Back to Journals » Journal of Inflammation Research » Volume 16
GBP5 Expression Predicted Prognosis of Immune Checkpoint Inhibitors in Small Cell Lung Cancer and Correlated with Tumor Immune Microenvironment
Authors Tong Q, Li D, Yin Y, Cheng L, Ouyang S
Received 14 December 2022
Accepted for publication 10 March 2023
Published 20 September 2023 Volume 2023:16 Pages 4153—4164
DOI https://doi.org/10.2147/JIR.S401430
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Qin Tong,1,* Deyu Li,2,* Yan Yin,1 Lifang Cheng,3 Shuming Ouyang4
1Department of Oncology, The First Affiliated Hospital, Hengyang Medical School, University of South China, Hengyang, People’s Republic of China; 2Department of Medical Oncology, Provincial Clinical College, Fujian Medical University, Fujian Provincial Hospital, Fuzhou, People’s Republic of China; 3Department of Hematology, Shenzhen Samii Medical Center, Shenzhen, People’s Republic of China; 4Gynecology & Obstetrics and Reproductive Medical Center, The First Affiliated Hospital, Hengyang Medical School, University of South China, Hengyang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lifang Cheng; Shuming Ouyang, Email [email protected]; [email protected]
Background: The discovery and development of immune checkpoint inhibitors (ICIs) has significantly enhanced the arsenal of immunotherapy treatments available for cancer patients. The identification of biomarkers that are indicative of an individual’s sensitivity to treatment with ICIs is useful for screening SCLC patients prior to commencement of any ICIs based immunotherapy. However, the relationship between GBP5 and the prognosis of SCLC immunotherapy is still unclear and requires further study.
Methods: We downloaded two SCLC datasets, namely the George-SCLC and Jiang-SCLC cohorts. We used the TIDE algorithm to predict the efficacy of immunotherapy for SCLC patients. The QuanTIseq, MCPcounter, and EPIC algorithms are used to calculate the proportions of immune cells in SCLC patients. Additionally, we retrospectively collected 35 SCLC samples from the first affiliated hospital of the Hengyang Medical school.
Results: Patients in each cohort were devided into two groups with high (GBP5-High) and low (GBP5-Low) expression of GBP5. In both cohorts, the GBP5-High population had a higher proportion of patients that responded well to immunotherapy (responders) (p < 0.05). In addition, both GBP5-High subgroups had significantly increased cytotoxicity, chemokines, antigen presenting, and TNF family related genes. We also determined that GBP5 was related to high-level infiltration of B cells, CD4+T cells, CD8+T cells and NK cells.
Conclusion: In this study, we found that GBP5 has the potential to be used as a biomarker of ICIs efficacy for SCLC patients. GBP5 is related to the quantity of inflammatory molecules, a high level of immune infiltration, and a highly activated immune response pathway.
Keywords: GBP5, small cell lung cancer, prognosis, tumor immune microenvironment, immune checkpoint inhibitors
Introduction
Lung cancer continues to have the highest morbidity and mortality in the world compared with other types of tumors.1 Specifically, small cell lung cancer (SCLC) is a highly malignant neuroendocrine tumor, accounting for ~15% of all lung cancers.2 Even if successfully treated, SCLC patients are prone to relapse. Thus, traditional combination therapy tends to have a poor outcome for patients with advanced SCLC, resulting in an average overall survival (OS) time of about 10 months.3 More than 70% of SCLC patients exhibit distant metastasis at the time of diagnosis, which has historically restricted the research and treatment options related to tumor occurrence and development. Consequently, the 5-year survival rate of SCLC patients has remained fundamentally unchanged for more than 20 years.4 However, with the recent improvements in the understanding of immune checkpoint inhibitors (ICIs), including programmed cell death protein-1 (PD-1) inhibitor and programmed death ligand-1 (PD-L1) inhibitor, the prognosis of SCLC patients is likely to improve.5
Recently, a number of potential biomarkers for ICIs sensitivity in SCLC patients, including tumor mutation burden (TMB)6–8 and T cell inflammatory gene expression profile (GEP),9,10 have been the focus of extensive research. Studies have shown that most SCLC lack PD-L1 expression. A recent study determined that only 2% of SCLC patients showed amplification of the CD274 gene and that only this small minority were potentially sensitive to ICI immunotherapy.11 However, this does contradict historic clinical trial results, which do not support these findings regarding PD-L1 expression as a marker of the immune efficacy of SCLC patients.12–15 TMB has yet to be fully verified by means of clinical research as a predictive biomarker. This is mainly because of the lack of a proven standard to determine the application of TMB.16 Therefore, further investigations of predicative biomarkers for sensitivity of SCLC patients to ICI therapy is required to allow more effective screening and treatment, which we look to undertake in this study.
