Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
Human Mesenchymal Stem Cell-Derived Exosomes Promote the Proliferation and Melanogenesis of Primary Melanocytes by Attenuating the H2O2-Related Cytotoxicity in vitro
Authors Wang Y, He Z, Luo B, Wong H, Wu L, Zhou H
Received 26 October 2023
Accepted for publication 13 February 2024
Published 18 March 2024 Volume 2024:17 Pages 683—695
DOI https://doi.org/10.2147/CCID.S446676
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Yexiao Wang,1,* Zibin He,2,* Bingqin Luo,2 Hioteng Wong,1 Liangcai Wu,3 Hui Zhou1
1Department of Dermatology, the First Affiliated Hospital of Sun Yat-sen University, Guangzhou, People’s Republic of China; 2MOE Key Laboratory of Gene Function and Regulation, State Key Laboratory of Biocontrol, School of Life Sciences, Sun Yat-sen University, Guangzhou, People’s Republic of China; 3Department of Dermatology, the Sixth Affiliated Hospital of Sun Yat-sen University, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Liangcai Wu; Hui Zhou, Tel +86 13380019222 ; +86 13660389658, Email [email protected]; [email protected]
Background: Mesenchymal stem cell-derived exosomes (MSC-Exo) have therapeutic potential. However, the impact of MSC-Exo on the survival and melanogenesis of human primary melanocytes following H2O2-induced damage has not been clarified. We therefore investigated the effects of MSC-Exo on the H2O2-affected survival of human primary melanocytes and their proliferation, apoptosis, senescence, and melanogenesis in vitro.
Methods: MSC-Exo were prepared from human MSCs by sequential centrifugations and characterized by Transmission Electron Microscopy, Western blot and Nanoparticle Tracking Analysis. Human primary melanocytes were isolated and treated with different concentrations of MSC-Exo, followed by exposing to H2O2. Furthermore, the impact of pretreatment with MSC-Exo on the proliferation, apoptosis, senescence and melanogenesis of melanocytes were tested by CCK-8, flow cytometry, Western blot, L-Dopa staining, tyrosinase activity and RT-qPCR.
Results: Pretreatment with lower doses of MSC-Exo protected human primary melanocytes from the H2O2-triggered apoptosis, while pretreatment with higher doses of MSC-Exo enhanced the H2O2-induced melanocyte apoptosis. Compared with the untreated control, pretreatment with a lower dose (1 μg/mL) of MSC-Exo enhanced the proliferation of melanocytes, abrogated the H2O2-increased p53, p21, IL-1β, IL-6 and IL-8 expression and partially rescued the H2O2-decreased L-dopa staining reaction, tyrosinase activity, MITF and TRP1 expression in melanocytes.
Conclusion: Our findings indicate that treatment with a low dose of MSC-Exo promotes the proliferation and melanogenesis of human primary melanocytes by ameliorating the H2O2-induced apoptosis and senescence of melanocytes. MSC-Exo may be a promising therapeutic strategy of vitiligo.
Keywords: mesenchymal stem cell-derived exosomes, melanocytes, apoptosis, proliferation, senescence
Introduction
Vitiligo is one of the common skin diseases and characterized by the selective destruction of epidermal melanocytes, leading to depigmented patches in the skin or mucous membranes. Vitiligo was first documented as far back as 3500 years ago,1 and currently affects about 0.5–2% of people in the world.2,3 Vitiligo affects people of all Fitzpatrick skin types,4 but it may be more noticeable on darker skin.5 Vitiligo can involve any part of the body, particularly for the visible areas, such as the face and arms, and even spread to whole body, causing great psychological distress to patients and seriously affecting quality of life of both the patients and their family members.6,7
In recent years, scientific researchers have made significant progress in understanding the pathogenesis of vitiligo and helped in developing better therapeutic strategies.1,8 Oxidative stress and autoimmunity-induced melanogenesis impairment and death of melanocyte have been considered to be the two key factors that contribute to the pathogenesis of vitiligo.2 It is currently believed that oxidative stress plays a pivotal role in initiating the autoimmune response associated with vitiligo9 and IFN-γ-CXCL9/CXCL10-CXCR3 Axis leads to melanocyte destruction in vitiligo.10–12 Furthermore, premature senescence of melanocytes is also crucial for the development and progression of vitiligo.13,14 Although a variety of treatments are available, including phototherapy,15 topical medications,2 systemic drugs,16 surgery17 and targeted therapies,8 the efficacy of these treatments still remains unsatisfactory.2,18 Nearly 40% of vitiligo patients relapse, due to lesional CD8+ resident memory T cells (TRM),19 within one year after discontinuation of treatment.20 Therefore, there is an urgent need to explore more new treatments.
Mesenchymal stem cells (MSCs) have unique characteristics with immunomodulatory, pro-survival, pro-angiogenic, anti-apoptotic, and anti-inflammatory activities,21 and have potential to be used as a novel therapeutic strategy for the intervention of a variety of diseases.22 Mechanistically, MSCs can secrete many soluble factors and release extracellular vesicles (EVs).23–25 Exosome is one of the main classes of EVs. Interestingly, the functions of MSC-derived exosomes (MSC-Exo) are similar to MSCs, whereas more stable, less immunogenic and with little risk of tumorigenesis.26 Recent studies have shown that MSC-Exo can protect and promote the regeneration of a variety of cells and tissues.27–29 MSC-Exo can also have immunomodulatory, anti-apoptotic, antioxidant, and anti-senescence activities.30–33 In the field of dermatology, MSC-Exo have been shown to be a promising novel therapeutic option for the treatment of wound healing, atopic dermatitis, hair loss and aging.34 However, the impact of MSC-Exo on the survival and function of melanocytes is not clarified.
