Back to Journals » Journal of Pain Research » Volume 16
Is Exercise Rehabilitation an Effective Adjuvant to Clinical Treatment for Myofascial Trigger Points? A Systematic Review and Meta-Analysis
Authors Zhou Y, Lu J, Liu L, Wang HW
Received 25 October 2022
Accepted for publication 17 January 2023
Published 28 January 2023 Volume 2023:16 Pages 245—256
DOI https://doi.org/10.2147/JPR.S390386
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Alaa Abd-Elsayed
Yu Zhou,1 Jiao Lu,2 Lin Liu,2 Hao-Wei Wang2
1School of Sports Sciences, Nanjing Normal University, Nanjing, Jiangsu, People’s Republic of China; 2School of Sport and Health, Nanjing Sport Institute, Nanjing, Jiangsu, People’s Republic of China
Correspondence: Lin Liu, School of Exercise and Health, Nanjing Sport Institute, Linggusi Road No. 8, Nanjing, 210014, People’s Republic of China, Tel +86 18817873543, Email [email protected]
Purpose: To systematically evaluate the effect of exercise rehabilitation as an adjuvant to clinical treatment for myofascial trigger points (MTrPs).
Patients and Methods: ESBCO, PubMed, Science Direct, Web of Science, China Knowledge Network (CNKI), and Wanfang databases were comprehensively searched from database inception date through July 2022. Randomized controlled trials comparing MTrPs treatments that included exercise rehabilitation with a single clinical treatment. Two researchers independently screened articles using inclusion/exclusion criteria, scored methodologic quality, and extracted data including patient demographics, interventions, and outcomes.
Results: We included 14 RCTs (N = 734). Results showed short-term (mean difference [MD], − 2.25; 95% confidence interval [CI], − 4.08 to − 0.41; Z = 2.40; P = 0.02) and long-term (MD = − 0.47; 95% CI: − 0.80 to − 0.17; Z = 3.05; P = 0.02) adjuvant exercise rehabilitation treatments were superior in reducing musculoskeletal pain intensity to single clinical treatment in controls, but long-term versus short-term effectiveness was not significantly different. The exercise rehabilitation group more effectively increased the range of motion (ROM) (standardized mean difference [SMD], 1.04; 95% CI: 0.32 to 1.77; Z = 2.84; P = 0.005) and decreased dysfunction (SMD = − 0.93; 95% CI: − 1.82 to − 0.05; Z = 2.06; P = 0.04) than controls; no significant difference was observed in the pressure pain threshold (PPT) between two groups.
Conclusion: Exercise rehabilitation as an adjuvant to clinical treatment for MTrPs was moderately effective in relieving pain intensity, increasing ROM, and improving dysfunction versus single clinical intervention. These findings must be validated by larger, higher-quality studies.
Keywords: trigger points, exercise, rehabilitation, meta-analysis, randomized controlled trial
Introduction
Myofascial trigger points (MTrPs) are hypersensitive nodules of contracture that are palpable to affected muscles and produce localized pain in and around the affected muscle or trigger distant referred pain.1 The trigger point theory, which comprises the concepts of potential MTrPs and activated MTrPS, was proposed in 1942 by the American clinical professor Janet Travell.2 In clinical, MTrPs were usually diagnosed by the gold standard of the presence of discrete focal tenderness located in a palpable taut band, which produces both referred pain and a local twitch response.2 Limited joint range of motion (ROM), skeletal muscle pain, and fatigue are associated with the development of myofascial pain syndrome (MPS) but effective deactivation of MTrPs is the most important treatment for MPS.3 Although the exact prevalence of MPS in the general population and between sexes has rarely been described in the literature, some researchers estimate 30–85% of musculoskeletal pain is due to MPS, which is most common in patients aged 27–50 years.4 In addition, myofascial pain has a variable presentation and several studies have determined the prevalence of MPS in multiple patient types. A recent prospective study showed the prevalence of MPS in 126 patients with chronic, non-specific neck pain was 88.9%.5 Another study found 51.9% of 137 patients treated for multiple sclerosis had been diagnosed with MPS.6 Therefore, the development of strategies for relief of pain related to MTrPs is a critical public health issue.
