Back to Journals » ImmunoTargets and Therapy » Volume 13
Inhibition Effect of Pancreatic Exocrine Insufficiency on Immune Checkpoint Inhibitor Treatment in Pancreatic Cancer: A Retrospective Study
Authors Luo Q , Dong Y, Liu P, He C, Chen L, Zhang K, Pan C, Gao Y, Qin T
Received 27 September 2023
Accepted for publication 26 January 2024
Published 1 February 2024 Volume 2024:13 Pages 45—54
DOI https://doi.org/10.2147/ITT.S442247
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sarah Wheeler
Qiankun Luo,1,* Yifei Dong,1,* Pan Liu,1,* Chao He,2 Lei Chen,1 Kailun Zhang,1 Changjie Pan,1 Yahui Gao,1 Tao Qin1
1Department of Hepatobiliary and Pancreatic Surgery, Zhengzhou University People’s Hospital, Henan Provincial People’s Hospital, Zhengzhou, Henan, People’s Republic of China; 2Department of Hepatobiliary and Pancreatic Surgery, Henan University People’s Hospital, Henan Provincial People’s Hospital, Zhengzhou, Henan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Tao Qin, Department of Hepatobiliary and Pancreatic Surgery, Zhengzhou University People’s Hospital, Henan Provincial People’s Hospital, No. 7, Weiwu Road, Jinshui District, Zhengzhou, Henan, 450003, People’s Republic of China, Tel +86 13949116088, Email [email protected]
Introduction: Chemotherapy combined with immune checkpoint inhibitors (ChIM) is used to treat advanced pancreatic ductal adenocarcinoma (PDAC). However, the efficacy of ChIM is similar to that of chemotherapy alone.
Methods: To assess potential factors affecting the effectiveness of ChIM, we analyzed the clinical data of 359 patients with PDAC who visited the hospital during June 2017 to December 2022.
Results: Surgical resection, diabetes, and ChIM were risk factors for pancreatic exocrine insufficiency (PEI). The adjusted odds ratio of ChIM was 2.63 (95% confidence interval (CI) 1.492– 4.626) (P = 0.001). The incidence of PEI in the ChIM group (76.9%) was significantly higher than that of the chemotherapy group (60.2%) (P = 0.004). Survival analysis showed that ChIM did not improve the survival rate of patients with PDAC (hazard ratio (HR) 0.92, 0.707– 1.197) (P = 0.534) in comparison with that of the chemotherapy group. However, in patients without PEI, those receiving ChIM showed a higher 1-year overall survival (OS) rate of 70.8% (two-sided, P = 0.045) and a median OS of 22.0 months (95% CI 11.5– 32.5). Moreover, pancreatic enzyme replacement therapy significantly improved the OS of patients with PDAC (HR = 0.73, 95% CI = 0.561– 0.956) (P = 0.022).
Conclusion: Immune checkpoint inhibitors (ICIs) increased the incidence of PEI in patients with PDAC. The OS was not different between patients receiving chemotherapy and ChIM due to irregular PERT treatment. The finding show that pancreatic enzyme replacement therapy may improve the response rate of patients with PDAC to ICIs.
Keywords: pancreatic cancer, pancreatic exocrine insufficiency, immune checkpoint inhibitors, pancreatic enzyme replacement therapy
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most challenging digestive tract malignancies. The 5-year survival rate of patients with the condition is less than 11%.1 Surgical resection is the main technique for radical treatment of PDAC. However, more than 80% of patients cannot receive surgical resection due to local advancement and metastasis. Therefore, systemic treatments, especially chemotherapy, are the main strategies to improve survival in patients with PDAC. However, chemotherapy has a limited response in this patient population.2
In recent years, immune checkpoint inhibitors (ICIs) have produced noticeable improvements in the treatment of cancer patients.3,4 However, treatment with single-agent ICIs has shown disappointing results in PDAC.5 Researchers considered that the poor clinical outcomes may be attributed to the complex immunosuppressive tumor microenvironment and intrinsically non-immunogenetic features of PDAC.6 To this end, novel systemic treatments are urgently required. A few studies have reported that chemotherapy combined with immunotherapy (ChIM) increases the response rates of patients with PDAC,7,8 while others showed similar efficacies between chemotherapy alone and ChIM treatment.9,10 Therefore, elucidating the factors that contribute to the poor response of patients with PDAC to ChIM is an urgent need.