Guanylate-binding protein (GBP)-5 (GBP5) is an activator of NLRP3 inflammation and plays a key role in innate immune system inflammation and macrophage activation.17 A positive correlation between immune cell infiltration and GBP5 expression in the stromal and epithelial cells of gastric tumors has been found, suggesting that GBP5 plays an important role in tumor pathobiology.18,19 In glioblastoma, the high expression of GBP5 has been shown to be negatively correlated with patient prognosis. Studies show that high expression of GBP5 can promote the migration, invasion and proliferation of tumor cells. The underlying mechanism is believed to be the activation of Src/ERK1/2MAPK pathway by GBP5 to induce the expression of matrix metalloproteinase-3 (MMP-3), which plays a key role in the growth and invasion of glioblastoma.20 Recently, Qian et al found that GBP5 may promote anti-PD-1/PD-L1 therapy of glioma by acting as an interferon-γ (IFN-γ) induced gene in the tumor microenvironment (TME).21 Additional studies have found that the expression of GBP5 is increased in colon cancer (CRC),22 pancreatic cancer,23 and it associates with prognosis of patients. However, the relationship between GBP5 and the prognosis of SCLC immunotherapy is still unclear.
In this study, we used the TIDE algorithm to predict the therapeutic effect ICI immunotherapy had on patients using data from two SCLC cohorts. Additionally, we analyzed the relationship between GBP5 and the activity of immune cells, immune-related molecules, and immune-related pathways of SCLC.
Methods
SCLC Cohort Download
We downloaded two SCLC cohorts with transcriptome data and clinical data from the Supplementary Files of the previously published research, namely the George-SCLC cohort24 and the Jiang-SCLC cohort.25 For details on the transcriptome sequencing methods of SCLC samples in the George-SCLC cohort and the Jiang-SCLC cohort, please refer to the previously published research.25,26 The baseline characteristics of the George-SCLC and Jiang-SCLC cohorts were detailed in Supplementary Tables 1 and 2, respectively. Additionally, we retrospectively collected 35 samples from the first affiliated hospital of the Hengyang Medical school, University of South China, relating to patients diagnosed with SCLC between 2015 and 2022. Our study was approved by the Institutional Review Board of the first affiliated hospital of the Hengyang Medical school, University of South China. All SCLC patients provided informed consent for their samples to be used. Details of immunohistochemistry (IHC) and imaging of the SCLC patients are provided in “Supplementary Methods”. See Table 1 for the clinical characteristics of these 35 SCLC patients of the first affiliated hospital of the Hengyang Medical school, University of South China.
 |
Table 1 GBP5 Expression and Its Relationship with the Clinical Characteristics of 35 Extensive SCLC Patients of the First Affiliated Hospital of University of South China |
Evaluation of Immune Response
We used the TIDE algorithm27 (http://tide.dfci.harvard.edu/) to predict the responsiveness and non-responsiveness of SCLC patients (including the George-SCLC and Jiang-SCLC cohorts). For this analysis, TIDE parameters selected were “cancer type: other” and “previous immunity: no”. To further verify the influence of GBP5 on the prognosis of tumor patients treated with ICIs, we downloaded the expression data and clinical prognosis data of urothelial cancer patients treated with ICIs from a study published by Mariathasan et al.28 The clinical characteristics of the ICIs cohort (Mariathasan et al) were detailed in the Supplementary Table 3. Additionally, we download the expression data and clinical prognosis data of melanoma patients treated with ICIs from a study published by Liu et al.29
Evaluation of Immune Infiltration and Pathway Enrichment
We calculated the immune cells present in the tumor microenvironment of each patient using three published immune infiltration algorithms (quanTIseq,30 MCPcounter31 and EPIC32,33 Additionally, the gene list of the immune checkpoint molecules and immune inflammatory molecules are taken from published literature.33 The pathway enrichment scores from the Reactome, KEGG, and GO databases were evaluated by GSEA and ssGSEA algorithm.34–36
Statistical Analysis
We used Mann–Whitney U-test to calculate the statistical differences between the continuous variables of the different groups. Fisher’s exact test was used to calculate the categorical variables in each group. The Kaplan-Meier (KM) method and Log rank test were used to compare the survival time of the different groups. Statistical analysis and visual analysis in this study are based on R software (version 3.6). In this study, the p-value is bilateral and p < 0.05 is regarded as statistically significant.
