Back to Journals » Research and Reports in Urology » Volume 14
Eriochloa villosa Alleviates Progression of Benign Prostatic Hyperplasia in vitro and in vivo
Authors Baek EB, Hwang YH, Park S, Hong EJ , Won YS, Kwun HJ
Received 13 July 2022
Accepted for publication 9 September 2022
Published 24 September 2022 Volume 2022:14 Pages 313—326
DOI https://doi.org/10.2147/RRU.S381713
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Guglielmo Mantica
Eun Bok Baek,1 Youn-Hwan Hwang,2 Suyoung Park,1 Eun-Ju Hong,1 Young-Suk Won,3 Hyo-Jung Kwun1
1Department of Veterinary Pathology, College of Veterinary Medicine, Chungnam National University, Daejeon, Korea; 2Herbal Medicine Research Division, Korean Institute of Oriental Medicine, Daejeon, Korea; 3Laboratory Animal Resource Center, Korean Research Institute of Bioscience and Biotechnology, Chungbuk, Korea
Correspondence: Hyo-Jung Kwun, Department of Veterinary Pathology, College of Veterinary Medicine, Chungnam National University, 220 Gung-dong, Yuseong-gu, Daejeon, 34134, Korea, Tel +82-42-821-6751, Fax +82-42-821-8903, Email [email protected]
Introduction: Benign prostatic hyperplasia (BPH) is a non-neoplastic proliferative disease of the prostate. Eriochloa villosa (EV) reportedly possesses various pharmacological activities, including anti-lipase activity and modulation of various antioxidative enzymes. In this study, we investigate the therapeutic potential of EV against BPH in a testosterone-induced BPH rat model.
Methods: Rats were subjected to a daily subcutaneous injection of testosterone (3 mg kg− 1) for 4 weeks to induce BPH. Along with testosterone, rats in the treatment group were administered finasteride (10 mg kg− 1) or EV (150 mg kg− 1) via oral gavage. Prostatic cancer (LNCaP) cell line was used to examine the effect of EV.
Results: Finasteride and EV significantly decrease the relative prostate weight, serum levels of dihydrotestosterone and testosterone, and prostate epithelial thickness. Testosterone injection induced prostatic hyperplasia and proliferating cell nuclear antigen expression; however, EV treatment significantly attenuated these effects. Moreover, finasteride- and EV-treated rats exhibit an increase in the number of TUNEL-positive cells and reduced Bcl-2 expression in the prostate tissues compared with the testosterone-treated animals. Furthermore, EV suppresses inflammatory cytokines, including interleukin (IL)-6 and IL-8, in the prostate tissues. Meanwhile, the expression of inflammatory mediator cyclooxygenase-2 is consistently upregulated in testosterone-treated rats, whereas EV treatment significantly reverses this effect. Notably, EV treatment suppresses malondialdehyde (MDA) levels and upregulates testosterone-induced catalase (CAT) expression. In addition, EV suppresses expression of androgen receptor (AR) and prostate-specific antigen (PSA) induced by testosterone in LNCaP cells.
Conclusion: The present study results suggest that EV regulates prostatic proliferation, apoptosis, response to inflammation, and oxidative stress in the BPH rat model, and may, therefore, serve as a useful therapeutic agent for BPH.
Keywords: benign prostatic hyperplasia, Eriochloa villosa, proliferation, apoptosis, inflammation, oxidative stress
Introduction
Benign prostatic hyperplasia (BPH) is one of prevalent diseases in men and its prevalence increases to nearly 70% in men over 80 years.1 BPH is characterized by an enlarged prostate and lower urinary tract symptoms (LUTS), which can significantly impact the quality of life (QOL) and requires medical intervention.1
Although many reports have suggested pathophysiology of BPH, precise molecular mechanisms of BPH are yet to be elucidated. However, androgens, such as testosterone and dihydrotestosterone (DHT), stimulate prostatic growth, while 5α-reductase plays an important role in BPH pathogenesis.1 In addition, an imbalance between the cell cycle and apoptotic cell death can result in an increase in cell number, ultimately resulting in uncontrolled prostatic growth.2 Moreover, considering that chronic inflammation is associated with the histological changes occurring in the prostatic tissue of patients with BPH,3 we hypothesized that inflammation contributes to BPH development. Local inflammatory process may be triggered by infection, which produce cytokines and chemokines involved in the inflammatory response, with consequent growth of epithelial and stromal prostatic cells.3,4