Increasing evidence shows that ICIs have adverse effects on the pancreas and increase the morbidity of diabetes mellitus, pancreatitis, and pancreatic exocrine insufficiency (PEI),11,12 which results in poor quality of life and prognosis. Notably, PEI-induced malnutrition has been confirmed to have a significant effect on quality of life, overall survival (OS), and tumor progression in patients with PDAC.13 Pancreatic enzyme replacement therapy (PERT) is widely used in the clinical treatment of patients with PEI and plays an important role in the improvement of OS.14 However, there is no study reporting whether PEI affects the efficacy of ChIM in PDAC.
Here, we report clinical data from a retrospective cohort study of patients with PDAC receiving chemotherapy and ChIM treatment. This study aimed to evaluate the risk factors for PEI and the effects of PEI on the efficacy of ChIM treatment in patients with PDAC.
Materials and Methods
Study Design and Patients
This single-center, retrospective cohort study was approved by the Ethics Committee of Zhengzhou University People’s Hospital (Approval No. 2021-69). The study complies with the Declaration of Helsinki. Written informed consent was obtained from all the participants prior to the enrollment of this study. From June 2017 to December 2022, 1088 patients with PDAC, diagnosed by surgical pathology or biopsy, were enrolled. None of the patients received any antitumor treatment before diagnosis. The exclusion criteria were OS of < 1 month, presence of other malignant tumors that affected life span, other potential causes of death, incomplete follow-up information, and other types of pancreatic tumors (Figure 1).
 |
Figure 1 Flow diagram of patient selection. |
Data Collection and Definitions
General information of the participants that was recorded included their personal information, medical history, laboratory indicators, and medical imaging. Personal information included age, sex, smoking history, drinking history, weight, height, and family history of malignant tumors (Figure 1). Weight and height were used for body mass index (BMI) calculation. Medical history included diabetes, cardiovascular, and cerebrovascular diseases, and treatment history after diagnosis (surgical resection, chemotherapy, ICIs, and PERT). Laboratory indicators, including liver function and serum CA 19-9, were recorded. CA 19-9 was dichotomized as > 1000 U/mL or < 1000 U/mL at diagnosis. Enhanced pancreatic computed tomography (CT) was performed to evaluate tumor location.
Follow-Up and Endpoints
CA-19-9 and pancreatic CT were performed to evaluate tumor recurrence and metastasis. Patient information regarding chemotherapy, ChIM, and PERT was also recorded. The occurrence of PEI was evaluated based on PEI symptoms at the final follow-up, including post-treatment weight loss (> 10% weight loss at 3 months), diarrhea, other dyspepsia-related symptoms, and nutritional status.13 The primary endpoints were the 1-year survival rate and OS of each group. OS was defined as the time from the date of pathological diagnosis to death or the final follow-up.
Statistical Analysis
SPSS 22.0 and R version 4.2.3 were used for statistical analysis. Continuous variables were analyzed using the unpaired Student’s t-test (normal distributions) (data are expressed as mean ± SD) or the Mann–Whitney U-test (non-normal distributions) (data are expressed as median interquartile range). Qualitative variables were evaluated using the chi-squared test and data are expressed as percentages (%). Logistic regression analysis was used to assess risk factors for PEI. Risk ratios are presented as odds ratios (OR) and 95% confidence intervals (CI). Cox proportional hazards regression analysis was used to assess the risk factors for OS in patients with PDAC. Risk ratios are presented as hazard ratios (HR) and 95% CI. Forward selection Wald was used in multivariate analyses. The level of statistical significance for all statistical tests was set at P < 0.05.
