients with HCC |
Patients were followed up with second-line therapy after disease progression, including regorafenib alone (n = 9, 15.5%), PD-1 alone (n = 2, 3.4%), regorafenib plus PD-1 (n = 7, 12.1%), and atezolizumab plus bevacizumab (n = 3, 5.2%), followed by camrelizumab and apatinib (n = 5, 8.6%) after further progression.
Treatment
In addition, in the study, 58 patients were treated with a total of 288 cycles of TACE (median 5 cycles). Patients received oral lenvatinib as described above 3–7 days prior to the initial TACE to confirm tolerability. A dose reductions due to lenvatinib-related toxicities (to 8 mg or 4 mg/day or to 4 mg every other day) were allowed. Dose adjustment of lenvatinib were observed 10 patients but no patients discontinued the target drugs. The median duration of lenvatinib was 8.3 months. Sintilimab as ICIs, was administered intravenously at a dose of 200 mg one day after TACE. 58 patients were treated with a total of 491 cycles of sintilimab (median 7 cycles).
Overall PFS and OS
The median follow-up duration of patients was 23.5 months (range, 5.8–36.2 months). At the final follow-up, 30 patients had disease progression, of whom 28 survived. Tumor assessments were based on the mRECIST criteria and 8.6% (5/58), 39.7% (23/58), 43.1% (25/58), and 8.6% (5/58) patients achieved CR, PR, SD, and PD, respectively (Table 2). The ORR was 48.3% [95% confidence interval (CI): 35.0–61.8%)], and the DCR was 91.4% (95% CI: 72.6–92.7%). The median OS (mOS) and median PFS (mPFS) were 17.3 months (95% CI: 13.7–20.9) and 13.0 months (95% CI: 11.7–14.3) (Figure 2a and b). The mOS was 25.8 months (95% CI: 19.2–32.4) in patients without PVTT and 13.6 months (95% CI: 11.6–15.6) in patients with PVTT (95% CI: 1.53–7.86, p = 0.003) (Figure 2c). The mOS for patients with type I/II and type III tumor thrombus was 15.3 months (95% CI: 12.5–18.1) and 21.0 months (95% CI: 11.1–30.9), respectively, with no significant difference between the two groups (95% CI: 0.26–1.15, p = 0.112) (Figure 2d). The mOS was 25.3 months (95% CI: 19.0–31.6) for patients without metastases compared to 15.3 months (95% CI: 21.6–19.0) for patients with metastases (95% CI: 1.02–4.69, p=0.044) (Figure 2e). The OS was 25.3 months (95% CI: 20.2–30.4) and 14.7 months (95% CI: 10.8–18.6) in the response and non-response groups of IVC/RA TT, respectively (Figure 2f). Reductions in tumor size are shown in Figure 3a.
 |
Table 2 Intra-Hepatic Tumor Response Was Evaluated in 58 Patients |
Evaluation of IVC and/or RATT
According to Cheng’s IVCTT classification, three cases were type I, 19 were type II, and 36 were type III. The IVCTT was supplied by the hepatic artery in 40 patients and by the inferior phrenic artery in 20 patients. In another 13 patients, no definite feeding artery was found. The remaining patients had extrahepatic feeding arteries, including the internal mammary artery (IMA) and the left gastric artery (LGA).
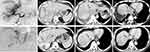
The PFS of IVC/RA TT was defined as the time from the first TACE treatment to the IVC/RATT progression or the last follow-up. The response of the IVC/RA TT was defined as CR and PR, and non-response was defined as SD and PD. The reduction details during follow-up are shown in Figure 3b, and the mPFS of IVC/RA TT was 14.3 months (95% CI: 10.1–18.5) (Figure 3c). The response of IVC/RA TT to combination therapy was assessed at three months. Of the 58 patients evaluated for the efficacy of IVC/RATT, 15.5% (9/58), 46.6% (27/58), 32.8% (19/58), and 5.1% (3/58) achieved CR, PR, SD, and PD, respectively; the ORR was 62.1% (95% CI: 48.4–74.5%), and the DCR was 94.9% (95% CI: 78.8–96.1%). A patient with a high tumor burden was evaluated as PR after treatment with TACE combined with lenvatinib plus sintilimab as shown in Figure 4 (imaging scans of 12 representative participants are shown in Figure S1–S13). Furthermore, of 58 patients in the present study, 19 had differential responses for target tumors versus IVC/RA TT, of whom 14 achieved a better response in the tumor thrombus versus the target tumors.