Results
The Relationship Between GBP5 and the Efficacy and Prognosis of Immunotherapy
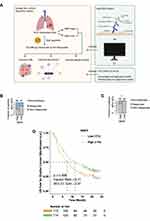
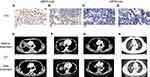
In this study, we explored the relationship between GBP5 expression and i) the efficacy of immunotherapy and ii) patient prognosis post-treatment, and we look to explain the underlying molecular mechanism from the perspective of the immune microenvironment (Figure 1A). In order to evaluate the relationship between GBP5 expression and the efficacy of immunotherapy in SCLC, we use the TIDE algorithm to predict the response of SCLC patients to the treatment. We found that patients with high levels of GBP5 (GBP5-High) in George-SCLC had a higher proportion of responders (patients with favorable treatment outcome) than those classified as GBP5-Low (Figure 1B, p < 0.05). These findings were echoed by the results of a similar analysis using the Jiang-SCLC cohort data (Figure 1C, p < 0.05). To further validate our assertion that elevated GBP5 levels are indicative of a better prognosis following ICI immunotherapy, we conducted the same analysis using data from urothelial cancer patients that had been treated with ICIs. The KM curve shows that the GBP5-High population has significantly longer overall survival (OS) time than the GBP5-Low population (Figure 1D, log-rank p = 0.008, HR = 0.71, 95% CI: 0.54–0.91). To further verify the relationship between GBP5 and ICI immunotherapy, we used IHC to evaluate the molecular level of GPB5 in SCLC patients (Figure 2A–D). The curative effect of an immune therapy is indicated by the degree of lesion regression (calculated by measuring tumor size in computed tomography (CT) scans before and after treatment). We found that GBP5-High patients (Figure 2A and B) had rapid tumor regression (Figure 2E and F) after receiving 6 cycles of combination therapy (Etoposide-cisplatin (EP) and ICIs (Durvalumab). Conversely, SCLC patients with GBP5-Low status (Figure 2C and D) did not show any obvious tumor regression after receiving the same regimen (Figure 2G and H). Due to the limited numbers of the SCLC patients received ICIs, we used another ICIs-Melanoma cohort (Liu et al)29 to explore whether the target gene GBP5 was an independent prognosis factor. In the univariable Cox regression model, we found that GBP5-high melanoma patients treated ICIs had longer PFS time compared GBP5-low melanoma patients treated ICIs (P < 0.05, Supplementary Figure 1). Additionally, we found GBP5 was an independent prognosis factor for melanoma patients received ICIs (P < 0.05, Supplementary Figure 1).
The Relationship Between GBP5 and i) Immune Checkpoint Molecules and ii) Immune-Related Genes
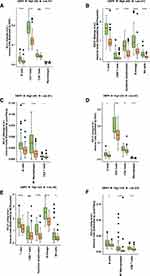
To achieve our objective, we analyzed the impact of GBP5 status on both the expression of immune checkpoint molecules and immune-related genes. In the George-SCLC cohort, GBP5-High patients had significantly higher levels of immune checkpoint molecules (Figure 3A, p < 0.05) (including PD-L1, HAVCR2, LAG3, IDP1, TIGIT, PD-1 and PDCD1LG2) compared with GBP5-Low patients. We observed similar results, in terms of increased expression of immune checkpoint molecules, for the GBP5-High population of the Jiang-SCLC cohort (Figure 3B, P < 0.05). In additional, in both cohorts (George-SCLC: Figure 3C, Jiang-SCLC: Figure 3D), the GBP5-High population exhibited significantly increased expression of cytotoxicity-related genes (CD8A, GZMA and GZMB), chemokine-related genes (CX3CL1, CXCL9 and CXCR3) and antigen presentation related gene (HLA-A) and tumor necrosis factor (TNF) family related genes (TNFRSF18, TNFRSF8, TNFRSF9, TNFSF10, TNFSF14, TNFSF18, TNFSF4 and TNFSF9) compared with GBP5-Low populations.