Currently, BPH treatment are aimed to ameliorate LUTS, inhibit disease progression, and reduce complications.5 Currently available drugs for the treatment of BPH include alpha-adrenergic blockers (α1-blockers), 5α-reductase inhibitors (5-ARIs), muscarinic receptor antagonists (MRAs), phosphodiesterase 5 inhibitors (PDE5Is) and β3-adrenoceptor agonists. Furthermore, and herbal extracts, such as saw palmetto extract is an herbal product used in the treatment of symptoms related to benign prostatic hyperplasia. Among these, the most commonly used are α1-blockers, 5-ARIs, and a combination of α1-blockers and 5-ARIs (dutasteride and tamsulosin).5
Eriochloa villosa (EV), also known as woolly cupgrass, is a member of the Poaceae family, which originates from Asia.6 In a previous report, methanol EV extracts exhibited strong (>80%) in vitro anti-lipase activity.7 The shoots and roots of EV also exhibit allelopathic effects for various antioxidative enzymes, such as ascorbate peroxidase, guaiacol peroxidase, and superoxide dismutase (SOD), while also impacting malondialdehyde (MDA) content.6 Among constituents of EV extracts, chlorogenic acid have shown antioxidant, hepatoprotective, cardioprotective, anti-inflammatory, anti-obesity, antiviral, antimicrobial, and lipid metabolism modulation properties and is a dietary constituent.8 Hwang et al demonstrated that chlorogenic acid attenuates LPS-induced cytokine release in RAW 264.7 macrophages, suggesting anti-inflammatory activity.9 Meanwhile, orientin and vitexin have been isolated from various medicinal plants and exhibit multiple biological activities, including anti-inflammatory, antioxidant, antiviral, antibacterial, and cardioprotective properties.10,11 Lastly, dihydroactinidiolide possesses acetylcholinesterase inhibitory, antioxidant, and anti-aggregation activity, and reduces ROS generation in N2A cells.12 However, the protective effects of EV against BPH have not yet been studied; therefore, in the present study, we investigate the protective effects of EV in a testosterone-induced BPH rat model.
Materials and Methods
Plants, Chemicals, and Reagents
EV extract (KPM037-022) was purchased from the Korea Plant Extract Bank at the Korea Research Institute of Bioscience and Biotechnology (Daejeon, Korea). The plant was collected from Sunchang-gun, Jeollabuk-do, KOREA in 2009. A voucher specimen (KRIB 0029775) is kept in the herbarium of the Korea Research Institute of Bioscience and Biotechnology. The plant (85 g) dried in the shade and powdered was added to 1L of Methyl alcohol 99.9% (HPLC grade) and extracted through 30 cycles (40KHz, 1500W, 15 min. ultrasonication-120 min. standing per cycle) at room temperature using an ultrasonic extractor (SDN-900H, SD-ULTRASONIC CO., LTD). After filtration (Qualitative Filter No.100, HYUNDAI MICRO CO., LTD) and drying under reduced pressure, EV extract (7.51 g) was obtained. For identification of EV constituents, chlorogenic acid, orientin, vitexin, and dihydroactinidiolide were provided from ChemFaces (Wuhan, China).
Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry (UHPLC-MS/MS) Analyses of EV
UHPLC-MS/MS analysis was performed according to previously described methods, with slight modifications to identify the constituents of the EV extract.13,14 The analysis was performed using a Dionex UltiMate 3000 system equipped with a Thermo Q-Exactive mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Chromatographic separation was achieved using an Acquity BEH C18 column (100 × 2.1 mm, 1.7 µm, Waters Corp., Milford, MA, USA) and gradient elution consisting of 0.1% (v v−1) formic acid in water (A) and acetonitrile (B).13 The injection volume and flow rate were 3 μL and 0.25 mL min−1, respectively. MS analysis was conducted using the full-scan MS-ddMS2 method in both positive and negative ionization modes, using an electrospray ionization source. The MS/MS parameters were set as follows: capillary temperature, 320 °C; scan range, 100–1500 m z−1; full-scan MS1 resolution, 70,000; and data-dependent MS2 resolution, 13,500. Data acquisition and processing were carried out using the software Xcalibur v.3.2 and TraceFinder v.4.0 (Thermo Fisher Scientific). The mass spectral patterns of the identified compounds were consistent with those reported previously.14
Animals
Male Sprague–Dawley rats (7-weeks-old) were purchased from Orient Bio (Seongnam-si, Republic of Korea). Animals were maintained in constant environment (22 ± 2 °C; 50 ± 5% humidity and 12 h light/dark cycle) during experimental period. Animal experiments were performed in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The experimental animal study procedures were approved by the Animal Experimental Ethics Committee of Chungnam National University (approval number: CNU-00711).