In this study, we tested the effects of pretreatment with MSC-Exo on the H2O2-affected survival of human primary melanocytes and their proliferation, apoptosis, senescence, and melanogenesis in vitro. Our findings indicated that pretreatment with MSC-Exo protected from the H2O2-induced cytotoxicity against human primary melanocytes by promoting their survival and melanogenesis and inhibiting their apoptosis and senescence, suggesting that MSC-Exo may be promising for the treatment of vitiligo.
Materials and Methods
Preparation of Human Primary Melanocytes
Written informed consent was obtained from all donors, and the experimental protocol was approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University (approval number: [2023]215). Human foreskin tissues were obtained from individual male subjects (n=30, 20–28 years old), who underwent circumcision and their primary melanocytes were isolated. Individual specimens were sequentially washed with 75% ethanol and PBS solution containing 1% penicillin-streptomycin. The specimens were digested with 0.25% Dispase II solution at 4°C for 16–20 hours. The epidermis was treated with 0.25% trypsin for 15 minutes and cultured in melanocyte medium (iCell, Shanghai, China) supplemented with 0.5% foetal bovine serum (FBS) (iCell) and 1% human melanocyte growth supplement (iCell) at 37°C for 14 days. The morphology of melanocytes was observed under a microscope, and the melanocytes were identified by L-Dopa staining (2 mg/mL levodopa in PBS, Sigma, MO, USA) at a temperature of 37°C for 1 hour.
Isolation and Characterization of Exosomes
Human umbilical cord mesenchymal stem cells (hUC-MSCs) were kindly provided by Prof. Wenbin Ma at the School of Life Sciences, Sun Yat-sen University. The hUC-MSCs at passage of 1–2 were cultured in DMEM (Gibco, NY, USA) supplemented with 10% FBS at 37°C for 14 days. Subsequently, the cells were cultured in FBS-free hUC-MSCs exosome secretion-promoting medium Ultra CULTURETM (Lonza, Basel, Switzerland) for 48 hours, followed by collecting their culture supernatants for the isolation of exosomes by continual centrifugations.35 In brief, the supernatants were sequentially centrifuged at 4°C at 300×g for 10 min, at 2000×g for 10 min, at 10,000×g for 30 min and 100,000×g for 90 min. The pellets were resuspended in PBS and centrifuged at 100,000×g for 90 min, followed by re-suspending in PBS. The MSC-Exo were quantified using the BCA kit (Thermo Fisher, MA, USA) at a wave length of 562 nm and characterized by Transmission Electron Microscopy (TEM) (Hitachi, Tokyo, Japan) and their protein markers of CD9, CD63, and CD81 were analysed by Western blot. The concentrations and size distribution of MSC-Exo were determined using Nanoparticle Tracking Analysis (NTA) (Malvern Panalytical, WILT, UK).
Cell Treatment
Previous studies have shown that treatment with H2O2 can induce the senescence, caspase-3-dependent apoptosis and necrosis, dependent on the concentrations of H2O2.36,37 Based on previous studies38,39 and our preliminary experiments, treatment with 750 µmol/L of H2O2 reduced the viability and proliferation of human primary melanocytes, but did not cause excessive cell necrosis. Accordingly, human primary melanocytes (1x105 cells/well) were treated with, or without, 750 µmol/L of H2O2 (Sigma-Aldrich, MO, USA) for 24 hours. In addition, the melanocytes were pre-cultured with 1–40 µg/mL of MSC-Exo for 24 hours and exposed to 750 µmol/L of H2O2 for another 24 hours.
Western Blot (WB)
The different groups of cells were harvested and lyzed, followed by centrifugation. After determining the protein concentrations, the cell lysate samples (40 μg/lane) were separated by sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on 10% gels and transferred onto polyvinylidene fluoride (PVDF) membrane. The membrane was blocked with 5% fat-free dry milk and incubated with primary antibodies overnight at 4°C. The primary antibodies included anti-caspase-3 (Cell Signaling Technology, CST, MA, USA), anti-microphthalmia-associated transcription factor (MITF) (CST), anti-tyrosinase (TYR) (Abcam, Cambridge, UK) and anti-tyrosinase related protein 1 (TRP1) (Abcam) as well as anti-β-Actin (Abclonal, Wuhan, China) and anti-GAPDH (Abmart, Shanghai, China). After being washed, the target proteins were visualized using fluorescent secondary antibodies (LI-COR, NE, USA) and chemiluminescent secondary antibodies (Ray antibody, Beijing, China and Abcam). In addition, the prepared MSC-Exo were subjected to WB analysis using primary antibodies against CD9, CD63, and CD81 (Immunoway Biotechnology, TX, USA).
Cell Counting Kit 8 (CCK-8) Assay
The viability, proliferation, and survival of primary melanocytes were determined using the CCK-8 assay kit (Dojindo, Tokyo, Japan) according to the manufacturer’s instruction. Briefly, melanocytes at 3–4 passages (1x104 cells/well) were cultured in 96-well plates for varying time periods. During the last 3-hour culture, the cells in each well were exposed to 10 μL of CCK-8 reagent. The formed formazan in each well was quantified by measuring the absorbance at 450 nm using a multi-mode reader (BioTek, VT, USA).
Cell Apoptosis Assay
The percentages of apoptotic cells were quantified by flow cytometry (Beckman Coulter, CA, USA) using a PI/Annexin V-FITC apoptosis detection kit (Beyotime, Shanghai, China), following the manufacturer’s protocol. Briefly, the different groups of melanocytes were harvested and the cells (1x105 cells/tube) were stained with Annexin V-FITC and PI in the dark. After being washed, the cells were analysed by flow cytometry.