Relevant controlled studies have demonstrated the effectiveness of consensus clinical treatment strategies for MTrPs including dry needling,7 myofascial release therapy,8 ultrasound therapy,9 extracorporeal shock wave therapy,10 and ischemic compression techniques.11 Recently, exercise rehabilitation interventions have been proposed as a treatment modality for MTrPs because they are safe, non-invasive, non-pharmacological, and low-cost. Exercise interventions may include aerobic, stretching, or strength exercises or some combination of these types of activities. Exercise interventions can induce hyperalgesia and increase pressure pain thresholds (PPTs) by decreasing central sensitization, resulting in multi-segmental nociceptive inhibition while muscle contraction facilitates the discharge of sensitizing substances from the MTrPs micro-environment, thereby reducing the central and peripheral sensitizing substances that cause local or referred pain.12 Kalamir et al13 compared the effects of intraoral myofascial therapy plus self-exercise with a single intraoral myofascial therapy intervention for mandibular joint ROM and pain at a one-year follow-up and found significant differences between the two groups and noted the superiority of the combined intervention. However, Wilke et al14 did not observe differences in short-term (30 min post-treatment) MTrPs-related neck pain, mechanical pain threshold, and ROM between patients who received trigger point acupuncture plus stretching and those with single-intervention acupuncture treatment; all outcome measures were significantly improved in both groups compared to controls.
To date, although one systematic review and Meta-analysis15 had reported the effectiveness of various types of physical exercise programs for MTrPs, which only considered a single physical exercise as the primary intervention mode compared with the non-physical exercise group, clinical treatment of MTrPs typically begins with the use of acupuncture, dry needling, and physical therapy techniques to release contracture nodes in small areas with precision, followed by exercise prescriptions for implementation to relax the involved muscle groups and the whole body muscle groups. Clinical treatment supplemented by exercise may achieve more effective inactivation of MTrPs, relaxation of muscles, and enhancement of the healing effect, but it is still controversial. Meanwhile, only three databases were searched in this study, and a more thorough search is required for statistical analysis. Therefore, in our study, we deeply and comprehensively explore the clinical effect of exercise rehabilitation training as an adjuvant to other treatment modalities for MTrPs. We also discuss the possibility of the synergistic effects of exercise rehabilitation with clinical treatment modalities for MTrPs.
Materials and Methods
Search Strategy
Electronic databases such as EBSCO, PubMed, Science Direct, Web of Science, CNKI, and Wanfang were searched from database inception date to July 2022 for randomized controlled trials (RCTs) on the effects of adding exercise rehabilitation training to clinical treatment for patients with MTrPs. The search strategy prioritized the following combinations of MeSH and entry terms: (1) MTrPs or MPS; (2) exercise or motor activity; (3) clinical treatment; and (4) allocation or random (sampling). The details of the search strategy are presented in Appendix S1.
Inclusion and Exclusion Criteria
We used the following inclusion criteria for study selection: (1) Study design: RCT; (2) Participants: patients (regardless of sex or race) with MTrPs confirmed by expert diagnosis who had voluntarily participated in the study and signed informed consent forms; (3) Intervention: the control group had received a single-intervention clinical treatment for MTrPs (including dry acupuncture therapy, ultrasound therapy, extracorporeal shock wave therapy, and ischemic compression) and the experimental group had received the same single intervention as the control group, as well as an exercise rehabilitation program (including aerobic, stretching, or strength training exercises, or some combination of exercise types); (4) Main outcome measures: A) Pain intensity, assessed by visual analog scale; B) PPT; C) ROM; and D) Dysfunction, assessed by the Oswestry Disability Index, Neck Disability Index and Constant–Murley Scale.
We used the following exclusion criteria: (1) non-RCTs, such as reviews, case reports, and retrospective studies; (2) duplicate publications; (3) literature with non-compliant diagnostic criteria, interventions, or outcome indicators; (4) literature with full text that could not be obtained through various standard channels of inquiry; and (5) studies of poor quality or those with an uncritical design.