Induction of BPH in Rats and Treatment Design
Rats were 220–250 g at dosing starting day and were randomly separated into four groups (7–8 rats in each group) and subjected to the following dosage regimens: 1) normal control (NC) group, administered a PBS oral gavage and corn oil as a vehicle subcutaneously (SC); 2) BPH group, BPH was induced daily via SC injection of testosterone propionate (Tokyo Chemicals Ins. Co., Tokyo, Japan) at 3 mg kg−1 and administered PBS as a vehicle control via oral gavage; 3) BPH plus finasteride (BPH + F) group (positive control), received finasteride (10 mg kg−1, Sigma) orally and SC injection of testosterone propionate (3 mg kg−1); (4) BPH + EV group, administered EV (150 mg kg−1) via oral gavage and SC injection of testosterone propionate (3 mg kg−1). All treatments were administered daily for 4 weeks, and administration volumes were 5 mL kg−1 for oral doses (PBS, finasteride, and EV) and 3 mL kg−1 for subcutaneous injections. All treatment groups were shown in Table 1. The body weights of the experimental animals were recorded once per week during the experiment, as well as on the day of necropsy. After the last injection, the rats were euthanized and whole blood was collected from the vena cava. Isolated sera from whole blood were stored at −70 °C. The prostate tissues were isolated and weighed.
 |
Table 1 Group Design for Treatment |
Determination of DHT, Testosterone, and MDA Level
Commercially available assay kits were used to measure testosterone (Caymanchem, MI, USA) and DHT (ALPCO Diagnostics, NH, USA) levels in the serum; all procedures were performed according to the manufacturer’s instructions. MDA levels in the prostatic tissue were determined using an enzyme-linked immunosorbent assay (ELISA) kit (Cell Biolabs, Inc., San Diego, USA), according to the manufacturer’s instructions. Values are expressed per milligram of protein for each prostatic tissue and content of protein was determined using protein assay kit (Thermo Scientific, MA, USA).
Histological Examination
Prostatic tissue fixed in 10% buffered formalin was embedded in paraffin wax, cut into 4-μm-thick sections and stained with hematoxylin and eosin (H&E). Epithelial thickness was measured in the ventral lobe of each prostate, as described in a previous study.15
Proliferative Cell Nuclear Antigen (PCNA) Staining
To perform immunohistochemistry of PCNA, the tissue sections were treated with antibodies against PCNA (Abcam, Cambridge, UK) for 2 h, followed by biotinylated secondary antibody for 1 h. Diaminobenzidine (DAB; Vector Laboratories, CA, USA) was used for detection of immunological complexes, and then, the tissue samples were counterstained with hematoxylin. Five ventral lobe sections of the tissues from each rat were randomly analyzed. PCNA-positive cells were calculated as the percentage of total cells.
Terminal Deoxynucleotidyl Transferase dUTP Nick-End Labeling (TUNEL) Assay
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay was performed using a commercial kit (Merck Millipore Corporation, MA, USA). TUNEL-positive cells were considered as apoptotic cells and these cells were counted in randomly selected fields. The results were showed as a percentage of the total cells.
Western Blot Analysis
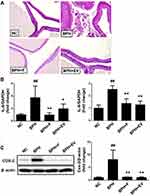
Equal amounts (15 µg) of total protein from rat prostatic tissues were resolved using SDS-PAGE (Bio-Rad, CA, USA). Total protein was determined using protein assay kit (Thermo Scientific, MA, USA). The transferred membranes were incubated overnight with the following primary antibodies: anti-COX-2, anti-catalase, anti-androgen receptor (AR), anti-prostate specific antigen (PSA), anti-β-actin (Cell Signaling Technology, MA, USA), and anti-B-cell lymphoma 2 (Bcl-2; Santa Cruz, CA, USA). The membranes were then incubated with secondary antibodies at room temperature for 2 h, and the signals were detected using a chemiluminescence detection kit and quantified using CSAnalyzer4 (Atto, Tokyo, Japan).