RNA Sample Preparation and Quantitative Real-Time PCR (RT-qPCR)
Total RNA was extracted from individual groups of cells using Trizol reagent (Gibco) and reverse-transcribed into cDNA using a PrimeScript™ RT reagent kit (Takara, Kyoto, Japan), according to manufacturer’s protocol. The RNA concentrations were measured using a spectrophotometer reader (NanoDrop Technologies, DE, USA). The relative levels of IL-8, IL-6 and IL-1β mRNA transcripts to the internal control GAPDH were measured by RT-qPCR using the power SYBR Green PCR master mix (Takara) and specific primers in a real-time PCR machine (Roche, Basel, Switzerland). The sequences of primers were forward 5’-ACTGAGAGTGATTGAGAGTGGAC-3’ and reverse 5’-AACCCTCTGCACCCAGTTTTC-3’ for IL-8; forward 5’-GCCCCAGTACCCCCAGGAG-3’ and reverse 5’-TCTGCCAGTGCCTCTTTGCT-3’ for IL-6; forward 5’-CCACAGACCTTCCAGGAGAATG-3’ and reverse 5’-GTGCAGTTCAGTGATCGTACAGG-3’ for IL-1β; and forward 5’-AAGATCCGAGAAGAATACCCTGA-3’ and reverse 5’-CTACCAACTGATGGACGGAGA-3’ for β-Tubulin. The data were normalized to β-Tubulin and analysed by 2−ΔΔCt.
Tyrosinase Activity Assay
The activity of tyrosinase in individual groups of melanocytes was measured by enzymatic assay using the Tyrosinase Activity Assay Kit (Solarbio, Beijing, China), per the supplier’s protocol. Briefly, melanocytes (1x105 cells/well) were cultured in 6-well plates for specific treatment, harvested and lyzed in lysis buffer on ice for 5 minutes. The tyrosinase activity was measured spectrophotometrically at an absorbance of 475 nm using a multi-mode reader (BioTek) and normalized to the protein concentration of each sample.
Statistical Analysis
The data are presented as mean ± standard deviation (SD). The difference among groups was analysed by one- or two-way ANOVA using SPSS 25.0. Graphs were created using GraphPad Prism 9.0. A P-value of <0.05 was considered statistically significant.
Results
Characterization of Primary Melanocytes and MSC-Exo
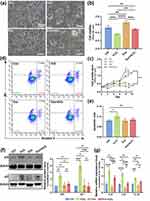
Following culture of melanocytes for 14 days, those cells displayed bipolar or multipolar morphology under a microscope (Figure 1a). L-Dopa staining revealed that the isolated melanocytes exhibited grey-brown cytosol and dendrites under a phase-contrast microscope, an indicative of positive L-Dopa staining (Figure 1b). The EVs of hUC-MSCs were isolated by sequential centrifugations and the isolated MSC-Exo had an average of about 100 nm in size (Figure 1c). TEM analysis unveiled that these MSC-Exo displayed a three-dimensional “teacup tray” shape and a double-layered membrane structure for a typical morphology (Figure 1c). Furthermore, WB revealed that these MSC-Exo contained CD9, CD63, and CD81, typical protein markers of exosome (Figure 1d). Moreover, NTA results indicated that these MSC-Exo had a peak diameter of 110 nm (Figure 1e). Hence, we successfully isolated human melanocytes and MSC-Exo.
Pretreatment with MSC-Exo Has Opposite Effects on the H2O2-Induced Apoptosis of Human Primary Melanocytes
Next, our study tested the effect of MSC-Exo on the H2O2-induced apoptosis of human primary melanocytes. Human primary melanocytes were pretreated with 1–40 µg/mL MSC-Exo for 24 hours and exposed to 750 µmol/L of H2O2 for another 24 hours. The percentages of apoptotic melanocytes were determined by flow cytometry after staining them with Annexin V-FITC and PI. Quantitative analysis indicated that compared with the untreated control, pretreatment with 1 μg/mL MSC-Exo significantly decreased the percentages of apoptotic melanocytes (Figure 2a and b). However, pretreatment with a higher dose of 5 or 10 μg/mL MSC-Exo did not change the H2O2-induced apoptosis of melanocytes and pretreatment with further increased doses (20, 40 μg/mL) of MSC-Exo significantly increased the frequency of apoptotic melanocytes in vitro.
Caspase-3 and MITF are important regulators of cell apoptosis and melanogenesis, respectively. To determine the dose effects of MSC-Exo, the levels of cleaved caspase-3 and MITF expression in the different groups of melanocytes were analysed by WB. Compared with the control, pretreatment with the indicated doses of MSC-Exo significantly reduced caspase-3 cleavage (Figure 2c). Pretreatment with a low concentration (1 µg/mL) of MSC-Exo had the most down-regulatory effect on caspase-3 cleavage while pretreatment with a high dose (40 µg/mL) of MSC-Exo achieved a little inhibitory effect on caspase-3 cleavage in the H2O2-exposed melanocytes (Figure 2c). In contrast, pretreatment with 1 µg/mL MSC-Exo obviously increased the levels of MITF expression, but pretreatment with 40 µg/mL MSC-Exo dramatically decreased MITF expression in the H2O2-exposed melanocytes (Figure 2c). Apparently, pretreatment with MSC-Exo had opposite effects on the H2O2-induced apoptosis of primary melanocytes, dependent on its doses and pre-treatment with low concentration of MSC-Exo protected strongly against the H2O2-induced apoptosis and promoted melanogenesis of melanocytes. Therefore, we chose 1 μg/mL as the intervention concentration for subsequent experiments.