The Relationship Between GBP5 and Immune Cells
In the George-SCLC cohort, the GBP5-High subset had significantly elevated levels of B cells, CD4+T cells, CD8+T cells, and macrophages compared to the GBP5-Low subset (Figure 4A, p < 0.05). We also used two other immune infiltration evaluation algorithms (MCPcounter: Figure 4B; quanTIseq: Figure 4C) to analyze the George-SCLC cohort and found that the GBP5-High population exhibited significantly higher levels of CD8+T cells, cytotoxic lymphocytes, B cells, and NKT cells than patients with GBP5-Low status. These analyses were repeated using the Jiang-SCLC cohort data and similar results were observed. The GBP5-High patients showed significantly higher levels of B cells, CD4+T cells, CD8+T cells and macrophages compared with their GBP5-Low counterparts (Figure 4D, p < 0.05). When MCPcounter (Figure 4E) and quanTIseq (Figure 4F) were used to analyze the Jiang-SCLC cohort, it was shown that once again that the GBP5-High population had significantly higher levels CD8+T cells, cytotoxic lymphocytes, B cells and NKT cells compared with the sup-group of GBP5-Low patients.
GBP5 and Pathway Enrichment Activity
The GSEA algorithm was used to analyze and compare the activity of the immune-related pathways in the GBP5-High and GBP5-Low groups. The results showed that the TME of the GBP5-High groups were significantly enriched with respect to the immune response and immune activation related pathways, including interferon gamma signaling, Interleukin-10 signaling, positive regulation of cytokine production involved in immune response, positive regulation of T cell mediated cytotoxicity, regulation of T cell mediated cytotoxicity, positive regulation of CD4-positive, alpha-beta T cell activation, antigen processing and presentation of endogenous antigen, B cell receptor signaling pathway, cytokine binding, chemokine activity, leukocyte activation involved in inflammatory response and positive regulation of chemokine secretion (Figure 5A: George-SCLC; Figure 5B: Jiang-SCLC). Similarly, the results of ssGSEA showed that the GBP5-High groups had significantly increased immune response signal pathway scores, including T cell receptor binding, interferon gamma signaling, MHC class I protein complex, natural killer cell activation, positive regulation of tumor necrosis factor superfamily cytokine production, positive regulation of tumor necrosis factor biosynthetic process, regulation of natural killer cell mediated immunity, regulation of lymphocyte activation, leukocyte mediated cytotoxicity, leukocyte proliferation, CD4 positive or CD8 positive alpha beta T cell lineage commitment, negative regulation of vascular endothelial cell proliferation (Figure 5C: George-SCLC; Figure 5D: Jiang-SCLC). On the contrary, the activity of fatty acid pathway was significantly down-regulated in both GBP5-High populations (Figure 5C: George-SCLC; Figure 5D: Jiang-SCLC).
Discussion
Although the combination therapy mode of SCLC has made considerable progress over the past 30 years, the stark reality is that only a small number of SCLC patients benefit from improved long-term survival.13,37,38 With the recent improvements in the understanding of ICIs, the prognosis of SCLC patients is likely to improve. Therefore, to optimize the treatment regimen, it is particularly important to identify predictive biomarkers that can be used to screen patient populations to select those most likely to benefit from immunotherapy. In both cohorts, having a GBP5-High status was indicative of having a better chance of responding to ICI treatment. Additionally, we found that compared with data from the GBP5-Low sub-groups, patients in the GBP5-High population had significantly increased inflammatory molecule expression, immune cell infiltration level, and immune response pathway. GBP5, the interferon (IFN)-inducible GTPases, can up-regulate antibacterial immunity and promote cell death.39 GBP-5 is increased in breast cancer with significantly prolonged survival time.2 These tumors were also up-regulated in IFNγ-induced chemokines.40 Additionally, GBP5 served as a good prognostic marker in PD1/PDL1 high-expressing basal-like breast cancer.41 Wang et al found that high expression of GBP5 was associated with a better prognosis of skin cutaneous melanoma patients, which indicated that GBP5 may be a novel prognostic biomarker.42 Therefore, we conclude that GBP5 may be a useful prognostic marker for the success of ICI immunotherapy as a treatment for SCLC patients.