Survival Analysis According to PCT
We also performed Kaplan–Meier analysis according to baseline PCT status. Based on the results calculated by the X-tile software, the optimal cutoff value of the PCT was set as 0.13 ng/mL. Among the 58 patients, 4 patients PCT value > 0.5 ng/mL, but positive blood culture were not find in these 4 patients. Our results showed that the mPFS of HCC with low PCT value (≤0.13 ng/mL) were significantly higher than these with high PCT value (>0.13 ng/mL) (15.5 vs 7.5 months, p = 0.001) (Figure 5a). The median OS of low PCT value and high PCT value were 25.3 and 15.3 months, respectively, p = 0.016 (Figure 5b).
 |
Figure 5 Median PFS (a) and median OS (b) stratified by the PCT level (0.13 ng/mL cutoff). Abbreviation: PCT; Procalcitonin. |
Factors Associated with OS
Univariate and multifactorial analyses included gender, age, alpha-fetoprotein (AFP) level, Child-Pugh classification, albumin-bilirubin (ALBI), extrahepatic metastases, portal vein invasion, type of IVC/RATT, and the response of IVC/RATT to treatment (Table 3). Univariate analysis found that Child-Pugh classification grade A [hazard ratio (HR) = 2.81, 95% CI: 1.24–6.40, p = 0.014] and absence of extrahepatic metastases (HR = 2.19, 95% CI: 1.02–4.69, p = 0.044) were associated with reduced mortality in patients. In contrast, non-response to IVC/RATT treatment (HR = 0.31, 95% CI: 0.15–0.65, p = 0.002) and the presence of PVTT (HR = 3.47, 95% CI: 1.53–7.86, p = 0.003) was associated with increased patient mortality. In multivariate analysis, the absence of PVTT (HR = 5.21, 95% CI: 1.96–13.89, p = 0.001) and extrahepatic metastases (HR = 2.40, 95% CI: 1.04–5.57, p = 0.041) were independent predictors associated with prolonged OS. Non-response to IVC/RATT treatment (HR = 0.35, 95% CI: 0.13–0.93, p = 0.035) was an independent predictor of reduced OS. Child-Pugh classification was not statistically significant. In addition, a statistical difference was detected in gender due to the bias of patients attending the clinic.
 |
Table 3 Univariate and Multivariate Analysis of the Clinical Characteristics Associated with OS |
Toxicity
A total of 84.5% (49/58) of patients receiving combination therapy experienced at least one treatment-related adverse event (TRAE) (Table 4). The most common TRAEs were abdominal pain 69.0% (40/58), fever 63.8% (37/58), hypertension 62.1% (36/58), increased liver enzymes 56.9% (33/58), fatigue 31.1% (18/58), and decreased appetite 27.6% (16/58). However, no patients discontinued the combination therapy. Dose adjustments were made in 12 patients due to lenvatinib-related adverse events. Grade 3 or higher TRAEs occurred in 56.9% (33/58) of patients.
 |
Table 4 Treatment-Related Adverse Events |
Immune-related adverse events of any grade were observed in 16 (27.6%) participants. The most common immune-related adverse events were hypothyroidism (20.7%). Immune-related hepatitis and pneumonitis occurred in 2 and 1 patients, respectively. Treatment-related grade 3 or 4 immune-related adverse events were seen in 4 (6.9%) participants. All immune-related adverse events disappeared after participants stopped ICIs and received hormone therapy.
The most common complication of TACE treatment was post-embolization syndrome, manifesting as pain, vomiting, fever, and elevated transaminases. With prompt symptomatic supportive treatment, the patient recovered within one week. Subsequently, one patient developed a liver abscess which improved with anti-infection and drainage. In addition, all RATTs were stable after TACE. No pulmonary embolism was detected on postoperative CT due to RATT detachment. No iodine oil deposits were observed in the lung parenchyma, and no heart failure or respiratory distress was detected.