MSC-Exo Attenuates the H2O2-Induced Cytotoxicity Against Melanocytes by Promoting Their Survival
To understand the role of MSC-Exo in regulating the survival of melanocytes, human primary melanocytes were pretreated with vehicle alone or 1 µg/mL MSC-Exo for 24 hours and treated with vehicle alone or 750 µmol/L of H2O2 for 24 hours. As a result, there were four groups of cells including the control (Ctrl), H2O2, Exo, Exo + H2O2 groups. Morphologically, melanocytes in the control and Exo groups displayed regular cytosolic and dendritic morphology with most cells with 3–6 dendrites and some cell fusion together (Figure 3a). In contrast, the H2O2 group exhibited a reduced number of cells, particularly with few cell fusion and thinner and shorter dendrites, and many cells only had 2–3 dendrites with membrane exfoliation and cell shrinkage, indicating that H2O2 exposure impaired melanocytes. Pretreatment with 1 μg/mL MSC-Exo obviously mitigated the damage of melanocytes and improved their morphology in the Exo+H2O2 group, reflecting that pretreatment with MSC-Exo protected against the H2O2-induced cytotoxicity against human primary melanocytes in vitro. Similarly, compared with the control, H2O2 exposure significantly decreased the viability of melanocytes while pretreatment with 1 μg/mL MSC-Exo significantly increased the viability of melanocytes and abrogated the H2O2-induced cytotoxicity against melanocytes (Figure 3b). A similar pattern of the dynamic melanocyte proliferation was observed by CCK-8 assays (Figure 3c). Furthermore, H2O2 exposure significantly increased the frequency of apoptotic melanocytes while pretreatment with 1 μg/mL MSC-Exo promoted the survival of melanocytes regardless of H2O2 exposure (Figure 3d and e). Moreover, while H2O2 exposure significantly increased the relative levels of p53 and p21 protein expression, pretreatment with 1 μg/mL MSC-Exo completely abrogated the H2O2-enhanced p53 and p21 expression in melanocytes (Figure 3f). Given that inflammatory cytokines, such as, IL-8, IL-6, and IL-1β, contribute to the process of melanocyte senescence, we tested the relative levels of IL-8, IL-6, and IL-1β mRNA transcripts in the different groups of melanocytes by RT-qPCR. The results indicated that H2O2 exposure significantly increased the relative levels of IL-8, IL-6, and IL-1β mRNA transcripts, relative to that in the control while pretreatment with 1 μg/mL MSC-Exo significantly decreased IL-6 mRNA transcripts and dramatically mitigated or abrogated the H2O2-enhanced IL-8, IL-6, and IL-1β mRNA transcripts in melanocytes (Figure 3g). Collectively, these data evidenced that pretreatment with MSC-Exo prevented the H2O2-induced cytotoxicity and senescence of melanocytes by promoting their survival in vitro.
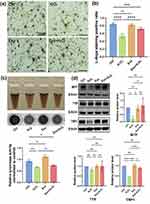
MSC-Exo Rescues the H2O2-Inhibited Melanogenesis in Primary Melanocytes
Finally, we tested the impact of pretreatment with MSC-Exo on melanogenesis of human primary melanocytes following H2O2 treatment. Following culture of the different groups of cells for 48 hours, the cells were subjected to L-Dopa staining, which can quantify the levels of melanin synthesis in melanocytes.40 The results displayed that the cells in the H2O2 group exhibited noticeably lighter cytosolic L-Dopa staining and fewer dendrites compared to the cells in the control group while pretreatment with MSC-Exo did not significantly increase the levels of L-Dopa staining in the Exo group, but partially rescued the H2O2-reduced L-Dopa staining in the Exo+H2O2 group of melanocytes (Figure 4a and b). A similar pattern of Tyrosinase activities was detected in the different groups of cells (Figure 4c). Furthermore, compared with the Control, H2O2 exposure significantly reduced the relative levels of MITF, TRP1, but not TYR expression while pretreatment with MSC-Exo significantly mitigated or abrogated the H2O2-reduced MITF and TRP1 expression in melanocytes in our experimental conditions (Figure 4d). Together, these data indicated that pretreatment with MSC-Exo promoted the melanogenesis of human primary melanocytes following H2O2 treatment.
Discussion
In this study, we investigated the effects of MSC-Exo on the H2O2-affected survival of human primary melanocytes and their proliferation, apoptosis, senescence, and melanogenesis in vitro. The data indicated that treatment with a low dose of MSC-Exo promoted the proliferation and melanogenesis of human primary melanocytes by ameliorating the H2O2-induced apoptosis and senescence of melanocytes. Our findings suggest that MSC-Exo may be a promising therapeutic agent for vitiligo.
Vitiligo clinically displays depigmented or hypopigmented patches due to progressive destruction of melanocytes. The aetiology of vitiligo is attributed to a high genetic vulnerability, oxidative stress, inflammation, and dysregulation of senescence or apoptosis.2,41 Early and aggressive treatment may prevent damage to melanocytes and/or improve their resistance to damage.
Exosome is one type of EVs and has a size of 40–160 nm in diameter. Exosomes can act as mediators of intercellular communication by carrying and exchanging bioactive components, such as proteins, cytokines and nucleic acids.22,42 A recent study indicates that MSC-Exo is now widely accepted as the next generation cell-free therapy for refractory diseases.30 In the field of dermatology, MSC-Exo has also been proven to have enormous potential for biomarkers and therapeutics.43 Treatment with MSC-Exo has protective and promotive effects on human keratinocytes44 and dermal fibroblasts45 in vitro. Actually, co-culture of MSC with melanocytes promotes the proliferation of melanocytes.46 In this study, we found for the first time, that treatment with low dose of MSC-Exo enhanced the proliferation of human primary melanocytes, besides mitigating the H2O2-induced cytotoxicity against melanocytes in vitro. These novel findings extended previous observation and support the notion that MSC-Exo protects melanocytes from the H2O2-induced cytotoxicity.