Results
Baseline Characteristics of the Patients
A total of 359 patients with PDAC were enrolled in this study, with a non-normally distributed age of 63.0 (55.0–70.0) years and a BMI of 22.5 (20.5–24.7) kg/m2. Among them, 205 (57.1%) were male, and 173 (48.2%) underwent radical surgical resection. Total PEI incidence was 67.7% (n = 243). There were 268 (74.7%) patients with tumors in the pancreatic head. However, only 149 patients (41.5%) underwent PERT. A total of 181 (50.4%) and 104 (29.0%) patients received chemotherapy alone and chemotherapy combined with immunotherapy (ChIM), respectively (Table 1).
 |
Table 1 Characteristics of Cohorts (n = 359) |
Comparison of the Baseline Characteristics of Patients with or Without PEI
The clinical characteristics and therapeutic information of patients with PDAC and PEI are shown in Table 2. There were no statistically significant differences between patients with and without PEI in terms of age, sex, BMI, smoking status, alcohol consumption, or serum CA 19-9. Although the incidence of PEI in patients with tumors in the head of the pancreas (70.1%) was higher than that (60.4%) in patients with tumors in the body-tail of the pancreas, they were not significantly different (P = 0.087) (Table 2).
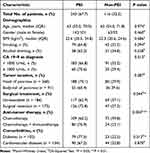 |
Table 2 Clinical Characteristics of Patients with or Without PEI (n = 359) |
Consistent with that of previous studies, our results showed that surgical resection increased PEI incidence (72.8%) in patients with PDAC compared with that in patients who received palliative treatment (62.9%) (P = 0.044). The occurrence rate in patients with diabetes was significantly higher than that in patients without diabetes (P = 0.013). Moreover, the incidence of PEI in patients who received ChIM was significantly higher (76.9%) than that in patients who received chemotherapy only (60.2%) (P = 0.004) (Table 2).
Risk Factors of PEI in Patients with PDAC
To confirm the risk factors for PEI, univariate logistic regression analysis of the data of 285 patients who received chemotherapy (n = 181) and ChIM (n = 104) was performed. The results showed that surgical resection, diabetes, and ChIM increased the morbidity risk of PEI with ORs of 1.65 (95% CI 1.003–2.699) (P = 0.048), 2.08 (95% CI 1.155–3.726) (P = 0.015), and 2.20 (95% CI 1.277–3.796) (P = 0.005), respectively. The adjusted OR values of surgical resection, diabetes, and ChIM were 1.85 (95% CI 1.102–3.099) (P = 0.020), 2.32 (95% CI 1.268–4.227) (P = 0.006), and 2.63 (95% CI 1.492–4.626) (P = 0.001), respectively (Table 3). Thus, ChIM is an independent risk factor for PEI.
 |
Table 3 Logistic Regression Variables Analysis of Risk Factors for PEI in PDAC (n = 285) |
Baseline Characteristics of Patients Receiving Chemotherapy or ChIM
To assess the effect of ChIM on PDAC, we divided patients into two groups: chemotherapy alone and ChIM. The baseline characteristics of the two groups are shown in Table 4. There were no statistically significant differences in age, sex, BMI, serum CA 19–9, tumor location, diabetes incidence, and PERT application between the two groups. The rate of ChIM treatment in patients who underwent surgical resection (31.2%) was lower than that in patients with unresectable tumors (42.7%) (P = 0.043). This may be because patients with advanced tumors tend to receive ChIM treatment. In the chemotherapy and ChIM groups, PEI incidences were 60.2% and 76.9%, respectively (P = 0.004) (Table 4). This indicated that ChIM treatment had a promoting effect on PEI occurrence (contingency coefficient = 0.168, P = 0.004). A previous report has shown that ICIs increase the injury of pancreatic acinar cells and ductal cells by activating infiltrated immune cells in the pancreas, thereby inducing PEI.11
 |
Table 4 Characteristics of Cohorts (n = 285) |
Risk Factors Associated with the OS of Patients with PDAC
Age and serum CA 19–9 level increased the risk of death in patients with PDAC (HR of 1.02, 95% CI, 1.005–1.031) (P = 0.006) and 1.72 (95% CI 1.273–2.317) (P = 0.006). In contrast, surgical resection decreased the risk of death (HR 0.43, 95% CI 0.331–0.555) (P < 0.001). Moreover, ChIM did not improve the survival rate of patients with PDAC (HR 0.92, 0.707–1.197) (P = 0.534) (Table 5), similar to findings of a recent clinical study.9 The death risk in patients with PEI was increased, however, due to irregular PERT treatment, there was no statistical difference between patients with PEI and those without PEI (P = 0.135).
 |
Table 5 Cox Proportional Hazard Regression Analysis of Risk Factors Associated with Overall Survival Among Patients with Pancreatic Cancer (n = 285) |
Interestingly, the association between ChIM treatment and OS was examined in the subgroups analyses according to age (> 63 or≤ 63 years), (BMI (≥ 22.5 or < 22.5 kg/m2), surgical treatment (yes or no), CA 19–9 (≥ 1000 or < 1000 U/mL), diabetes (yes or no), and PERT (yes or no). The results showed that there was no statistically significant interaction between age, BMI, diabetes status, surgical treatment, serum CA 19–9 level, and PERT. The statistical significance was observed only among patients without PEI (HR = 0.53, 95% CI 0.306–0.905, P = 0.020) (Figure 2). Patients receiving ChIM had a lower risk of death.