In this study, we found that GBP5 was associated with higher levels of inflammatory factors, including cytotoxic related genes (CD8A, GZMA and GZMB); chemokine related genes (CX3CL1, CXCL9 and CXCR3); antigen presentation related genes (HLA-A) and TNF family related genes (TNFRSF18, TNFRSF8, TNFRSF9, TNFSF10, TNFSF14, TNFSF18, TNFSF4 and TNFSF9). The chemokine (C-X-C motif) ligands (CXCL) are important members of the chemokine family, which mainly cause the directional migration of cells, but can induce the migration of immune cells (such as T cells, B cells, dendritic cells and natural killer cells) during inflammation.43 Yan et al observed that the cytokine gene IRF-1 can regulate CXCL10 and CXCR3 to induce anti-tumor immunity in hepatocellular carcinoma (HCC) cells.44 In addition, CXCL9 can predict the curative effect of the PD1/PDL1 immune checkpoint inhibitor in HCC patients.45 Other studies have shown that Granzyme A (GZMA) is related to the efficacy of ICIs in many cancer types.46,47 TNF-α is an effective inflammatory cytokine mainly produced by activated macrophages, lymphocytes, and other cell types. TNF-α plays an important role in inflammatory immune response.48–50
In addition to to up-regulation of inflammatory factors, we found that GBP5 was related to a high level of immune cell infiltration, including B cells, CD4+T cells, CD8+T cells, and NKT cells. Besides secreting cytokines such as interferon IFN-γ, NK cells also release lysosomes containing perforin and granzyme to kill target cells.51 Studies have shown that by increasing the expression level and activation of NK cells, the curative effect of ICIs treatment can be enhanced, thus prolonging the OS time of cancer patients.52 A randomized Phase II clinical trial (NCT02519322) found that the density of B cells was higher in the responders of melanoma treated with ICIs compared with the non-responders.53 Study of the anti-tumor response of B cells in clinical samples of patients with metastatic melanoma found that any increase of OS rate was associated with the co-existence of tumor-associated CD8+ T cells and CD20+ B cells, rather than other clinical features.54 The percentage of infiltrating CD8+ T cells in tissues increased, which was related to the prolonged progression-free survival (PFS) of patients with melanoma treated with ICIs.55
However, this study has some limitations. First, since the SCLC cohort receiving ICIs was very limited, we were only able to verify the relationship between GBP5 expression and the response of immunotherapy estimated by the TIDE algorithm in the George-SCLC and Jiang-SCLC cohorts. Secondly, we only used a local cohort containing 35 SCLC patients from the First affiliated hospital of the Hengyang Medical school, University of South China for verification, therefore, we hope to recruit more SCLC patients receiving ICIs for follow-up verification. Third, we have not conducted relevant cell experiments or animal experiments to directly prove our hypothesis. We hope to further explore the link between the GBP5 expression and immunotherapy prognosis. Finally, we hope that future work on this topic will focus on the collection of more cancer types in order to validate the role of GBP5 expression in the prognosis associated with ICIs.
Conclusions
In this study, we found that the high expression of GBP5 was related to a better response to ICI immunotherapy. Additionally, GBP5 is related to increased levels of inflammatory molecules, significantly enriched immune cell infiltration and increased activity of the immune response pathway. Therefore, GBP5 may be a biomarker of the therapeutic effect of immunotherapy on SCLC.
Data Sharing Statement
All the data generated or analyzed during this study are included in this article and its Supplementary Files.