Quantitative Real-Time PCR (RT-qPCR)
Total RNA was extracted from prostate tissue using TRIzol reagent (Sigma, MO, USA) and the absorbance of RNA samples were measured at 260 and 280 nm. Equal amounts of total RNA (1.4 µg) from the prostate tissue of each rat were subjected to reverse transcription (RT) using a commercially available RT kit (Toyobo, Osaka, Japan). Quantitative real-time PCR was performed using SYBR Green Master Mix (Thermo Scientific, MA, USA). The following primer sequences were used: Il6, 5ʹ-TAGTCCTTCCTACCCCAACT −3ʹ (forward) and 5ʹ-TTGGTCCTTAGCCACTCCTT-3ʹ (reverse); Il8, 5ʹ-CATTAATATTTAACGATGTGGATGCGTTTCA-3ʹ (forward) and 5ʹ-GCCTACCATCTTTAAACTGCACAAT-3ʹ (reverse); Gapdh, 5ʹ-ACAGCAACAGGGTGGTGGAC-3ʹ (forward) and 5ʹ-TTTGAGGGTGCAGCGAACTT-3ʹ (reverse). Relative expressions are presented as fold-change in the cDNA level of the target gene relative to that of the endogenous control (GAPDH), determined using the 2−ΔΔCt method, as described previously.16
Cell Culture and Treatment
The Lymph Node Carcinoma of the Prostate (LNCaP) was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). The cell line was incubated in RPMI-1640 medium (Gibco, CA, USA) with 10% fetal bovine serum (FBS). The cells were incubated in medium containing testosterone propionate (10 µM) and EV (50 to 200 µg mL−1) for 72h. After incubation, the cells were harvested for protein analysis. Cytotoxicity of EV was evaluated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as previously described.17
Statistical Analysis
Multiple comparisons were performed using one-way analysis of variance (ANOVA), followed by Dunnett’s post-hoc test for comparison between BPH group and the other groups. Statistical significance was set at p < 0.05. Statistical analyses were performed using GraphPad Prism 6 (GraphPad Software Inc., CA, USA). Values are expressed as the mean ± standard deviation (SD).
Results
Analysis of EV
UHPLC-MS/MS analysis was conducted to identify EV constituents. The base peak chromatograms of EV and the extracted ion chromatograms for each phytochemical are shown in Figure 1. The mass spectral characteristics of all identified compounds in EV are summarized in Table 2. In this study, four phytochemicals of EV, namely, chlorogenic acid, orientin, vitexin, and dihydroactinidiolide were identified from EV extract by comparisons with the mass spectra and retention times of reference standards. Most compounds except chlorogenic acid were identified in positive ion mode. The mass spectral patterns of the identified compounds were consistent with those reported previously.14
 |
Table 2 Phytochemicals Identified in Eriochloa villosa Using Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry (UHPLC-MS/MS) |
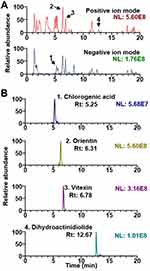 |
Figure 1 UHPLC-MS/MS analysis of EV. (A) Base peak ion chromatograms of EV in positive and negative ion modes. (B) Extracted ion chromatogram of the identified phytochemicals. Rt is retention time. |
Effect of EV Extracts on Prostatic Weight
Compared to the prostates of the animals in the NC group, those of rats administered testosterone exhibited a significant increase in relative weight. However, these increases were significantly alleviated in the finasteride- and EV-treated groups (Table 3).
 |
Table 3 Effect of Eriochloa villosa on Prostate Weight and Percentage Inhibition |
Effect of EV Extracts on the Level of Testosterone and DHT
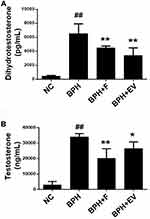
Considering that androgen hormones, such as testosterone and DHT, are critical mediators of BPH pathogenesis,18 we examined the effects of EV on androgen hormone levels. The serum DHT levels were higher in the BPH group (6559 ± 1509 pg/mL) than in the NC group (478 ± 116 pg/mL); however, the levels were significantly lower in the finasteride- (4479 ± 285 pg/mL) and EV-treated groups (3414± 1247 pg/mL) compared to those in the BPH group (Figure 2A). Similarly, testosterone was higher in the prostate of the BPH group than in that of the NC group; however, these levels were markedly lower in the finasteride- and EV-treated groups (Figure 2B).
Effects of EV on Histological Changes in Prostate Tissues in a Rat Model of Testosterone-Induced BPH
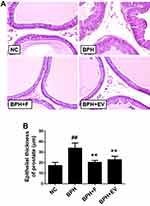
Within the NC group, the prostate tissue morphology exhibited normal features. In contrast, the tissues of the BPH group showed glandular hyperplasia and increased epithelial thickness with decreased glandular luminal area (Figure 3A). Meanwhile, epithelial thickness was markedly decreased in the finasteride- and EV-treated groups compared to that in the BPH group (Figure 3B).
Effects of EV on Prostatic Cell Proliferation in a Rat Model of Testosterone-Induced BPH
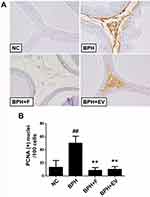
PCNA staining was performed to evaluate the effect of EV on cell proliferation in the prostatic tissues. The number of PCNA-positive cells was significantly higher in the BPH group (50.10 ± 10.88%) than in the NC group (13.42 ± 10.45%). Notably, this increase was significantly inhibited in both finasteride (8.80 ± 4.20%) and EV-treated groups (10.22 ± 4.62%) (Figure 4). The data suggest that EV prevents BPH progression through its antiproliferative activity.