Accumulated data support the hypothesis that apoptosis, but not necrosis, is responsible for the loss of melanocytes during the early process of vitiligo.47–49 Actually, high levels of CD95L (FasL) expression are detected in the epidermis and dermis of both perilesional and non-lesional skin of active vitiligo patients, suggesting that the FasL/Fas-related apoptosis may be crucial for melanocyte destruction and the pathogenesis of active vitiligo.50 Compared to healthy controls, perilesional melanocytes from unstable vitiligo display higher levels of caspase-3 expression following apoptos is stimuli.51 It is notable that MSC-Exo have potent anti-apoptotic effect in vitro52,53 and in vivo.54,55 In this study, we found that pretreatment of melanocytes with low dose of MSC-Exo had reversal effects on the H2O2-induced caspase-3 cleavage and melanocyte apoptosis. As previous studies found that MSC-Exo could repair oxidative stress-induced damage in keratinocytes and skin of animal model through adaptive regulation of NRF2 defense system and NRF2/HO-1 signaling pathway is involved in the mitigation of H2O2-mediated apoptosis in human keratinocytes,32,56 we suggest that MSC-Exo may mitigate the H2O2-triggered apoptosis of melanocytes through the NRF2/HO-1 pathway.
However, our flow cytometry results indicated that higher doses (20 and 40 μg/mL) of MSC-Exo had a pro-apoptotic effect on cells with H2O2 exposure, which was in accordance with a previous study in cardiomyocytes.52 Combined with our WB results that lower doses of exosomes inhibited the production of apoptosis-associated cleaved caspase-3 induced by H2O2, we hypothesized that the pro-apoptotic effect of high concentrations of MSC-Exo might be mediated through the caspase-3-independent pathway in melanocytes, such as excessive MSC-Exo intake by melanocytes leading to overloading of cells and thus activating other apoptotic pathways. More experiments are needed to prove this hypothesis.
Previous studies have shown that melanocyte senescence participates in the early pathogenic process of vitiligo and the persistent aggravation of damage will eventually lead to death and destruction of melanocytes.13,37 In this study, we found that pretreatment with MSC-Exo dramatically abrogated or mitigated the H2O2-enhanced P53 and p21 expression, IL-8, IL-6 and IL-1β mRNA transcription in melanocytes. The upregulated p53 expression is previously observed in melanocytes of non-lesional vitiligo skin,14,57 and p53 can induce downstream p21 expression. Furthermore, during the process of senescence, stimuli can also induce the overexpression of the senescence-associated secretory phenotype (SASP)-related cytokines including IL-6 and IL-8.58 Given that up-regulated p53 and downstream p21 expression are hallmarks of cell senescence, the significantly attenuated p53 and p21 expression by MSC-Exo, together with the decreased levels of IL-8, IL-6 and IL-1β expression, clearly indicated that pretreatment with MSC-Exo inhibited and prevented the H2O2-induced senescence of melanocytes. We speculate that MSC-Exo may inhibit the H2O2-induced p53-p21 expression and cell senescence in human melanocytes. We are interested in further investigating the hypothesis in the future studies.
Melanogenesis is a complicated process and regulated by multiple genes and signalling pathways.59 The MITF is a key transcription factor regulating the survival and melanogenesis of melanocytes and the expression of downstream proteins including TYR, TRP-1, and TRP-2.60 A previous study has shown that exosomes, such as keratinocyte derived-exosome (KC-Exo), can regulate melanogenesis and miRNA in KC-Exo regulates pigmentation in melanocytes, contributing to the pathogenesis of pigmentation-related diseases.61 KC-Exo containing miRNAs and other soluble factors can mediate the communication between keratinocytes and melanocytes in pigmentation modulation by regulating the expression of MITF, TYR, TRP1 and TRP2.62,63 In this study, we found that pretreatment with MSC-Exo significantly rescued the H2O2-decreased MITF and TRP1 expression as well as tyrosinase activities in melanocytes. Therefore, in the early stage of melanocyte injury process induced by oxidative stress in patients with vitiligo, external or systematic use of MSC-Exo may promote the functional recovery of melanocyte melanogenesis in patients with vitiligo.
These novel data indicated that MSC-Exo enhanced the melanogenesis of melanocytes following H2O2 treatment. Hence, therapeutic strategies to enhance MITF and other melanogenesis-related protein expression as well as their activities may be valuable for the intervention of vitiligo.64
Exosomes have already become promising multipotent frontiers in dermatology and cutaneous medical aesthetics.65 Cryogel wound dressing OxOBand loaded with exosomes from adipose-derived stem cells has been demonstrated to facilitate wound healing in rat model of diabetic wound ulcer.66 Microneedle device integrated with MSC-Exo has been shown to promote pigmentation and hair regrowth in a mouse model.67 The available findings suggest that exosomes, especially MSC-Exo, are highly bioactive and may be used as a potential therapeutic option for the treatment of vitiligo.
Conclusion
In summary, the results from this study indicated that pretreatment with MSC-Exo ameliorated the H2O2-induced cytotoxicity against human primary melanocytes by promoting the proliferation and melanogenesis of human primary melanocytes and attenuating the H2O2-induced apoptosis and senescence of melanocytes. The treatment of vitiligo has always been challenging. It is well known that the key to the treatment of vitiligo is the restoration of melanocyte activity and melanin synthesis. These findings indicate that MSC-Exo may be a potential novel cell-free therapeutic strategy for the intervention of vitiligo.
We recognized that our study had limitations. The current study only preliminarily explored the effect of MSC-Exo on melanocytes. Further elucidation of the therapeutic efficacy of MSC-Exo and the specific mechanisms underlying the action of MSC-Exo in regulating the survival and melanogenesis of melanocytes, particularly for those from vitiligo patients or in animal models of vitiligo are warranted.