Study Selection and Data Extraction
Two researchers independently screened the literature according to the inclusion and exclusion criteria. Duplicate studies were eliminated, and the titles and abstracts of the remaining articles were screened using the exclusion criteria. Finally, the full text of each article was read to ensure inclusion criteria had been met. The independent researchers periodically reviewed the extracted data, and discussed conflicting results or submitted them to a third party for arbitration to reach a consensus. The extracted data included the first author, publication time, study method, sample size, intervention measures, intervention frequency, outcome indicators, and measurement duration of the outcome indicators. If the original research data were incomplete, we emailed the corresponding author to supplement the missing information and the study was excluded if we were unsuccessful in obtaining a response.
Quality Assessment
The quality of the literature was evaluated using the RCT risk of bias (RoB) assessment tool recommended by the Cochrane Handbook.16 The main RoB domains include: (1) random assignment methods; (2) allocation protocol concealment; (3) blinding of study participants and personnel; (4) blinding of study outcome measures; (5) completeness of outcome data; (6) selective reporting of study results; and (7) other sources of bias. Each domain was divided into three levels: “low risk of bias”, “unclear risk”, and “high risk of bias”, and we represented the evaluation results using a risk of bias graph.
Statistical Analysis
Meta-analysis of the data extracted from the included literature was performed using Review Manager 5.4. A heterogeneity analysis between the results of the included studies was first performed using the chi-square test. When I2 ≤ 50% and P ≥ 0.10, homogeneity among the results was low and considered acceptable and the fixed-effects model was used; when I2 > 50% and/or P < 0.10, heterogeneity among the results was considered high, and sensitivity analysis was further used to determine the source of heterogeneity among the study results and if possible, reduce it. If the heterogeneity could still not be excluded, the random-effect model was used for the combined analysis. For continuous data, the mean difference (MD) and its 95% confidence interval (CI) were used, while the standardized mean difference (SMD) was used to describe continuous variables with different units of measurement and large differences in means. The Z-test was used to investigate pooled statistics for outcome indicators, and the probability P-value was calculated based on the Z-value. If P ≤ 0.05, the combined statistic was significant; if P > 0.05, the combined statistic has no significance. The Begg–Mazumdar rank correlation test were used to evaluate the risk of publication bias. Finally, the Grading of Recommendations in Assessment, Development, and Evaluation (GRADE) system was used to assess the quality of evidence on the effectiveness of adding exercise rehabilitation training to single clinical treatment regimens for MTrPs.
Results
Study Selection
A total of 1211 articles were initially identified from the ESBCO, PubMed, Science Direct, Web of Science, CNKI, and Wanfang electronic databases. We then removed the duplicates and screened the records. Twenty-seven full-text articles were reviewed for eligibility and ultimately, 14 studies17–30 met the eligibility criteria, with a total of 734 participants in the systematic evaluation. The flow chart for the literature search and its results are shown in Figure 1.
 |
Figure 1 Flowchart of search strategy and results. Abbreviations: MTrPs, myofascial trigger points; MPS, myofascial pain syndrome. Notes: Figure 1 adapted from Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.31 |
Study Characteristics
See Table 1 for details.
 |
Table 1 Characteristics of Included Studies |
Quality Evaluation of the Included Studies
All 14 studies used random assignment sampling, three provided further description on allocation concealment, only one involved double-blinding, and no studies mentioned the blinding of outcome evaluators. Further, we only included studies with complete datasets and the Cochrane RoB assessment showed the overall quality of the literature met the requirements. The quality evaluation of the included studies is shown in Figures 2 and 3.
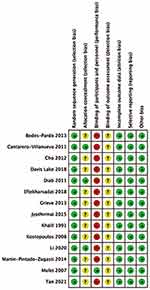 |
Figure 2 Risk assessment of bias in RCT. |
 |
Figure 3 Overall risk assessment of bias in RCT. |
Meta-Analysis Results
Pain Intensity
Eight studies17,19–22,24,27,28 involving 474 patients reported post-intervention changes in pain scores in patients with MTrPs. The effects of short-term (< 4 wks) and long-term (≥ 4 wks) interventions on pain intensity in patients with MTrPs were classified based on the duration of intervention between studies. A pooled analysis of the heterogeneous data showed a high heterogeneity among the study results for the short-term intervention (I2 = 94%, P < 0.00001); therefore, single studies were excluded one at a time for further sensitivity analysis. The heterogeneity did not change significantly after any of the literature was excluded, so a random-effects model was used for the meta-analysis. As shown in Figure 4A, the combined effect size under the random-effects model was −2.25, (95% CI: −4.08 to −0.41, Z = 2.40, P = 0.02), indicating a significantly better effect of the short-term intervention on musculoskeletal pain in the experimental group compared to the control group.