Effects of EV on Prostatic Cell Apoptosis in a Rat Model of Testosterone-Induced BPH
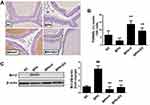
Next, we examined the effect of EV on apoptosis in the prostate using the TUNEL assay and Western blot analysis. Representative TUNEL staining is shown in Figure 5A. A higher number of TUNEL-positive cells was observed in the finasteride (13.82 ± 2.42%) and EV-treated groups (9.37 ± 2.02%) than in the BPH group (2.85 ± 1.77%) (Figure 5B). Protein expression of the anti-apoptotic protein, Bcl-2, was increased in the BPH group; in contrast, the finasteride- and EV-treated groups showed lower Bcl-2 expression (Figure 5C). These findings suggest that EV activates the apoptotic pathway via Bcl-2-dependent signaling.
Effects of EV on Inflammation in a Rat Model of Testosterone-Induced BPH
Considering that inflammation plays an important role in BPH,4 we sought to evaluate the involvement of inflammatory processes and the effects of EV on inflammatory cytokines within the BPH rat model. The morphology of the prostatic tissue was normal, with few inflammatory cells in the NC group, whereas more inflammatory cells were detected in the prostatic interstitial lesions of the BPH group animals. In contrast, the number of inflammatory cells in the prostatic tissues of finasteride- and EV-treated animals was similar to that in the NC group animals (Figure 6A).
Additionally, the relative expression of IL-6 and IL-8 was higher in prostatic tissues from the BPH group, while being significantly lower in tissue from the finasteride- and EV-treated groups (Figure 6B). Similarly, COX-2 expression increased in the BPH group, while being notably reduced in the finasteride- and EV-treated groups (Figure 6C).
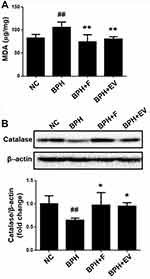
Effects of EV on Oxidative Stress
To examine the involvement of oxidative stress in the testosterone-induced BPH rat model and the effect of EV on oxidative stress, MDA levels were evaluated in the prostatic tissues. MDA level increased in the BPH group, and decreased in the finasteride- and EV-treated group (Figure 7A). Moreover, the expression of catalase (CAT), an antioxidant enzyme, decreased in the BPH group, and increased in the finasteride- and EV-treated groups (Figure 7B). These data suggest that EV prevents BPH progression, at least partially, through the antioxidative signaling pathway.
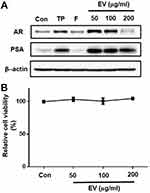
Effect of EV in LNCaP Cells
Androgens stimulate prostatic growth and PSA level is related to BPH pathogenesis.1 Thus, we examined the effect of EV on androgen receptor (AR) and prostate-specific antigen (PSA) expression in human prostatic cancer cell lines. Testosterone treatment significantly increased the expression of AR and PSA, and that treatment with finasteride or EV (50 to 200 µg mL−1) significantly attenuated this effect (Figure 8A). EV did not induce cytotoxicity up to 200 µg mL−1 as shown Figure 8B. These findings suggest that EV suppresses androgen signaling in prostatic cancer cells.
Discussion
BPH is characterized by an enlarged prostate and LUTS, which usually require medical consultation.1 Androgen hormones, such as testosterone and DHT, play a major role in the development and progression of BPH.18 The 5-ARIs, including finasteride and dutasteride, suppress the conversion of testosterone to DHT and are currently used for the treatment of BPH.19 In the present study, EV decreased relative prostatic weight and the levels of DHT and testosterone in serum, similar to finasteride-treated animals.
To date, the pathogenesis and etiology of BPH have not been clearly elucidated.20 However, an imbalance of prostate cell proliferation and apoptotic cell death contribute to BPH pathogenesis.21 In the present study, cell proliferation and apoptosis were analyzed to examine the underlying mechanism of BPH, using TUNEL assay, as well as quantifying PCNA and Bcl-2 proteins. PCNA protein has key role in DNA replication, repair of DNA damage, and cell-cycle progression, and various physiological conditions, including BPH.22,23 Similarly, Bcl-2 inhibits apoptosis and binds to, and inactivates, pro-apoptotic proteins such as Bax.24 In the present study, EV notably decreased the testosterone-induced increase in PCNA-positive cells. In addition, apoptotic indices, namely, TUNEL assay and Bcl-2 expression indicated that EV activated the apoptotic pathway. These results demonstrate that EV inhibits proliferation and promotes apoptosis of prostatic tissue in this BPH rat model, indicating an improvement in the balance of cell proliferation and apoptotic process.