Ethics Statement
The study was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University. The patients provided their written informed consent before participating in this study. Our study was complied with the Declaration of Helsinki.
Acknowledgments
The authors sincerely thank Professor Wenbin Ma and Research Assistant Ting Zeng, School of Life Sciences, Sun Yat-sen University for their excellent technical assistance.
Funding
This work was supported by a grant from the Natural Science Foundation of Guangdong Province (No.2021A1515012074 to L. Wu). All authors had full access to the full data in the study and accepted responsibility to submit for publication.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Frisoli ML, Essien K, Harris JE. Vitiligo: mechanisms of pathogenesis and treatment. Ann Rev Immunol. 2020;38(1):621–648. doi:10.1146/annurev-immunol-100919-023531
2. Picardo M, Dell’Anna ML, Ezzedine K, et al. Vitiligo. Nat Rev Dis Primers. 2015;1:15011. doi:10.1038/nrdp.2015.11
3. Iwanowski T, Kołkowski K, Nowicki RJ, Sokołowska-Wojdyło M. Etiopathogenesis and emerging methods for treatment of vitiligo. Int J Mol Sci. 2023;24(11):9749. doi:10.3390/ijms24119749
4. Zhang Y, Cai Y, Shi M, et al. The prevalence of vitiligo: a meta-analysis. PLoS One. 2016;11(9):e0163806. doi:10.1371/journal.pone.0163806
5. Ahn JS, Lim JG, Kim SD, Kim KH, Park KC. Vitiligo skin types in Koreans. J Dermatol. 2000;27(5):324–328. doi:10.1111/j.1346-8138.2000.tb02175.x
6. Rodrigues M, Ezzedine K, Hamzavi I, Pandya AG, Harris JE, Vitiligo Working G. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol. 2017;77(1):1–13. doi:10.1016/j.jaad.2016.10.048
7. Bin Saif GA, Al-Balbeesi AO, Binshabaib R, et al. Quality of life in family members of vitiligo patients: a questionnaire study in Saudi Arabia. Am J Clin Dermatol. 2013;14(6):489–495. doi:10.1007/s40257-013-0037-5
8. Feng Y, Lu Y. Advances in vitiligo: update on therapeutic targets. Front Immunol. 2022;13:986918. doi:10.3389/fimmu.2022.986918
9. Chang WL, Ko CH. The role of oxidative stress in vitiligo: an update on its pathogenesis and therapeutic implications. Cells. 2023;12(6):936. doi:10.3390/cells12060936
10. Tulic MK, Cavazza E, Cheli Y, et al. Innate lymphocyte-induced CXCR3B-mediated melanocyte apoptosis is a potential initiator of T-cell autoreactivity in vitiligo. Nat Commun. 2019;10(1):2178. doi:10.1038/s41467-019-09963-8
11. Hlaca N, Zagar T, Kastelan M, Brajac I, Prpic-Massari L. Current Concepts of Vitiligo Immunopathogenesis. Biomedicines. 2022;10(7):1639. doi:10.3390/biomedicines10071639
12. Liu H, Wang Y, Le Q, Tong J, Wang H. The IFN-γ-CXCL9/CXCL10-CXCR3 axis in vitiligo: pathological mechanism and treatment. Eur J Immunol. 2023;e2250281. doi:10.1002/eji.202250281
13. Bellei B, Picardo M. Premature cell senescence in human skin: dual face in chronic acquired pigmentary disorders. Ageing Res Rev. 2020;57:100981. doi:10.1016/j.arr.2019.100981
14. Bellei B, Pitisci A, Ottaviani M, et al. Vitiligo: a possible model of degenerative diseases. PLoS One. 2013;8(3):e59782. doi:10.1371/journal.pone.0059782
15. Bae JM, Jung HM, Hong BY, et al. Phototherapy for vitiligo: a systematic review and meta-analysis. JAMA Dermatol. 2017;153(7):666–674. doi:10.1001/jamadermatol.2017.0002
16. Searle T, Al-Niaimi F, Ali FR. Vitiligo: an update on systemic treatments. Clin Exp Dermatol. 2021;46(2):248–258. doi:10.1111/ced.14435
17. Ju HJ, Bae JM, Lee RW, et al. Surgical interventions for patients with vitiligo: a systematic review and meta-analysis. JAMA Dermatol. 2021;157(3):307–316. doi:10.1001/jamadermatol.2020.5756
18. Ezzedine K, Eleftheriadou V, Whitton M, van Geel N. Vitiligo. Lancet. 2015;386(9988):74–84. doi:10.1016/S0140-6736(14)60763-7
19. Shah F, Patel S, Begum R, Dwivedi M. Emerging role of tissue resident memory T cells in vitiligo: from pathogenesis to therapeutics. Autoimmun Rev. 2021;20(8):102868. doi:10.1016/j.autrev.2021.102868
20. Nicolaidou E, Antoniou C, Stratigos AJ, Stefanaki C, Katsambas AD. Efficacy, predictors of response, and long-term follow-up in patients with vitiligo treated with narrowband UVB phototherapy. J Am Acad Dermatol. 2007;56(2):274–278. doi:10.1016/j.jaad.2006.09.004
21. Song N, Scholtemeijer M, Shah K. Mesenchymal stem cell immunomodulation: mechanisms and therapeutic potential. Trends Pharmacol Sci. 2020;41(9):653–664. doi:10.1016/j.tips.2020.06.009
22. Xunian Z, Kalluri R. Biology and therapeutic potential of mesenchymal stem cell-derived exosomes. Cancer Sci. 2020;111(9):3100–3110. doi:10.1111/cas.14563
23. Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther. 2016;7(1):125. doi:10.1186/s13287-016-0363-7