The χ2 test showed no statistical heterogeneity among the results of the studies of long-term intervention (I2 = 0%, P = 0.64), so a fixed-effects model was used for the meta-analysis. As shown in Figure 4B, we found a significant improvement in musculoskeletal pain for the long-term intervention in the experimental group versus the control group (MD = −0.49, 95% CI: −0.80 to −0.17, Z = 3.05, P = 0.02).
Pressure Pain Threshold
A total of five studies17,20,22,24,28 involving 238 patients reported post-intervention changes in PPTs in patients with MTrPs. The data from the pooled studies in the random-effects model (Figure 5A) demonstrated no significant effects of clinical treatment plus exercise rehabilitation in the improvement of PPT compared with single clinical treatments (I2 = 64%, SMD = 0.31, 95% CI: −0.14 to 0.77, Z = 1.35, P = 0.18).
Range of Motion
Five studies17,22,25,27,29 reported post-intervention changes in ROM in patients with MTrPs, with a total of 134 patients in the experimental group (n = 67) and the control group (n = 67). There was statistical heterogeneity between the findings by χ2 test (I2 = 69%, P = 0.004), so the meta-analysis was performed using a random-effects model. As shown in Figure 5B, the combined effect size under the random-effects model was 1.04 (95% CI: 0.32 to 1.77, Z = 2.84, P = 0.005), indicating a significant difference in the increased ROM of patients with MTrPs. Due to the high heterogeneity among studies (I2 = 69%), the included studies were excluded one-by-one for sensitivity analysis. After we excluded a 2012 article by Cho,22 the heterogeneity was significantly reduced (I2 = 28%, P = 0.23), and further meta-analysis using a fixed-effects model showed significant differences in ROM between groups (SMD = 1.25, 95% CI: 0.72 to 1.78, P < 0.00001).
Dysfunction
Five studies21–24,26 reported post-intervention changes in functional impairment in patients with MTrPs, with a total of 186 patients in the experimental group (n = 93) and the control group (n = 93). There was high statistical heterogeneity among the results of these studies by the χ2 test (I2 = 86%, P < 0.00001), and heterogeneity was not reduced by the single-study exclusion method. Hence, a random-effects model was used for the combined analysis. As shown in Figure 5C, the combined effect size under the random-effects model was −0.93, (95% CI: −1.82 to −0.05, Z = 2.06, P = 0.04), indicating a significant difference in improved functional impairment in patients with MTrPs after an exercise rehabilitation intervention.
Sensitivity Analysis and Publication Bias
Sensitivity analysis was performed on the same set of data for each outcome using two effect models. The results showed great differences in the outcome indicator of PPT after the interchange of effect models, indicating that the small sample study had a greater impact on the combined effect size, and the Meta-analysis results should be used with caution in clinical practice. The differences in the data of short-term and long-term pain intensity, ROM, and dysfunction were small, indicating that the small sample study had little impact on the combined effect size, and the results of the meta-analysis were relatively stable, see Table 2. Next, we used the Begg–Mazumdar test to assess publication bias for the outcome indicators of pain intensity, PPT, ROM, and dysfunction and the resulting P values for the effect of adding exercise rehabilitation training to clinical treatment of MTrPs on pain intensity, PPT, ROM, and dysfunction were 0.206, 0.488, 0.138, and 0.370, respectively (all P > 0.05), suggesting no publication bias.
 |
Table 2 Sensitivity Analysis of the Effectiveness Comparison Results of Different Outcome Indicators |
Systematic Recommendation Grading
Based on the results of meta-analysis and methodological quality evaluation, the GRADE system was applied to grade each outcome index. We found that the quality of evidence was intermediate for the pain intensity and dysfunction indices and low for the PPT and ROM indices. The grading results are shown in Supplemental Figure S1.