Ethics Statement
The patients/participants provided their written informed consent to participate in this study and the research presented here has been performed in accordance with the Declaration of Helsinki and has been approved by the ethics committee of the first affiliated hospital of the Hengyang Medical school, University of South China.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by Project of Natural Science Foundation of Hunan Province (Grant No.2021JJ30622, 2021JJ70041), Clinical Medical Technology Innovation Guidance Project of Hunan Province (Grant No.2021SK51808, 2021SK51827), Project of Health Commission of Hunan Province (Grant No.20201992), Scientific Research Fund of Education Department of Hunan Province (Grant No.21B0430), The second batch of technological innovation plan projects of Hengyang City in 2021 (Study on the Mechanism of MYCN Targeting HES1 to Regulate Stem Cells and Chemotherapy Resistance of Small Cell Lung Cancer), Joint Funds for the Innovation of Science and Technology of Fujian Province, China (Grant No.2020Y9026).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Nicholson AG, Chansky K, Crowley J, et al. The international association for the study of lung cancer lung cancer staging project: proposals for the revision of the clinical and pathologic staging of small cell lung cancer in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(3):300–311. doi:10.1016/j.jtho.2015.10.008
3. Wang S, Zimmermann S, Parikh K, Mansfield AS, Adjei AA. Current diagnosis and management of small-cell lung cancer. Mayo Clin Proc. 2019;94(8):1599–1622. doi:10.1016/j.mayocp.2019.01.034
4. Lally BE, Urbanic JJ, Blackstock AW, Miller AA, Perry MC. Small cell lung cancer: have we made any progress over the last 25 years? Oncologist. 2007;12(9):1096–1104. doi:10.1634/theoncologist.12-9-1096
5. Reck M, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter Phase 2 trial. Ann Oncol. 2013;24(1):75–83. doi:10.1093/annonc/mds213
6. Peifer M, Fernández-Cuesta L, Sos ML, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet. 2012;44(10):1104–1110. doi:10.1038/ng.2396
7. Sabari JK, Lok BH, Laird JH, Poirier JT, Rudin CM. Unravelling the biology of SCLC: implications for therapy. Nat Rev Clin Oncol. 2017;14(9):549–561. doi:10.1038/nrclinonc.2017.71
8. Hellmann MD, Callahan MK, Awad MM, et al. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell. 2018;33(5):853–861.e4. doi:10.1016/j.ccell.2018.04.001
9. Ott PA, Bang Y-J, Piha-Paul SA, et al. T-cell-inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J Clin Oncol. 2019;37(4):318–327. doi:10.1200/JCO.2018.78.2276
10. Ayers M, Lunceford J, Nebozhyn M, et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127(8):2930–2940. doi:10.1172/JCI91190
11. Tian Y, Zhai X, Han A, Zhu H, Yu J. Potential immune escape mechanisms underlying the distinct clinical outcome of immune checkpoint blockades in small cell lung cancer. J Hematol Oncol. 2019;12(1):67. doi:10.1186/s13045-019-0753-2
12. Liu SV, Horn L, Mok T, et al. 1781MO IMpower133: characterisation of long-term survivors treated first-line with chemotherapy±atezolizumab in extensive-stage small cell lung cancer. Ann Oncol. 2020;31:S1032–S1033. doi:10.1016/j.annonc.2020.08.1543
13. Rudin CM, Awad MM, Navarro A, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, Phase III KEYNOTE-604 study. J Clin Oncol. 2020;38(21):2369–2379. doi:10.1200/JCO.20.00793
14. Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum–etoposide versus platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, Phase 3 trial. Lancet. 2019;394(10212):1929–1939. doi:10.1016/S0140-6736(19)32222-6
15. Pujol J-L, Greillier L, Audigier-Valette C, et al. A randomized non-comparative phase II study of anti-programmed cell death-ligand 1 atezolizumab or chemotherapy as second-line therapy in patients with small cell lung cancer: results from the IFCT-1603 trial. J Thorac Oncol. 2019;14(5):903–913. doi:10.1016/j.jtho.2019.01.008