24. Liang X, Ding Y, Zhang Y, Tse HF, Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014;23(9):1045–1059. doi:10.3727/096368913x667709
25. Keshtkar S, Azarpira N, Ghahremani MH. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther. 2018;9(1):63. doi:10.1186/s13287-018-0791-7
26. Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci. 2014;15(3):4142–4157. doi:10.3390/ijms15034142
27. Zhu LP, Tian T, Wang JY, et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics. 2018;8(22):6163–6177. doi:10.7150/thno.28021
28. Bruno S, Grange C, Deregibus MC, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20(5):1053–1067. doi:10.1681/ASN.2008070798
29. Psaraki A, Ntari L, Karakostas C, Korrou-Karava D, Roubelakis MG. Extracellular vesicles derived from mesenchymal stem/stromal cells: the regenerative impact in liver diseases. Hepatology. 2022;75(6):1590–1603. doi:10.1002/hep.32129
30. Ha DH, Kim HK, Lee J, et al. Mesenchymal stem/stromal cell-derived exosomes for immunomodulatory therapeutics and skin regeneration. Cells. 2020;9(5):1157. doi:10.3390/cells9051157
31. Cheng X, Zhang G, Zhang L, et al. Mesenchymal stem cells deliver exogenous miR-21 via exosomes to inhibit nucleus pulposus cell apoptosis and reduce intervertebral disc degeneration. J Cell Mol Med. 2018;22(1):261–276. doi:10.1111/jcmm.13316
32. Wang T, Jian Z, Baskys A, et al. MSC-derived exosomes protect against oxidative stress-induced skin injury via adaptive regulation of the NRF2 defense system. Biomaterials. 2020;257:120264. doi:10.1016/j.biomaterials.2020.120264
33. Liao CM, Luo T, von der Ohe J, de Juan Mora B, Schmitt R, Hass R. Human MSC-derived exosomes reduce cellular senescence in renal epithelial cells. Int J Mol Sci. 2021;22(24):13562. doi:10.3390/ijms222413562
34. Yang GH, Lee YB, Kang D, et al. Overcome the barriers of the skin: exosome therapy. Biomater Res. 2021;25(1):22. doi:10.1186/s40824-021-00224-8
35. Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in Exosome Isolation Techniques. Theranostics. 2017;7(3):789–804. doi:10.7150/thno.18133
36. Demelash A, Karlsson JO, Nilsson M, Björkman U. Selenium has a protective role in caspase-3-dependent apoptosis induced by H2O2 in primary cultured pig thyrocytes. Eur J Endocrinol. 2004;150(6):841–849. doi:10.1530/eje.0.1500841
37. Hou X, Shi J, Sun L, et al. The involvement of ERK1/2 and p38 MAPK in the premature senescence of melanocytes induced by H(2)O(2) through a p53-independent p21 pathway. J Dermatol Sci. 2022;105(2):88–97. doi:10.1016/j.jdermsci.2022.01.002
38. Zhang B, Wang J, Zhao G, et al. Apigenin protects human melanocytes against oxidative damage by activation of the Nrf2 pathway. Cell Stress Chaperones. 2020;25(2):277–285. doi:10.1007/s12192-020-01071-7
39. Tang L, Fang W, Lin J, Li J, Wu W, Xu J. Vitamin D protects human melanocytes against oxidative damage by activation of Wnt/β-catenin signaling. Lab Invest. 2018;98(12):1527–1537. doi:10.1038/s41374-018-0126-4
40. Kim YJ, Kim MJ, Kweon DK, Lim ST, Lee SJ. Quantification of Hypopigmentation Activity In Vitro. J Vis Exp. 2019;145. doi:10.3791/58185
41. Migayron L, Boniface K, Seneschal J. Vitiligo, from physiopathology to emerging treatments: a review. Dermatol Ther. 2020;10(6):1185–1198. doi:10.1007/s13555-020-00447-y
42. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478). doi:10.1126/science.aau6977
43. McBride JD, Rodriguez-Menocal L, Badiavas EV. Extracellular vesicles as biomarkers and therapeutics in dermatology: a focus on exosomes. J Invest Dermatol. 2017;137(8):1622–1629. doi:10.1016/j.jid.2017.04.021
44. Zhang B, Wang M, Gong A, et al. HucMSC-exosome mediated-wnt4 signaling is required for cutaneous wound healing. Stem Cells. 2015;33(7):2158–2168. doi:10.1002/stem.1771
45. Kim S, Lee SK, Kim H, Kim TM. Exosomes secreted from induced pluripotent stem cell-derived mesenchymal stem cells accelerate skin cell proliferation. Int J Mol Sci. 2018;19(10):3119. doi:10.3390/ijms19103119
46. Zhu L, Lin X, Zhi L, et al. Mesenchymal stem cells promote human melanocytes proliferation and resistance to apoptosis through PTEN pathway in vitiligo. Stem Cell Res Ther. 2020;11(1):26. doi:10.1186/s13287-019-1543-z
47. Huang CL, Nordlund JJ, Boissy R. Vitiligo: a manifestation of apoptosis? Am J Clin Dermatol. 2002;3(5):301–308. doi:10.2165/00128071-200203050-00001
48. Tian J, Wang Y, Ding M, et al. The Formation of melanocyte apoptotic bodies in vitiligo and the relocation of vitiligo autoantigens under oxidative stress. Oxid Med Cell Longev. 2021;2021:1–13. doi:10.1155/2021/7617839
49. Xie H, Zhou F, Liu L, et al. Vitiligo: how do oxidative stress-induced autoantigens trigger autoimmunity? J Dermatological Sci. 2016;81(1):3–9. doi:10.1016/j.jdermsci.2015.09.003