Discussion
The Necessity for the Study and Its Evidence
In recent years, with the accelerated pace of life, sedentary behavior is a contributing factor to MPS.32 The long and indolent course of MPS aggravates its medical burden, and exercise interventions have been proposed for their proactivity, safety, and acceptability to patients. The clinical application of active exercise in MTrPs not only alleviates the burden of medical resources but also reflects the modern, holistic value of the “physical medicine integration” approach to healthcare. There is still a paucity of research on whether enhanced efficacy in clinical MPS treatment can be achieved with exercise rehabilitation as an adjuvant to other clinical interventions. The results of this meta-analysis showed the addition of exercise rehabilitation interventions to single-intervention clinical treatment significantly improved pain intensity, PPT, ROM, and functional impairment in patients with MTrPs compared to single-intervention clinical treatment. However, we saw no evidence for the superiority of clinical interventions containing exercise rehabilitation in reducing PPT in patients with MTrPs.
Effect Analysis and Physiological Mechanisms of Adjuvant Exercise Rehabilitation
Our findings suggest that the addition of exercise rehabilitation training to clinical treatment is effective in reducing pain intensity, increasing ROM, and improving dysfunction in patients with MTrPs. These findings are consistent with those of previous studies reporting the benefits of combined interventions. Dembowski et al33 treated a pole vaulter with a hamstring injury with MTrPs dry needle therapy combined with centrifugal exercise training, and found that the athlete’s pain intensity and functional status were significantly improved and could be fully restored to the pre-training, pain-free state. The muscle strength of the trained side was higher than that of the opposite side, and the injury did not relapse. Zhang et al34 adopted acupuncture therapy with stretching techniques when inactivating knee trigger points. The sensitivity of activated MTrPs was controlled, while the strong acupuncture sensation was reduced, which both consolidated the efficacy and increased knee joint ROM. The results of a double-blinded RCT showed the use of acupuncture and sham acupuncture with exercise to treat patients with knee osteoarthritis with thigh MTrPs in the short term was beneficial in improving their pain and dysfunction.35
Despite these promising results, the physiological mechanisms underlying the additive effect of exercise rehabilitation to the clinical treatment of MTrPs are not clear. Due to their contracted state, MTrPs receive an inadequate supply of oxygen and nutrients from the blood, which are necessary for energy production and muscle relaxation. The reduction of oxygen and nutrient delivery prolongs the contracted state within the MTrPs. The pathological tissue changes caused by this condition stimulate the release of neurovascularly active substances from the vasculature into the tissue interstitium, where various nerve endings and receptors are sensitized by a variety of active substances, which are transported through afferent nerve fibers to the center of the contraction, producing pain and autonomic responses at trigger points.36 The application of clinical techniques inactivates trigger points, relaxes contracture nodes within the muscle, and puts the musculoskeletal system in a state of equilibrium while the implementation of different exercise types adds to the therapeutic effect.37 Exercise may increase the blood supply and metabolic substrate of MTrPs through the mechanical displacement of muscle fibers. Intensive and aerobic exercise may lead to an increase in local blood pressure, improving both blood flow to the resistance site and vascular bed resistance. Rice et al38 suggested aerobic exercise achieves its effect by increasing blood flow, blood pressure, and oxygen saturation, allowing more blood and metabolic substrates to enter MTrPs. In addition, aerobic exercise helps to prevent central sensitization by its ability to reduce circulating levels of pro-inflammatory markers such as interleukin (IL)-6 and IL-8 to normal levels and to reduce substance P. Exercise also promotes the production of anti-inflammatory cytokines such as IL-10, enhances the release of endogenous opioids, catecholamines, and endorphins, and reduces pain. Stretching exercises improve blood flow and energy metabolism in muscles while reorganizing the cellular structure of muscle fibers.39 Notably, premature exercise may enhance muscle tension, so well-timed, moderate, repetitive training is needed to consolidate treatment effects.