Discussion
Large vascular tumor thrombus is a common feature in the advanced stages of HCC, and the portal and hepatic veins are the most commonly involved vessels.20 Several previous studies on these patients have achieved satisfactory results.21 The hepatic vein can progress along the venous wall to the inferior vena cava or the right atrium. Compared to PVTT, IVC/RATT occurs rarely and is only detected in about 3–4% of HCC patients who undergo imaging examinations.5 Since it carries an increased risk of systemic metastasis and a threat of impending death due to pulmonary embolism or sudden cardiac arrest, HCC patients presenting IVC/RATT have an extremely poor prognosis, and the survival time is about three months.22 Although several therapeutic methods have been applied, such as TACE, surgery, radiotherapy, and targeted drugs, the treatment required is yet unmet.23,24
TACE is considered safe and effective and has become an acceptable treatment option for most patients with advanced HCC. TACE is more frequently applied in Asia to treat HCC with macrovascular invasion.25,26 However, most of these tumors show an infiltrative growth pattern, with extrahepatic collateral arteries supplying the tumor, making them refractory to TACE.27,28 Thus, in recent years, TACE combined with systemic therapy (eg, sorafenib or lenvatinib) has been applied to treat unresectable HCC, achieving good results.29,30 Also, TACE has been widely applied in treating IVC/RA TT patients. TACE monotherapy in HCC patients with a tumor thrombus in the IVC and RA showed a median OS of 4.2–10.9 months.31 The results were not satisfactory, and consequently, it is necessary to explore a combination therapy with TACE to prolong the OS of these patients.
Atezolizumab plus bevacizumab is recommended as the preferred first-line treatment for advanced HCC by the National Comprehensive Cancer Network (NCCN) Guidelines.32 Recently, a few immunotherapy combined therapies have emerged as the first-line option for HCC, such as lenvatinib plus pembrolizumab (KEYNOTE-524),33 camrelizumab plus apatinib (RESCUE),34 and lenvatinib combined with toripalimab plus hepatic arterial infusion chemotherapy (NCT04044313).35 Most studies showed promising anti-tumor activity with a tolerable safety profile, with a median OS of 17.9–22.1 months, a median PFS of 5.6–10.4 months, and an ORR was 34.3–63.9%. Although lenvatinib plus pembrolizumab phase III trial LEAP-00236 did not meet its primary endpoint, it showed better OS compared to lenvatinib monotherapy (21.2 vs 19.0 months). These data suggested that further exploration of TKIs combined with ICIs is essential. In this study, TACE combined with lenvatinib and sintilimab achieved an ORR of 48.3%, median PFS of 13.0 months, and median OS of 17.3 months. Interestingly, a previous study by Cao et al30 reported that 60 unresectable HCC patients were administered combination therapy with TACE and lenvatinib plus sintilimab; the PFS and OS were 13.3 months and 23.6 months, respectively, and the ORR reached 46.7% (28/60). The present study showed a similar PFS and shorter OS compared to the study mentioned above. In addition to IVC/RA TT, patients in this study were relatively late in staging; 55.2% (32/58) had PVTT, and 53.4% (31/58) had distant metastases, which might be attributed to short OS. These results suggest that TACE combined with lenvatinib plus PD-1 inhibition may improve the anti-tumor activity, although the exact mechanism driving these phenomena needs to be further explored. The reasons could be speculated as follows: TACE induces an ischemic-hypoxic microenvironment leading to tumor necrosis and tumor-specific antigen release, and the combination of PD1 inhibitors enhances the production of tumor antigen-specific memory T cells and maintains the patient’s anti-tumor response.37 The hypoxic microenvironment promotes the upregulation of hypoxia-inducible factor-1, bFGF and VEGF, while the combination of lenvatinib inhibits the activity of tumor angiogenic factors.38 Anti-angiogenic targeting drugs can enhance tumor blood filling through vascular normalization and increase lymphocyte infiltration in tumor tissue,37 reversing the immunosuppressed state and increasing PD-L1 expression.39 Secondly, lenvatinib plus PD-1 inhibitors exert a unique immunomodulatory effect by blocking FGFR-4, reducing Treg differentiation, and inhibiting TGF signaling.40