50. Hassan AS, Kohil MM, Sayed SSE, Mahmoud SB. Immunohistochemical study of perforin and apoptosis stimulation fragment ligand (FasL) in active vitiligo. Arch Dermatol Res. 2021;313(6):453–460. doi:10.1007/s00403-020-02117-7
51. Kumar R, Parsad D, Kanwar AJ. Role of apoptosis and melanocytorrhagy: a comparative study of melanocyte adhesion in stable and unstable vitiligo. Br J Dermatol. 2011;164(1):187–191. doi:10.1111/j.1365-2133.2010.10039.x
52. Wen Z, Mai Z, Zhu X, et al. Mesenchymal stem cell-derived exosomes ameliorate cardiomyocyte apoptosis in hypoxic conditions through microRNA144 by targeting the PTEN/AKT pathway. Stem Cell Res Ther. 2020;11(1):36. doi:10.1186/s13287-020-1563-8
53. Huang B, Lu J, Ding C, Zou Q, Wang W, Li H. Exosomes derived from human adipose mesenchymal stem cells improve ovary function of premature ovarian insufficiency by targeting SMAD. Stem Cell Res Ther. 2018;9(1):216. doi:10.1186/s13287-018-0953-7
54. Xu J, Feng Z, Wang X, et al. hUC-MSCs exert a neuroprotective effect via anti-apoptotic mechanisms in a neonatal HIE rat model. Cell Transplant. 2019;28(12):1552–1559. doi:10.1177/0963689719874769
55. Shao M, Xu Q, Wu Z, et al. Exosomes derived from human umbilical cord mesenchymal stem cells ameliorate IL-6-induced acute liver injury through miR-455-3p. Stem Cell Res Ther. 2020;11(1):37. doi:10.1186/s13287-020-1550-0
56. Park C, Lee H, Noh JS, et al. Hemistepsin A protects human keratinocytes against hydrogen peroxide-induced oxidative stress through activation of the Nrf2/HO-1 signaling pathway. Arch Biochem Biophys. 2020;691:108512. doi:10.1016/j.abb.2020.108512
57. Kovacs D, Bastonini E, Ottaviani M, et al. Vitiligo skin: exploring the dermal compartment. J Invest Dermatol. 2018;138(2):394–404. doi:10.1016/j.jid.2017.06.033
58. Xu S, Wu W, Huang H, et al. The p53/miRNAs/Ccna2 pathway serves as a novel regulator of cellular senescence: complement of the canonical p53/p21 pathway. Aging Cell. 2019;18(3):e12918. doi:10.1111/acel.12918
59. Zhou S, Zeng H, Huang J, et al. Epigenetic regulation of melanogenesis. Ageing Res Rev. 2021;69:101349. doi:10.1016/j.arr.2021.101349
60. Wan P, Hu Y, He L. Regulation of melanocyte pivotal transcription factor MITF by some other transcription factors. Mol Cell Biochem. 2011;354(1–2):241–246. doi:10.1007/s11010-011-0823-4
61. Wong PM, Yang L, Yang L, et al. New insight into the role of exosomes in vitiligo. Autoimmun Rev. 2020;19(11):102664. doi:10.1016/j.autrev.2020.102664
62. Liu Y, Xue L, Gao H, et al. Exosomal miRNA derived from keratinocytes regulates pigmentation in melanocytes. J Dermatol Sci. 2019;93(3):159–167. doi:10.1016/j.jdermsci.2019.02.001
63. Lo Cicero A, Delevoye C, Gilles-Marsens F, et al. Exosomes released by keratinocytes modulate melanocyte pigmentation. Nat Commun. 2015;6(1):7506. doi:10.1038/ncomms8506
64. Niu C, Aisa HA. Upregulation of melanogenesis and tyrosinase activity: potential agents for vitiligo. Molecules. 2017;22(8):1303. doi:10.3390/molecules22081303
65. Xiong M, Zhang Q, Hu W, et al. The novel mechanisms and applications of exosomes in dermatology and cutaneous medical aesthetics. Pharmacol Res. 2021;166:105490. doi:10.1016/j.phrs.2021.105490
66. Shiekh PA, Singh A, Kumar A. Exosome laden oxygen releasing antioxidant and antibacterial cryogel wound dressing OxOBand alleviate diabetic and infectious wound healing. Biomaterials. 2020;249:120020. doi:10.1016/j.biomaterials.2020.120020
67. Yang G, Chen Q, Wen D, et al. A therapeutic microneedle patch made from hair-derived keratin for promoting hair regrowth. ACS Nano. 2019;13(4):4354–4360. doi:10.1021/acsnano.8b09573
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
A 5`-tRNA Derived Fragment NamedtiRNA-Val-CAC-001 Works as a Suppressor in Gastric Cancer
Zheng J, Li C, Zhu Z, Yang F, Wang X, Jiang P, Yan F
Cancer Management and Research 2022, 14:2323-2337
Published Date: 4 August 2022
Eriochloa villosa Alleviates Progression of Benign Prostatic Hyperplasia in vitro and in vivo
Baek EB, Hwang YH, Park S, Hong EJ, Won YS, Kwun HJ
Research and Reports in Urology 2022, 14:313-326
Published Date: 24 September 2022
TP63 Functions as a Tumor Suppressor Regulated by GAS5/miR-221-3p Signaling Axis in Human Non-Small Cell Lung Cancer Cells
Shen Q, Wang H, Zhang L
Cancer Management and Research 2023, 15:217-231
Published Date: 24 February 2023
Cold Air Plasma Inhibiting Tumor-Like Biological Behavior of Rheumatoid Arthritis Fibroblast-Like Synovial Cells via G2/M Cell Cycle Arrest
Ni LY, Ding CB, Deng JM, Wu ZW, Zhou Y
Open Access Rheumatology: Research and Reviews 2024, 16:75-85
Published Date: 14 May 2024