Although most of the relevant studies that included PPTs as an outcome indicator reported positive effects of adjuvant exercise rehabilitation on improving pain, pooled estimates did not reach statistical significance. More effective exercise rehabilitation programs may require longer interventions and follow-up to observe changes in patients’ PPTs. Tan et al21 found the recurrence rate of low back pain was 8.70% in the experimental group and 19.05% in the control group by following up with patients for six months after treatment. The study showed MTrPs acupuncture combined with suspension exercise therapy was more effective than treatment with MTrPs acupuncture alone in reducing chronic lower back pain, increasing pain threshold, and reducing recurrence rate. They posited their treatment was effective because the MTrPs were first needled to weaken hyperactive muscle activity so that the majority of symptoms were quickly relieved. Next, suspension exercise training was performed to further reduce the activity of hyperactive muscles in order that inactive muscles were activated and the sensitivity and responsiveness of the body’s self-perception were improved, thus improving the long-term efficacy and reducing the recurrence rate. However, Eftekharsadat et al24 found that both acupuncture alone and aerobic exercise combined with acupuncture were effective in the treatment of MPS, with significant improvements in pain, mechanical PPTs, neck disability index, and quality of life in both groups, but the differences between the two groups were not significant and there was no superiority between the two approaches.
This meta-analysis has certain limitations. First, we found few eligible studies with limited sample sizes and a large amount of heterogeneity. Second, gray literature sources were not included, and the selection of studies evaluating the effectiveness of clinical treatment techniques for interventions with MTrPs containing exercise rehabilitation may not have been complete. Third, the lack of uniform diagnostic criteria for MTrPs and the subjectivity of the examiners may muddy the diagnosis of MTrPs. Moreover, variations in clinical treatment modality, exercise type, and exercise frequency among studies and inconsistent measurement of various outcome indicators may have contributed to heterogeneity.
Conclusion
Current evidence demonstrates that clinical interventions incorporating exercise rehabilitation have a positive impact on patients with MTrPs and are superior to single interventions, and that exercise can be used as a complementary alternative therapy to provide a synergistic treatment effect and consolidate long-term outcomes. The superimposed effect of exercise rehabilitation on clinical interventions can be used as a reference point for treatment of MTrPs. Since the specific clinical treatment modality, type of exercise, duration, intensity, and frequency of exercise varied among studies included in this meta-analysis, more high-quality and large-sample RCTs are needed to validate our findings.
Abbreviations
CI, confidence interval; CNKI, China national knowledge infrastructure; MD, mean difference; MPS, myofascial pain syndrome; MTrPs, myofascial trigger points; PPT, pressure pain threshold; RCTs, randomized controlled trials; ROM, range of motion; SMD, standardized mean difference.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was funded by the Natural Science Foundation of the Jiangsu higher Education Institutions of China (grant number 19KJB310006), the National Natural Science Foundation of China (grant number 32000829) and “Qinglan Project” of the Jiangsu higher Education Institutions of China.
Disclosure
The authors have no conflicts of interest to declare.
References
1. Zheng B, Zhu J, Zhao L, et al. Advances in the assessment of myofascial trigger points. Chin J Rehabil Med. 2021;37(01):117–120.
2. Simons DG, Travell JG, Simons LS. Myofascial pain and dysfunction: the trigger point manual. In: Johnson E, Napora L, editors. Pain Patterns. Maryland: Lippincott Williams & Wilkins; 1983:398–418.
3. Ahmed S, Khattab S, Haddad C, et al. Effect of aerobic exercise in the treatment of myofascial pain: a systematic review. J Exerc Rehabil. 2018;14:902–910. doi:10.12965/jer.1836406.205