In the present study, the combination therapy has a satisfactory ORR and DCR on the IVC/RATT (62.1% and 94.9%, respectively). Also, 15.5% (9/58) patients achieved CR and 46.6% (27/58) patients achieved PR. Furthermore, we found that the IVC/RA TT responders had an obvious survival benefit over non-responders (25.3 vs.14.7 months), and the response to IVC/RATT was identified as an independent predictor of mortality by multivariable analysis. This phenomenon was attributed to the synergistic anti-tumor effect of TKIs combined with ICIs. Previous study reported that almost 100% IVC/RATT exists in extrahepatic vessels. Therefore, during the TACE procedure in our hospital, any vessels suspected of supplying blood to the IVC/RATT should be assessed carefully, such as the left and right inferior phrenic artery, left gastric artery, internal mammary, and other angiographic examinations. In this cohort, 77.6% (45/58) of patients had definite arteries feeding the IVC/RATT. Therefore, accurate embolization of the cancer emboli might result in a high local control rate and response rate. Furthermore, our study found a higher ORR in IVC/RATT than in target lesions (62.1% vs 48.3%). Of 58 patients in the present study, 19 had differential responses for target tumors versus IVC/RATT, of whom 14 achieved a better response in the tumor thrombus versus the target tumors. Higher ORR and DCR can help prevent pulmonary embolism and cardiac arrest, which can provide survival benefits.
Several biomarkers at the molecular, genomic, transcriptional, and gut microbiome levels have been explored to predict the efficacy of HCC immunotherapy and targeted drugs.41 Biomarkers of inflammation and inflammatory gene signatures have also been associated with response to immunotherapy.42 However, no validated biomarker is available to guide clinical decision-making. Serum C-reactive protein (CRP) is a biomarker of an acute inflammatory response and has been successfully used as a prognostic predictor for several malignancies. Recently, some studies43 demonstrated the prognostic value of pretreatment serum CRP levels for advanced HCC patients and treatment with PD-1 inhibitors. In addition Scheiner al. developed a novel prognostic score named “CRAFITY” based on serum CRP and alpha-fetoprotein levels that predicts the outcome of HCC patients receiving atezolizumab plus bevacizumab.44 PCT has been considered an excellent marker of bacterial infection and acute inflammatory for over two decades. Currently, some studies reported for the first time that PCT is a prognostic factor in lung cancer patients receiving ICIs.45 Therefore, we speculated whether PCT, as an indicator of inflammation, could also predict the efficacy of immunotherapy. Our results showed that the PFS and OS of HCC with low PCT value were significantly higher than those with high PCT value (15.5 vs 7.5 months, p = 0.001; 25.3 vs 15.3 months, p = 0.016). In addition, the pretreatment level of serum PCT were an independent predictors associated with reduced PFS and OS. To our knowledge, no study has reported an association between serum PCT levels and prognosis in HCC. Further research is needed to determine the specificity of PCT in patients with HCC and in particular to explore the potential usefulness of PCT to monitor the response to treatment and predict prognosis.
A tolerable safety profile was observed with combination therapy in this study. Also, no unexceptional toxicities were detected in the triple treatment group, which was consistent with the known AEs of each drug. Abdominal pain 69.0% (40/58), fever 63.8% (37/58), hypertension 62.1% (36/58), increased liver enzymes 56.9% (33/58), fatigue 31.1% (18/58) and decreased appetite 27.6% (16/58) were the most frequent any-grade treatment-related AEs, and 56.9% (33/58) of patients presented grade ≥ 3 AES. In addition, we found a high incidence of elevated transaminases, which might have resulted from TACE and a high proportion of patients with hepatitis B virus (HBV) infection. Moreover, precise embolization could stabilize the tumor thrombus, but detached IVC/RATT was observed in none of the patients. Overall, the tolerable safety profile ensures a long-term survival benefit through appropriate management.