In patients without PEI, the 1-year survival rate was 70.8% (two-side P = 0.045) and median OS was 22.0 months (95% CI 11.5–32.5) in the ChIM group, which was higher than those of the chemotherapy group (1-year survival rate = 47.2%, median OS = 11.0 months, 95% CI 8.0–14.0) (Figure 3A).
Moreover, Cox proportional hazard regression analysis showed that PERT was a significant protective factor for the OS of patients with PDAC in both univariate (HR 0.63, 95% CI 0.487–0.818) (P = 0.001) and multivariate analyses (HR 0.73, 95% CI 0.561–0.956) (P = 0.022) (Table 5). Kaplan-Meier survival analysis showed that the median OS of patients who received PERT (n = 123) was 16.0 months (95% CI 12.3–19.7), which was significantly longer than that of non-PERT patients (n = 162) (median OS = 11.0 months, 95% CI 9.6–12.4) (P < 0.001) (Figure 3B).
Discussion
ICIs, such as inhibitors of programmed cell death protein 1 (PD-1) or its ligand 1 (PD-L1), have been widely used in many solid tumor treatments and have achieved significant improvements in OS in many advanced cancers.15 However, PDAC is highly resistant to ICI therapy because of its complex tumor stroma and immune microenvironment.16 Chemotherapy with gemcitabine plus nab-paclitaxel and FOLFIRINOX can remodel the tumor stroma and immune cell infiltration. Thus, its combination with chemotherapy is thought to be a potential way to improve the therapeutic efficacy of ICIs in PDAC.17,18
In a mouse model of PDAC, PD-1 inhibitors combined with gemcitabine significantly inhibited the recurrence rate of resected PDAC compared to that with gemcitabine alone.19 In a clinical study, the PD-1 inhibitor nivolumab combined with nab-paclitaxel and gemcitabine achieved improved OS, progression-free survival (PFS), and manageable toxicity.20 However, another Phase I study showed insufficient median PFS and OS in patients with advanced or metastatic PDAC who received nivolumab combined with gemcitabine and nab-paclitaxel.21 Therefore, it is necessary to elucidate the factors that affect ICI efficacy.
PEI is a common complication of PDAC, particularly in patients who have undergone surgical resection. A previous meta-analysis reported that the total prevalence of PEI was 72% in advanced PDAC.22 PEI is thought to be an important factor; however, it is often overlooked by surgeons, potentially impacting the prognosis of patients with PDAC.13,23 Studies have shown that ICIs are associated with pancreatic adverse events, including pancreatitis and diabetes. Moreover, ICIs may induce PEI.12 Some studies have reported that the endocrine and exocrine pancreas may be destroyed by increased activated CD8+ T cell infiltration from ICIs treatment. To date, no studies have confirmed whether PEI affects the clinical effectiveness of ICIs.
Our clinical data showed that the total incidence of PEI was 72.8%, and tumors located at the head of the pancreas and surgical resection were risk factors for PEI. This is consistent with the results of a previous study.24 We also found that patients with diabetes were more likely to develop PEI. Moreover, the data showed that chemotherapy combined with ICIs is an independent risk factor for PEI (OR 2.63, 95% CI 1.492–4.626) (P = 0.001). However, the PERT rate was only 41.5%, which is far below the PEI occurrence rate. This indicates that many patients with PEI do not receive PERT. PEI has been reported to be underdiagnosed and undertreated in many countries.25–27 Moreover, the role of PEI in the management of PDAC has not yet been elucidated.