4. Tantanatip A, Chang KV. Myofascial Pain Syndrome. Treasure Island (FL): StatPearls Publishing; 2022.
5. Ezzati K, Ravarian B, Saberi A, et al. Prevalence of cervical myofascial pain syndrome and its correlation with the severity of pain and disability in patients with chronic non-specific neck pain. Arch Bone Jt Surg. 2021;9:230–234. doi:10.22038/abjs.2020.48697.2415
6. Tutuncu M, Ertem DH, Soysal A. Prevalence and impact of myofascial pain syndrome in relapsing-remitting multiple sclerosis and the effects of local anesthetic injections for short-term treatment. Mult Scler Relat Disord. 2020;46:102528. doi:10.1016/j.msard.2020.102528
7. Kietrys D, Palombaro K, Azzaretto E, et al. Effectiveness of dry needling for upper-quarter myofascial pain: a systematic review and meta-analysis. J Orthop Sports Phys Ther. 2013;43(9):620–634. doi:10.2519/jospt.2013.4668
8. Laimi K, Makila A, Barlund E, et al. Effectiveness of myofascial release in treatment of chronic musculoskeletal pain: a systematic review. Clin Rehabil. 2018;32:440–450. doi:10.1177/0269215517732820
9. Xia P, Wang X, Lin Q, et al. Effectiveness of ultrasound therapy for myofascial pain syndrome: a systematic review and meta-analysis. J Pain Res. 2017;10:545–555. doi:10.2147/JPR.S131482
10. Yoo JI, Oh MK, Chun SW, et al. The effect of focused extracorporeal shock wave therapy on myofascial pain syndrome of trapezius: a systematic review and meta-analysis. Medicine. 2020;99:e19085. doi:10.1097/MD.0000000000019085
11. Li X, Chen N, Yang J, et al. Efficacy of ischemic compression at the provocation point in the treatment of cervical and shoulder myofascial pain syndrome. J Rehabil. 2020;30:140–144.
12. Stroth S, Hille K, Spitzer M, et al. Aerobic endurance exercise benefits memory and affect in young adults. Neuropsychol Rehabil. 2009;19(2):223–243. doi:10.1080/09602010802091183
13. Kalamir A, Bonello R, Graham P, et al. Intraoral myofascial therapy for chronic myogenous temporomandibular disorder: a randomized controlled trial. J Manipulative Physiol Ther. 2012;35(1):26–37. doi:10.1016/j.jmpt.2011.09.004
14. Wilke J, Vogt L, Niederer D, et al. Short-term effects of acupuncture and stretching on myofascial trigger point pain of the neck: a blinded, placebo-controlled trial. Complement Ther Med. 2014;22:835–841. doi:10.1016/j.ctim.2014.09.001
15. Guzmán-Pavón MJ, Cavero-Redondo I, Martínez-Vizcaíno V, et al. Effect of physical exercise programs on myofascial trigger points-related dysfunctions: a systematic review and meta-analysis. Pain Med. 2020;21(11):2986–2996. doi:10.1093/pm/pnaa253
16. Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi:10.1136/bmj.d5928
17. Bodes-Pardo G, Pecos-Martin D, Gallego-Izquierdo T, et al. Manual treatment for cervicogenic headache and active trigger point in the sternocleidomastoid muscle: a pilot randomized clinical trial. J Manipulat Physiol Ther. 2013;36(7):403–411. doi:10.1016/j.jmpt.2013.05.022
18. Cantarero-Villanueva I, Fernandez-Lao C, Del MR, et al. Effectiveness of core stability exercises and recovery myofascial release massage on fatigue in breast cancer survivors: a randomized controlled clinical trial. Evid Based Complement Alternat Med. 2012;2012:620619. doi:10.1155/2012/620619
19. Diab AA, Moustafa IM. The efficacy of forward head correction on nerve root function and pain in cervical spondylotic radiculopathy: a randomized trial. Clin Rehabil. 2012;26:351–361. doi:10.1177/0269215511419536
20. Martin-Pintado ZA, Rodriguez-Fernandez AL, Garcia-Muro F, et al. Effects of spray and stretch on postneedling soreness and sensitivity after dry needling of a latent myofascial trigger point. Arch Phys Med Rehabil. 2014;95:1925–1932. doi:10.1016/j.apmr.2014.05.021
21. Tan Y, Liu C. Efficacy of acupuncture myofascial trigger points combined with suspension exercise therapy in the treatment of chronic lower back pain. J Pract Tradit Chin Med. 2021;37:1586–1588.