The present study has several limitations. First, this was a retrospective, single-hospital study, which might cause an inherent information and selection bias. Second, this was a single-arm design, limited by the lack of a control group. Therefore, a multicenter, randomized, controlled clinical trial is required to validate the efficacy and safety of the different combinations. Third, the sample size of this study was small. However, our findings highlighted the potential for future expansion of the indication for TACE combination with lenvatinib plus PD-1 inhibitors in patients with advanced unresectable HCC with high-risk tumors and macrovascular invasion.
In conclusion, our data suggest that TACE combined with lenvatinib plus sintilimab has promising efficacy in treating patients with IVC/RA TT. Furthermore, this combination therapy is well-tolerated and could be a treatment option for this patient group.
Abbreviations
PVTT, Portal vein tumor thrombus; IVC, Inferior vena cava; RA, Right atrium; TACE, Transarterial chemoembolization; HAIC, Hepatic arterial infusion chemotherapy; RT, Radiotherapy; HIFU, High-intensity focused ultrasound; RFA, Radiofrequency ablation; MWA, Microwave ablation; TKI, Tyrosine kinase inhibitor; ICI, Immune checkpoint inhibitor; MDCT, Multi-detector computed tomography; DSA, Digital subtraction angiography; ALBI, Albumin-bilirubin; TRAE, Treatment-related adverse event.
Data Sharing Statement
The dataset used for this study is available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
Informed consent was obtained from the patient to publish, and approval for this study was provided by the Research Ethics Committee of The Affiliated Cancer Hospital of Shandong First Medical University.
Consent for Publication
Written informed consent for publication of this study was obtained from the patient. A copy of the written consent form is available for review by the Editor of this journal.
Funding
This research was supported by Natural Science Foundation of Shandong Province ZR2020QH177 (the Efficacy of TACE combined with TGF-β blockade in the treatment of hepatocellular carcinoma and its impact on the immune microenvironment).
Disclosure
The authors declare that they have no competing interests.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Ouyang X, Lv L, Zhao Y, et al. ASF1B serves as a potential therapeutic target by influencing cell cycle and proliferation in hepatocellular carcinoma. Front Oncol. 2022;11:801506. doi:10.3389/fonc.2021.801506
3. Zhang Y, Wu JL, Li LQ. Efficacy comparison of optimal treatments for hepatocellular carcinoma patients with portal vein tumor thrombus. Ann Hepatol. 2022;27:100552. doi:10.1016/j.aohep.2021.100552
4. Ding X, Sun W, Li W, et al. Transarterial chemoembolization plus lenvatinib versus transarterial chemoembolization plus sorafenib as first-line treatment for hepatocellular carcinoma with portal vein tumor thrombus: a prospective randomized study. Cancer. 2021;127(20):3782–3793. doi:10.1002/cncr.33677
5. Zhang XP, Liu YC, Chen ZH, et al. Postoperative adjuvant transarterial chemoembolization improves outcomes of hepatocellular carcinoma associated with hepatic vein invasion: a propensity score matching analysis. Ann Surg Oncol. 2019;26(5):1465–1473. doi:10.1245/s10434-019-07223-z
6. Wang Y, Yuan L, Ge RL, Sun Y, Wei G. Survival benefit of surgical treatment for hepatocellular carcinoma with inferior vena cava/right atrium tumor thrombus: results of a retrospective cohort study. Ann Surg Oncol. 2013;20(3):914–922. doi:10.1245/s10434-012-2646-2
7. Chao WS, Shen CH, Luo SC, et al. Concomitant hepatectomy and atrial thrombectomy under cardiopulmonary bypass versus staged hepatectomy in the treatment for hepatocellular carcinoma with large right atrial tumor thrombi. J Clin Med. 2022;11(8):2140.
8. Shirono T, Koga H, Niizeki T, et al. Usefulness of a novel transarterial chemoinfusion plus external-beam radiation therapy for advanced hepatocellular carcinoma with tumor thrombi in the inferior vena cava and right atrium: case study. Cancer Rep. 2022;5(8):e1539.