16. Lin A, Qiu Z, Zhang J, Luo P. Effect of NCOR1 mutations on immune microenvironment and efficacy of immune checkpoint inhibitors in patient with bladder cancer. Front Immunol. 2021;12:630773. doi:10.3389/fimmu.2021.630773
17. Fujiwara Y, Hizukuri Y, Yamashiro K, et al. Guanylate-binding protein 5 is a marker of interferon-γ-induced classically activated macrophages. Clin Transl Immunol. 2016;5(11):e111. doi:10.1038/cti.2016.59
18. Blakely AM, Matoso A, Patil PA, et al. Role of immune microenvironment in gastrointestinal stromal tumours. Histopathology. 2018;72(3):405–413. doi:10.1111/his.13382
19. Patil PA, Blakely AM, Lombardo KA, et al. Expression of PD-L1, indoleamine 2,3-dioxygenase and the immune microenvironment in gastric adenocarcinoma. Histopathology. 2018;73(1):124–136. doi:10.1111/his.13504
20. Yu X, Jin J, Zheng Y, et al. GBP5 drives malignancy of glioblastoma via the Src/ERK1/2/MMP3 pathway. Cell Death Dis. 2021;12(2):203. doi:10.1038/s41419-021-03492-3
21. Qian J, Wang C, Wang B, et al. The IFN-γ/PD-L1 axis between T cells and tumor microenvironment: hints for glioma anti-PD-1/PD-L1 therapy. J Neuroinflammation. 2018;15(1):290. doi:10.1186/s12974-018-1330-2
22. Friedman K, Brodsky AS, Lu S, et al. Medullary carcinoma of the colon: a distinct morphology reveals a distinctive immunoregulatory microenvironment. Mod Pathol. 2016;29(5):528–541. doi:10.1038/modpathol.2016.54
23. He Q-L, Jiang H-X, Zhang X-L, Qin S-Y. Relationship between a 7-mRNA signature of the pancreatic adenocarcinoma microenvironment and patient prognosis (a STROBE-compliant article). Medicine. 2020;99(29):e21287. doi:10.1097/MD.0000000000021287
24. George J, Lim JS, Jang SJ, et al. Comprehensive genomic profiles of small cell lung cancer. Nature. 2015;524(7563):47–53. doi:10.1038/nature14664
25. Jiang L, Huang J, Higgs BW, et al. Genomic landscape survey identifies SRSF1 as a key oncodriver in small cell lung cancer. PLoS Genet. 2016;12(4):e1005895. doi:10.1371/journal.pgen.1005895
26. Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14(1):26–38. doi:10.5114/pg.2018.80001
27. Jiang P, Gu S, Pan D, et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat Med. 2018;24(10):1550–1558. doi:10.1038/s41591-018-0136-1
28. Mariathasan S, Turley SJ, Nickles D, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554(7693):544–548. doi:10.1038/nature25501
29. Liu D, Schilling B, Liu D, et al. Integrative molecular and clinical modeling of clinical outcomes to PD1 blockade in patients with metastatic melanoma. Nat Med. 2019;25(12):1916–1927. doi:10.1038/s41591-019-0654-5
30. Plattner C, Finotello F, Rieder D. Deconvoluting tumor-infiltrating immune cells from RNA-seq data using quanTIseq. Methods Enzymol. 2020;636:261–285. doi:10.1016/bs.mie.2019.05.056
31. Becht E, Giraldo NA, Lacroix L, et al. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol. 2016;17(1):218. doi:10.1186/s13059-016-1070-5
32. Racle J, Gfeller D. EPIC: a tool to estimate the proportions of different cell types from bulk gene expression data. Methods Mol Biol. 2020;2120:233–248. doi:10.1007/978-1-0716-0327-7_17
33. Lin A, Qi C, Wei T, et al. CAMOIP: a web server for comprehensive analysis on multi-omics of immunotherapy in pan-cancer. Brief Bioinform. 2022;23(3). doi:10.1093/bib/bbac129
34. Subramanian A, Kuehn H, Gould J, Tamayo P, Mesirov JP. GSEA-P: a desktop application for gene set enrichment analysis. Bioinformatics. 2007;23(23):3251–3253. doi:10.1093/bioinformatics/btm369
35. Hänzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013;14(1):7. doi:10.1186/1471-2105-14-7
36. Liberzon A, Subramanian A, Pinchback R, Thorvaldsdottir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27(12):1739–1740. doi:10.1093/bioinformatics/btr260