Overall, age, surgical resection and CA 19–9 levels were key factors related to patient survival. ChIM did not improve patient OS. However, among patients without PEI, the 1-year survival rate and median OS of patients who received ChIM were superior to those of patients who received chemotherapy alone. Additionally, patients who received PERT had a longer OS and better prognosis than those who did not receive PERT, although the use of PERT may not be accurate. The findings of this study indicate that the OS of PDAC patients with PDAC was affected by a variety of factors including age, chemotherapy, immunotherapy, surgical resection and PERT. More reliable global prospective studies still need to be developed to confirm the effect of PEI and ICI treatment on the prognosis of patients with PDAC.
In conclusion, our findings suggest that ICIs increase the incidence of PEI in patients with PDAC. ChIM may improve the OS of patients with PDAC without PEI. In the clinical management of PDAC, PEI and PERT should be seriously considered, as they may improve the response rate of PDAC to ICI therapy.
Data Sharing Statement
All the data in this study are available upon request from the corresponding author.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31671440) and Key Science and Technology Research Project of Henan Province (212102310151).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that this study was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi:10.3322/caac.21708
2. Renouf DJ, Loree JM, Knox JJ, et al. The CCTG PA.7 Phase II trial of gemcitabine and nab-paclitaxel with or without durvalumab and tremelimumab as initial therapy in metastatic pancreatic ductal adenocarcinoma. Nat Commun. 2022;13(1):5020. doi:10.1038/s41467-022-32591-8
3. Cortes J, Rugo HS, Cescon DW, et al. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N Engl J Med. 2022;387(3):217–226. doi:10.1056/NEJMoa2202809
4. Schonfeld SJ, Tucker MA, Engels EA, et al. Immune-related adverse events after immune checkpoint inhibitors for melanoma among older adults. JAMA Network Open. 2022;5(3):e223461. doi:10.1001/jamanetworkopen.2022.3461
5. Henriksen A, Dyhl-Polk A, Chen I, Nielsen D. Checkpoint inhibitors in pancreatic cancer. Cancer Treat Rev. 2019;78:17–30. doi:10.1016/j.ctrv.2019.06.005
6. Kabacaoglu D, Ciecielski KJ, Ruess DA, et al. Immune checkpoint inhibition for pancreatic ductal adenocarcinoma: current limitations and future options. Front Immunol. 2018;9:1878. doi:10.3389/fimmu.2018.01878
7. Aglietta M, Barone C, Sawyer MB, et al. A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann Oncol. 2014;25(9):1750–1755. doi:10.1093/annonc/mdu205
8. Padrón LJ, Maurer DM, O’Hara MH, et al. Sotigalimab and/or nivolumab with chemotherapy in first-line metastatic pancreatic cancer: clinical and immunologic analyses from the randomized Phase 2 PRINCE trial. Nat Med. 2022;28(6):1167–1177. doi:10.1038/s41591-022-01829-9
9. Kamath SD, Kalyan A, Kircher S, et al. Ipilimumab and gemcitabine for advanced pancreatic cancer: a Phase Ib study. Oncologist. 2020;25(5):e808–e815. doi:10.1634/theoncologist.2019-0473
10. Schizas D, Charalampakis N, Kole C, et al. Immunotherapy for pancreatic cancer: a 2020 update. Cancer Treat Rev. 2020;86:102016. doi:10.1016/j.ctrv.2020.102016
11. Prasanna T, McNeil CM, Nielsen T, Parkin D. Isolated immune-related pancreatic exocrine insufficiency associated with pembrolizumab therapy. Immunotherapy. 2018;10(3):171–175. doi:10.2217/imt-2017-0126
12. Liu Y, Zhang H, Zhou L, et al. Immunotherapy-associated pancreatic adverse events: current understanding of their mechanism, diagnosis, and management. Front Oncol. 2021;11:627612. doi:10.3389/fonc.2021.627612