9. Zheng K, Zhu X, Fu S, et al. Sorafenib plus hepatic arterial infusion chemotherapy versus sorafenib for hepatocellular carcinoma with major portal vein tumor thrombosis: a randomized trial. Radiology. 2022;303(2):455–464. doi:10.1148/radiol.211545
10. Lou J, Li Y, Liang K, et al. Hypofractionated radiotherapy as a salvage treatment for recurrent hepatocellular carcinoma with inferior vena cava/right atrium tumor thrombus: a multi-center analysis. BMC Cancer. 2019;19(1):668. doi:10.1186/s12885-019-5870-3
11. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
12. Kudo M. Durvalumab plus tremelimumab in unresectable hepatocellular carcinoma. Hepatobiliary Surg Nutr. 2022;11(4):592–596. doi:10.21037/hbsn-22-143
13. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, Phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi:10.1016/S0140-6736(17)31046-2
14. Kudo M, Finn RS, Edeline J, et al. Updated efficacy and safety of KEYNOTE-224: a Phase II study of pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. Eur J Cancer. 2022;167:1–12.
15. Khalili K, Kim TK, Jang HJ, et al. Optimization of imaging diagnosis of 1–2 cm hepatocellular carcinoma: an analysis of diagnostic performance and resource utilization. J Hepatol. 2011;54(4):723–728. doi:10.1016/j.jhep.2010.07.025
16. Sangiovanni A, Manini MA, Iavarone M, et al. The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut. 2010;59(5):638–644. doi:10.1136/gut.2009.187286
17. Kkf C, Mitchell SA, Chan N, Ang E, Tam W, Kanesvaran R. Linguistic validation of the simplified Chinese version of the US National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE™). BMC Cancer. 2020;20(1):1153. doi:10.1186/s12885-020-07631-5
18. Chen ZH, Wang K, Zhang XP, et al. A new classification for hepatocellular carcinoma with hepatic vein tumor thrombus. Hepatobiliary Surg Nutr. 2020;9(6):717–728.
19. Llovet JM, Lencioni R. mRECIST for HCC: performance and novel refinements. J Hepatol. 2020;72(2):288–306. doi:10.1016/j.jhep.2019.09.026
20. Schlachterman A, Craft WW
21. Alrashidi I, Chu HH, Kim JH, et al. Combined chemoembolization and radiotherapy versus chemoembolization alone for hepatocellular carcinoma invading the hepatic vein or inferior vena cava. Cardiovasc Intervent Radiol. 2021;44(7):1060–1069. doi:10.1007/s00270-021-02815-3
22. Nakazawa T, Adachi S, Kitano M, et al. Potential prognostic benefits of radiotherapy as an initial treatment for patients with unresectable advanced hepatocellular carcinoma with invasion to intrahepatic large vessels. Oncology. 2007;73(1–2):90–97. doi:10.1159/000120996
23. Kokudo T, Hasegawa K, Matsuyama Y, et al. Liver resection for hepatocellular carcinoma associated with hepatic vein invasion: a Japanese nationwide survey. Hepatology. 2017;66(2):510–517. doi:10.1002/hep.29225
24. Chern MC, Chuang VP, Cheng T, Lin ZH, Lin YM. Transcatheter arterial chemoembolization for advanced hepatocellular carcinoma with inferior vena cava and right atrial tumors. Cardiovasc Intervent Radiol. 2008;31(4):735–744. doi:10.1007/s00270-008-9342-4
25. Kim JH, Shim JH, Yoon HK, Ko HK, Kim JW, Gwon DI. Chemoembolization related to good survival for selected patients with hepatocellular carcinoma invading segmental portal vein. Liver Int. 2018;38(9):1646–1654. doi:10.1111/liv.13719
26. Kim GA, Shim JH, Yoon SM, et al. Comparison of chemoembolization with and without radiation therapy and sorafenib for advanced hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. J Vasc Interv Radiol. 2015;26(3):320–9.e6. doi:10.1016/j.jvir.2014.10.019
27. Lee IJ, Chung JW, Kim HC, et al. Extrahepatic collateral artery supply to the tumor thrombi of hepatocellular carcinoma invading inferior vena cava: the prevalence and determinant factors. J Vasc Interv Radiol. 2009;20(1):22–29. doi:10.1016/j.jvir.2008.09.030
28. Kojiro M, Nakahara H, Sugihara S, Murakami T, Nakashima T, Kawasaki H. Hepatocellular carcinoma with intra-atrial tumor growth. A clinicopathologic study of 18 autopsy cases. Arch Pathol Lab Med. 1984;108(12):989–992.