Inflammatory process of prostatic tissue may be critical point of BPH progression.25 However, the origin of prostate inflammation has not been fully elucidated and various factors could influence the generation of inflammation.26 Both acute and chronic inflammation lead to recruitment of inflammatory cells into prostatic tissue, such as T lymphocytes and macrophages, neutrophils, eosinophils, and mast cells and recruited inflammatory cell type is dependent on the stimulant.26 Prostate epithelial, stromal, and inflammatory cells produce cytokines, including CCL family (CCL-5, CCL-2), IL-1, IL-6, IL-18, and hypoxia-inducible factor-1α (HIF-1α), inducing local inflammation.27,28 IL-6 could be released by epithelial and stromal cells during inflammation and published report showed that IL-6 was upregulated in prostatic tissues of BPH compared to the normal prostate.29 IL-8 is a proinflammatory cytokine and secretion of IL-8 by prostate epithelial cells and is involved in leukocyte chemotaxis. The levels of IL-8 was elevated in prostatic tissue of BPH patient and men with chronic prostatitis.30 Moreover, cyclooxygenase (COX) activity plays a key role in the association of inflammation and proliferation of prostatic tissue.20 Elevated COX-2 activity was found in various inflammatory cells of the epithelium and interstitium of human prostate and was observed in proliferative inflammatory lesions.31,32 Consistent with previous reports, in the present study, inflammatory cell infiltration was observed in the prostatic stroma and the levels of IL-6 and IL-8, as well as the expression of COX-2 were significantly higher in the BPH group animals; meanwhile, EV treatment significantly reversed these effects.
Alterations in the redox status play a role in the pathophysiology of inflammation. Previous studies have suggested that oxidative stress provoked chronic inflammation inducing various chronic prostatic diseases, such as chronic prostatitis and prostate cancer.33 MDA levels in the plasma or serum reflect the value of lipid peroxidation acts as a biomarker of oxidative stress.34 Serum MDA levels are significantly higher in BPH patients and showed strong correlation with PSA levels.35 Meanwhile, the activity of antioxidant enzymes, including CAT and SOD, is downregulated in most prostatic tissue of BPH patient than normal prostatic tissues.36 Similarly, the overall antioxidant activity is decreased in BPH patients compared to controls, and negative correlation has been reported between the levels of plasma peroxides and overall antioxidant activity of patients with BPH.37 In the present study, EV notably alleviated the increase in MDA levels and increased CAT expression, suggesting antioxidative activity.
Androgen hormone has a key role in prostatic growth, and AR and PSA level is related to BPH pathogenesis.1 In our previous reports, several extracts inhibited expressions of AR and PSA induced by testosterone and these extract showed therapeutic effect in BPH rat model.38,39 Consistently, in this study, testosterone increased expression of AR and PSA in LNCaP cells. Notably, EV treatment attenuated expression of AR and PSA. These findings suggest that EV suppresses androgen signaling in this cell system.
The present study results suggest that EV attenuated BPH progression by cell growth, anti-inflammation and anti-oxidative stress in testosterone-induced rat model.
Conclusion
This study demonstrates that EV treatment significantly prevents the progression of testosterone-induced prostatic enlargement and subsequent histological alterations in a rat model of BPH. More specifically, EV lowers the levels of DHT and testosterone and ameliorates the testosterone-induced imbalance between prostatic proliferation and apoptotic cell death. Furthermore, the expression of testosterone-induced inflammatory cytokine and oxidative stress markers is significantly suppressed in EV-treated animals. In LNCaP cells, EV significantly inhibited expression of AR and PSA induced by testosterone treatment. Collectively, these results suggest that EV exhibits therapeutic potential for preventing BPH progression.
Abbreviations
5-ARIs, 5α-reductase inhibitors; α1-blockers, alpha1-adrenergic antagonists; Bcl-2, B-cell lymphoma 2; BPH, benign prostatic hyperplasia; COX-2, cyclooxygenase-2; DHT, dihydrotestosterone; EV, Eriochloa villosa; H&E, hematoxylin and eosin; IL, interleukin; LUTS, lower urinary tract symptoms; MDA, malondialdehyde; MRA, muscarinic receptor antagonists; PCNA, proliferating cell nuclear antigen; PDE5Is, phosphodiesterase 5 inhibitors; QOL, quality of life; SC, subcutaneous; TUNEL, terminal deoxynucleotidyl transferase dUTP nick-end labeling; UHPLC-MS/MS, ultra-high performance liquid chromatography-tandem mass spectrometry.
Data Sharing Statement
All the data generated and analyzed in this study are included in this manuscript.