22. Cho YPS, Jang S, Choi Y, et al. Effects of the combined treatment of extracorporeal shock wave therapy (ESWT) and stabilization exercises on pain and functions of patients with myofascial pain syndrome. J Phys Ther Sci. 2012;24(12):1319–1323. doi:10.1589/jpts.24.1319
23. Davis Lake A, Myers H, Aefsky B, et al. Immediate and short term effect of dry needling on triceps surae range of motion and functional movement: a randomized trial. Int J Sports Physic Ther. 2018;13(2):185–195. doi:10.26603/ijspt20180185
24. Eftekharsadat B, Porjafar E, Eslamian F, et al. Combination of exercise and acupuncture versus acupuncture alone for treatment of myofascial pain syndrome: a randomized clinical trial. J Acupunct Meridian Stud. 2018;11:315–322. doi:10.1016/j.jams.2018.04.006
25. Grieve R, Cranston A, Henderson A, et al. The immediate effect of triceps surae myofascial trigger point therapy on restricted active ankle joint dorsiflexion in recreational runners: a crossover randomised controlled trial. J Bodyw Mov Ther. 2013;17(4):453–461. doi:10.1016/j.jbmt.2013.02.001
26. Jyothirmai B, Senthil Kumar K, Raghavkrishna S, et al. Effectiveness of integrated neuromuscular inhibitory technique (INIT) with specific strength training exercises in subjects with upper trapezius trigger points. Int J Phys. 2015;2(5):759–763.
27. Khalil TM, Asfour SS, Martinez LM, et al. Stretching in the rehabilitation of low-back pain patients. Spine. 1992;17:311–317.
28. Kostopoulos D, Nelson AJ, Ingber RS, et al. Reduction of spontaneous electrical activity and pain perception of trigger points in the upper trapezius muscle through trigger point compression and passive stretching. J Musculoskelet Pain. 2009;16(4):266–278. doi:10.1080/10582450802479594
29. Li L, Huang F, Huang Q, et al. Compression of myofascial trigger points with a foam roller or ball for exercise-induced anterior knee pain: a randomized controlled trial. Altern Ther Health Med. 2020;26:16–23.
30. Mulet M, Decker KL, Look JO, et al. A randomized clinical trial assessing the efficacy of adding 6 x 6 exercises to self-care for the treatment of masticatory myofascial pain. J Orofac Pain. 2007;21:318–328.
31. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71
32. Xue Y. Current status of research on static behavior in patients with chronic diseases. J Tianjin Nurs. 2021;29:613–616.
33. Dembowski S, Westrick R, Zylstra E, et al. Treatment of hamstring strain in a collegiate pole-vaulter integrating dry needling with an eccentric training program: a resident's case report. Int J Sports Phys Ther. 2013;8(3):328–339.
34. Zhang H, Huang QM, Nguyen TT, et al. Clinical observation of effectiveness in the treatment of senile knee osteoarthritis with the inactivation of myofascial trigger points--108 cases reports. Zhongguo Gu Shang. 2016;29:782–786. doi:10.3969/j.issn.1003-0034.2016.09.002
35. Sánchez-Romero EA, Pecos-Martín D, Calvo-Lobo C, et al. Effects of dry needling in an exercise program for older adults with knee osteoarthritis. Medicine. 2018;97(26):e11255. doi:10.1097/MD.0000000000011255
36. Kuan T, Chen J, Chen S, et al. Effect of botulinum toxin on endplate noise in myofascial trigger spots of rabbit skeletal muscle. Am J Phys Med Rehabil. 2002;81(7):512–520. doi:10.1097/00002060-200207000-00008
37. Huang Q, Ao L, Liu Y. Point analysis of myofascial trigger point pain characteristics. Chin J Clin Rehabil. 2004;48:22–24.
38. Rice D, Nijs J, Kosek E, et al. Exercise-induced hypoalgesia in pain-free and chronic pain populations: state of the art and future directions. J Pain. 2019;20:1249–1266. doi:10.1016/j.jpain.2019.03.005
39. Majlesi J, Unalan H. Effect of treatment on trigger points. Curr Pain Headache Rep. 2010;14:353–360. doi:10.1007/s11916-010-0132-8
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