13. Domínguez-Muñoz JE, Nieto-Garcia L, López-Díaz J, et al. Impact of the treatment of pancreatic exocrine insufficiency on survival of patients with unresectable pancreatic cancer: a retrospective analysis. BMC Cancer. 2018;18(1):534. doi:10.1186/s12885-018-4439-x
14. Pezzilli R, Caccialanza R, Capurso G, Brunetti O, Milella M, Falconi M. Pancreatic enzyme replacement therapy in pancreatic cancer. Cancers. 2020;12(2):275. doi:10.3390/cancers12020275
15. Melaiu O, Lucarini V, Giovannoni R, Fruci D, Gemignani F. News on immune checkpoint inhibitors as immunotherapy strategies in adult and pediatric solid tumors. Semin Cancer Biol. 2022;79:18–43. doi:10.1016/j.semcancer.2020.07.001
16. Li X, Gulati M, Larson AC, et al. Immune checkpoint blockade in pancreatic cancer: trudging through the immune desert. Semin Cancer Biol. 2022;86(Pt 2):14–27. doi:10.1016/j.semcancer.2022.08.009
17. Dias Costa A, Väyrynen SA, Chawla A, et al. Neoadjuvant chemotherapy is associated with altered immune cell infiltration and an anti-tumorigenic microenvironment in resected pancreatic cancer. Clin Cancer Res. 2022;28(23):5167–5179. doi:10.1158/1078-0432.CCR-22-1125
18. Michelakos T, Cai L, Villani V, et al. Tumor microenvironment immune response in pancreatic ductal adenocarcinoma patients treated with neoadjuvant therapy. J Natl Cancer Inst. 2021;113(2):182–191. doi:10.1093/jnci/djaa073
19. Brooks J, Fleischmann-Mundt B, Woller N, et al. Perioperative, spatiotemporally coordinated activation of T and NK cells prevents recurrence of pancreatic cancer. Cancer Res. 2018;78(2):475–488. doi:10.1158/0008-5472.CAN-17-2415
20. Weiss GJ, Blaydorn L, Beck J, et al. Phase Ib/II study of gemcitabine, nab-paclitaxel, and pembrolizumab in metastatic pancreatic adenocarcinoma. Invest New Drugs. 2018;36(1):96–102. doi:10.1007/s10637-017-0525-1
21. Wainberg ZA, Hochster HS, Kim EJ, et al. Open-label, Phase I study of nivolumab combined with nab-paclitaxel plus gemcitabine in advanced pancreatic cancer. Clin Cancer Res. 2020;26(18):4814–4822. doi:10.1158/1078-0432.CCR-20-0099
22. Iglesia D, Avci B, Kiriukova M, et al. Pancreatic exocrine insufficiency and pancreatic enzyme replacement therapy in patients with advanced pancreatic cancer: a systematic review and meta-analysis. United Eur Gastroenterol J. 2020;8(9):1115–1125. doi:10.1177/2050640620938987
23. Roeyen G, Berrevoet F, Borbath I, et al. Expert opinion on management of pancreatic exocrine insufficiency in pancreatic cancer. ESMO Open. 2022;7(1):100386. doi:10.1016/j.esmoop.2022.100386
24. Vujasinovic M, Valente R, Del Chiaro M, Permert J, Löhr JM. Pancreatic exocrine insufficiency in pancreatic cancer. Nutrients. 2017;9(3):183. doi:10.3390/nu9030183
25. Ru N, Zou WB, Wu H, et al. Chinese guidelines for the diagnosis and treatment of pancreatic exocrine insufficiency (2018 edition). J Dig Dis. 2019;20(11):567–571. doi:10.1111/1751-2980.12753
26. Phillips ME, Hopper AD, Leeds JS, et al. Consensus for the management of pancreatic exocrine insufficiency: UK practical guidelines. BMJ Open Gastroenterol. 2021;8(1):e000643. doi:10.1136/bmjgast-2021-000643
27. Lan X, Robin G, Kasnik J, Wong G, Abdel-Rahman O. Challenges in diagnosis and treatment of pancreatic exocrine insufficiency among patients with pancreatic ductal adenocarcinoma. Cancers. 2023;15(4):1331. doi:10.3390/cancers15041331
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Construction of a Pyroptosis-Related Genes Signature to Improve the Prognostic Prediction and Therapeutic Drugs Selection in Patients with Pancreatic Cancer
Li C, Wang M, Wei J, Zhang W, Liu H, Zhao D
International Journal of General Medicine 2022, 15:6387-6403
Published Date: 2 August 2022
GBP5 Expression Predicted Prognosis of Immune Checkpoint Inhibitors in Small Cell Lung Cancer and Correlated with Tumor Immune Microenvironment
Tong Q, Li D, Yin Y, Cheng L, Ouyang S
Journal of Inflammation Research 2023, 16:4153-4164
Published Date: 20 September 2023