Funding
This work was supported by the research fund of Chungnam National University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Madersbacher S, Sampson N, Culig Z. Pathophysiology of benign prostatic hyperplasia and benign prostatic enlargement: a mini-review. Gerontology. 2019;65(5):458–464. doi:10.1159/000496289
2. Kyprianou N, Tu H, Jacobs SC. Apoptotic versus proliferative activities in human benign prostatic hyperplasia. Hum Pathol. 1996;27(7):668–675. doi:10.1016/s0046-8177(96)90396-2
3. Soler R, Andersson KE, Chancellor MB, et al. Future direction in pharmacotherapy for non-neurogenic male lower urinary tract symptoms. Eur Urol. 2013;64(4):610–621. doi:10.1016/j.eururo.2013.04.042
4. Chughtai B, Lee R, Te A, Kaplan S. Role of inflammation in benign prostatic hyperplasia. RevUrol. 2011;13(3):147–150.
5. Yu ZJ, Yan HL, Xu FH, et al. Efficacy and side effects of drugs commonly used for the treatment of lower urinary tract symptoms associated with benign prostatic hyperplasia. FrontPharmacol. 2020;11:658. doi:10.3389/fphar.2020.00658
6. Szilágyi A, Radócz L, Hájos MT, et al. The impacts of woolly Cupgrass on the antioxidative system and growth of a maize hybrid. Plants. 2021;10(5):982. doi:10.3390/plants10050982
7. Sharma N, Sharma VK, Seo SY. Screening of some medicinal plants for anti-lipase activity. J Ethnopharmacol. 2005;97(3):453–456. doi:10.1016/j.jep.2004.11.009
8. Naveed M, Hejazi V, Abbas M, et al. Chlorogenic acid (CGA): a pharmacological review and call for further research. Biomed Pharmacother. 2018;97:67–74. doi:10.1016/j.biopha.2017.10.064
9. Hwang SJ, Kim YW, Park Y, Lee HJ, Kim KW. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm Res. 2014;63(1):81–90. doi:10.1007/s00011-013-0674-4
10. He M, Min JW, Kong WL, He XH, Li JX, Peng BW. A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia. 2016;115:74–85. doi:10.1016/j.fitote.2016.09.011
11. Lam KY, Ling AP, Koh RY, Wong YP, Say YH. A review on medicinal properties of Orientin. Adv Pharmacol Sci. 2016;2016:4104595. doi:10.1155/2016/4104595
12. Das M, Prakash S, Nayak C, et al. Dihydroactinidiolide, a natural product against Abeta25-35 induced toxicity in Neuro2a cells: synthesis, in silico and in vitro studies. Bioorg Chem. 2018;81:340–349. doi:10.1016/j.bioorg.2018.08.037
13. Kim YJ, Jeon WY, Hwang YH, Lee MY. Inhibitory effects of Gyeji-tang on MMP-9 activity and the expression of adhesion molecules in IL-4- and TNF-alpha-stimulated BEAS-2B cells. Plants. 2021;10(5):951. doi:10.3390/plants10050951
14. Seo CS, Song KH. Phytochemical characterization for quality control of Phyllostachys pubescens Leaves using high-performance liquid chromatography coupled with diode array detector and tandem mass detector. Plants. 2021;11(1):50. doi:10.3390/plants11010050
15. Rho J, Seo CS, Park HS, et al. Ulmus macrocarpa Hance improves benign prostatic hyperplasia by regulating prostatic cell apoptosis. J Ethnopharmacol. 2019;233:115–122. doi:10.1016/j.jep.2018.11.042
16. Park HS, Wijerathne CUB, Jeong HY, Seo CS, Ha H, Kwun HJ. Gastroprotective effects of Hwanglyeonhaedok-tang against Helicobacter -induced gastric cell injury. J Ethnopharmacol. 2018;216:239–250. doi:10.1016/j.jep.2018.01.025
17. Jiang R, Xu XH, Wang K, et al. Ethyl acetate extract from panax ginseng C.A. Meyer and its main constituents inhibit alpha-melanocyte-stimulating hormone-induced melanogenesis by suppressing oxidative stress in B16 mouse melanoma cells. J Ethnopharmacol. 2017;208:149–156. doi:10.1016/j.jep.2017.07.004
18. Andriole G, Bruchovsky N, Chung LW, et al. Dihydrotestosterone and the prostate: the scientific rationale for 5alpha-reductase inhibitors in the treatment of benign prostatic hyperplasia. J Urol. 2004;172(4 Pt 1):1399–1403. doi:10.1097/01.ju.0000139539.94828.29
19. Wurzel R, Ray P, Major-Walker K, Shannon J, Rittmaster R. The effect of dutasteride on intraprostatic dihydrotestosterone concentrations in men with benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 2007;10(2):149–154. doi:10.1038/sj.pcan.4500931
20. Minciullo PL, Inferrera A, Navarra M, Calapai G, Magno C, Gangemi S. Oxidative stress in benign prostatic hyperplasia: a systematic review. Urol Int. 2015;94(3):249–254. doi:10.1159/000366210
21. Pawlicki B, Zieliński H, Dabrowski M. Znaczenie apoptozy i przewlekłego zapalenia gruczołu krokowego w patogenezie łagodnego rozrostu. [Role of apoptosis and chronic prostatitis in the pathogenesis of benign prostatic hyperplasia]. Pol Merkur Lekarski. 2004;17(100):307–310. Polish.