29. Liu J, Li Z, Zhang W, et al. Comprehensive treatment of trans-arterial chemoembolization plus lenvatinib followed by camrelizumab for advanced hepatocellular carcinoma patients. Front Pharmacol. 2021;12:709060. doi:10.3389/fphar.2021.709060
30. Cao F, Yang Y, Si T, et al. The efficacy of TACE combined with lenvatinib plus sintilimab in unresectable hepatocellular carcinoma: a multicenter retrospective study. Front Oncol. 2021;11:783480. doi:10.3389/fonc.2021.783480
31. Chung SM, Yoon CJ, Lee SS, et al. Treatment outcomes of transcatheter arterial chemoembolization for hepatocellular carcinoma that invades hepatic vein or inferior vena cava. Cardiovasc Intervent Radiol. 2014;37(6):1507–1515. doi:10.1007/s00270-014-0841-1
32. Pal SK, McDermott DF, Atkins MB, et al. Patient-reported outcomes in a Phase 2 study comparing atezolizumab alone or with bevacizumab vs sunitinib in previously untreated metastatic renal cell carcinoma. BJU Int. 2020;126(1):73–82. doi:10.1111/bju.15058
33. Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38(26):2960–2970. doi:10.1200/JCO.20.00808
34. Xu J, Shen J, Gu S, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): a nonrandomized, open-label, phase II trial. Clin Cancer Res. 2021;27(4):1003–1011. doi:10.1158/1078-0432.CCR-20-2571
35. Lai Z, He M, Bu X, et al. Lenvatinib, toripalimab plus hepatic arterial infusion chemotherapy in patients with high-risk advanced hepatocellular carcinoma: a biomolecular exploratory, phase II trial. Eur J Cancer. 2022;174:68–77. doi:10.1016/j.ejca.2022.07.005
36. Finn RS, Kudo M, Merle P. Primary results from the Phase 3 LEAP-002 study: lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2022;33:S1401. doi:10.1016/j.annonc.2022.08.031
37. Cheu JW, Wong CC. Mechanistic rationales guiding combination hepatocellular carcinoma therapies involving immune checkpoint inhibitors. Hepatology. 2021;74(4):2264–2276. doi:10.1002/hep.31840
38. Wu JY, Yin ZY, Bai YN, et al. Lenvatinib combined with anti-PD-1 antibodies plus transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: a multicenter retrospective study. J Hepatocell Carcinoma. 2021;8:1233–1240. doi:10.2147/JHC.S332420
39. Allen E, Jabouille A, Rivera LB, et al. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci Transl Med. 2017;9(385):eaak9679. doi:10.1126/scitranslmed.aak9679
40. Yi C, Chen L, Lin Z, et al. Lenvatinib targets FGF receptor 4 to enhance antitumor immune response of anti-programmed cell death-1 in HCC. Hepatology. 2021;74(5):2544–2560. doi:10.1002/hep.31921
41. Sangro B, Sarobe P, Hervás-Stubbs S, et al. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18(8):525–543. doi:10.1038/s41575-021-00438-0
42. Llovet JM, Castet F, Heikenwalder M, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19(3):151–172.
43. Zhang Y, Lu L, He Z, et al. C-reactive protein levels predict responses to PD-1 inhibitors in hepatocellular carcinoma patients. Front Immunol. 2022;13:808101. doi:10.3389/fimmu.2022.808101
44. Scheiner B, Pomej K, Kirstein MM, et al. Prognosis of patients with hepatocellular carcinoma treated with immunotherapy - development and validation of the CRAFITY score. J Hepatol. 2022;76(2):353–363. doi:10.1016/j.jhep.2021.09.035
45. Ichikawa K, Watanabe S, Miura S, et al. Prognostic significance of procalcitonin in small cell lung cancer. Transl Lung Cancer Res. 2022;11(1):43–52. doi:10.21037/tlcr-21-838
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.