37. Leal T, Wang Y, Dowlati A, et al. Randomized phase II clinical trial of cisplatin/carboplatin and etoposide (CE) alone or in combination with nivolumab as frontline therapy for extensive-stage small cell lung cancer (ES-SCLC): ECOG-ACRIN EA5161. J Clin Oncol. 2020;38(suppl15):9000. doi:10.1200/JCO.2020.38.15_suppl.9000
38. Reck M, Luft A, Szczesna A, et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol. 2016;34(31):3740–3748. doi:10.1200/JCO.2016.67.6601
39. Liang C, Fan J, Liang C, Guo J. Identification and validation of a pyroptosis-related prognostic model for gastric cancer. Front Genet. 2021;12:699503. doi:10.3389/fgene.2021.699503
40. Hachim MY, Hachim IY, Talaat IM, Yakout NM, Hamoudi R. M1 polarization markers are upregulated in basal-like breast cancer molecular subtype and associated with favorable patient outcome. Front Immunol. 2020;11:560074. doi:10.3389/fimmu.2020.560074
41. Cheng S-W, Chen P-C, Lin M-H, Ger T-R, Chiu H-W, Lin Y-F. GBP5 repression suppresses the metastatic potential and PD-L1 expression in triple-negative breast cancer. Biomedicines. 2021;9(4):371. doi:10.3390/biomedicines9040371
42. Wang Q, Wang X, Liang Q, et al. Distinct prognostic value of mRNA expression of guanylate-binding protein genes in skin cutaneous melanoma. Oncol Lett. 2018;15(5):7914–7922. doi:10.3892/ol.2018.8306
43. Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702. doi:10.1146/annurev-immunol-032713-120145
44. Yan Y, Zheng L, Du Q, et al. Interferon regulatory factor 1(IRF-1) activates anti-tumor immunity via CXCL10/CXCR3 axis in hepatocellular carcinoma (HCC). Cancer Lett. 2021;506:95–106. doi:10.1016/j.canlet.2021.03.002
45. Hsu C-L, Ou D-L, Bai L-Y, et al. Exploring markers of exhausted CD8 T cells to predict response to immune checkpoint inhibitor therapy for hepatocellular carcinoma. Liver Cancer. 2021;10(4):346–359. doi:10.1159/000515305
46. Inoue H, Park J-H, Kiyotani K, et al. Intratumoral expression levels of PD-L1, GZMA, and HLA-A along with oligoclonal T cell expansion associate with response to nivolumab in metastatic melanoma. Oncoimmunology. 2016;5(9):e1204507. doi:10.1080/2162402X.2016.1204507
47. Ruf M, Moch H, Schraml P. Interaction of tumor cells with infiltrating lymphocytes via CD70 and CD27 in clear cell renal cell carcinoma. Oncoimmunology. 2015;4(12):e1049805. doi:10.1080/2162402X.2015.1049805
48. Willeaume V, Kruys V, Mijatovic T, Huez G. Tumor necrosis factor-alpha production induced by viruses and by lipopolysaccharides in macrophages: similarities and differences. J Inflamm. 1995;46(1):1–12.
49. de Silva DG, Mendis LN, Sheron N, et al. TNF alpha in stool as marker of intestinal inflammation. Lancet. 1992;340(8815):372. doi:10.1016/0140-6736(92)91446-f
50. Grell M, Wajant H, Zimmermann G, Scheurich P. The type 1 receptor (CD120a) is the high-affinity receptor for soluble tumor necrosis factor. Proc Natl Acad Sci U S A. 1998;95(2):570–575. doi:10.1073/pnas.95.2.570
51. Colonna M. innate lymphoid cells: diversity, plasticity, and unique functions in immunity. Immunity. 2018;48(6):1104–1117. doi:10.1016/j.immuni.2018.05.013
52. Zhang C, Targeting LY. NK cell checkpoint receptors or molecules for cancer immunotherapy. Front Immunol. 2020;11:1295. doi:10.3389/fimmu.2020.01295
53. Helmink BA, Reddy SM, Gao J, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577(7791):549–555. doi:10.1038/s41586-019-1922-8
54. Cabrita R, Lauss M, Sanna A, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020;577(7791):561–565. doi:10.1038/s41586-019-1914-8
55. Daud AI, Loo K, Pauli ML, et al. Tumor immune profiling predicts response to anti-PD-1 therapy in human melanoma. J Clin Invest. 2016;126(9):3447–3452. doi:10.1172/JCI87324
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.