22. Glover M, Soni S, Ren Q, Maclennan GT, Fu P, Gupta S. Influence of chronic inflammation on Bcl-2 and PCNA expression in prostate needle biopsy specimens. Oncol Lett. 2017;14(4):3927–3934. doi:10.3892/ol.2017.6668
23. Schönenberger F, Deutzmann A, Ferrando-May E, Merhof D. Discrimination of cell cycle phases in PCNA-immunolabeled cells. BMC Bioinform. 2015;16:180. doi:10.1186/s12859-015-0618-9
24. Tzifi F, Economopoulou C, Gourgiotis D, Ardavanis A, Papageorgiou S, Scorilas A. The role of BCL2 family of apoptosis regulator proteins in acute and chronic leukemias. AdvHematol. 2012;524308. doi:10.1155/2012/524308
25. Robert G, Descazeaud A, Nicolaïew N, et al. Inflammation in benign prostatic hyperplasia: a 282 patients’ immunohistochemical analysis. Prostate. 2009;69(16):1774–1780. doi:10.1002/pros.21027
26. Krušlin B, Tomas D, Džombeta T, Milković-Periša M, Ulamec M. Inflammation in prostatic hyperplasia and carcinoma-basic scientific approach. FrontOncol. 2017;7:77. doi:10.3389/fonc.2017.00077
27. Mishra VC, Allen DJ, Nicolaou C, et al. Does intraprostatic inflammation have a role in the pathogenesis and progression of benign prostatic hyperplasia? BJU Int. 2007;100(2):327–331. doi:10.1111/j.1464-410X.2007.06910.x
28. Nickel JC. Prostatic inflammation in benign prostatic hyperplasia—the third component? Can J Urol. 1994;1(1):1–4.
29. Mechergui YB, Ben Jemaa A, Mezigh C, et al. The profile of prostate epithelial cytokines and its impact on sera prostate specific antigen levels. Inflammation. 2009;32(3):202–210. doi:10.1007/s10753-009-9121-7
30. Liu LR, Li QJ, Han P, et al. Evaluation of interleukin-8 in expressed prostatic secretion as a reliable biomarker of inflammation in benign prostatic hyperplasia. Urology. 2009;74(2):340–344. doi:10.1016/j.urology.2009.02.064
31. Palapattu GS, Sutcliffe S, Bastian PJ, et al. Prostate carcinogenesis and inflammation: emerging insights. Carcinogenesis. 2009;26(7):1170–1181. doi:10.1093/carcin/bgh317
32. Sugar LM. Inflammation and prostate cancer. Can J Urol. 2006;13(suppl 1):46–47.
33. Paulis G. Inflammatory mechanisms and oxidative stress in prostatitis: the possible role of antioxidant therapy. Res RepUrol. 2018;10:75–87. doi:10.2147/RRU.S170400
34. Meagher EA, FitzGerald GA. Indices of lipid peroxidation in vivo: strengths and limitations. Free Radic Biol Med. 2000;28(12):1745–1750. doi:10.1016/s0891-5849(00)00232-x
35. Merendino RA, Salvo F, Saija A, et al. Malondialdehyde in benign prostate hypertrophy: a useful marker? Mediators Inflamm. 2003;12(2):127–128. doi:10.1080/0962935031000097745
36. Olinski R, Zastawny TH, Foksinski M, Barecki A, Dizdaroglu M. DNA base modifications and antioxidant enzyme activities in human benign prostatic hyperplasia. Free Radic Biol Med. 1995;18(4):807–813. doi:10.1016/0891-5849(94)00171-f
37. Pace G, Di Massimo C, De Amicis D, et al. Oxidative stress in benign prostatic hyperplasia and prostate cancer. Urol Int. 2010;85(3):328–333. doi:10.1159/000315064
38. Ub Wijerathne C, Park HS, Jeong HY, et al. Quisqualis indica improves benign prostatic hyperplasia by regulating prostate cell proliferation and apoptosis. Biol Pharm Bull. 2017;40(12):2125–2133. doi:10.1248/bpb.b17-00468
39. Park HS, Seo CS, Wijerathne CU, et al. Effect of veratrum maackii on testosterone propionate-induced benign prostatic hyperplasia in rats. Biol Pharm Bull. 2019;42(1):1–9. doi:10.1248/bpb.b18-00313
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.