Back to Journals » Patient Preference and Adherence » Volume 17
Health-Related Behaviors and Psychological Status of Adolescent Patients with Atopic Dermatitis: The 2019 Korea Youth Risk Behavior Web-Based Survey
Authors Park JH , Prochnow T, Chang J, Kim SJ
Received 27 January 2023
Accepted for publication 15 March 2023
Published 18 March 2023 Volume 2023:17 Pages 739—747
DOI https://doi.org/10.2147/PPA.S406125
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Jeong-Hui Park,1 Tyler Prochnow,1 Jongwha Chang,2 Sun Jung Kim3– 5
1Texas A&M University, School of Public Health, College Station, TX, USA; 2Texas A&M University, Irma Lerma Rangel School of Pharmacy, College Station, TX, USA; 3Soonchunhyang University, College of Medical Sciences, Asan, Republic of Korea; 4Center for Healthcare Management Science, Soonchunhyang University, Asan, Republic of Korea; 5Department of Software Convergence, Soonchunhyang University, Asan, Republic of Korea
Correspondence: Jongwha Chang, Texas A&M University, Irma Lerma Rangel School of Pharmacy, Department of Pharmaceutical Sciences, College Station, TX, 77843, USA, Email [email protected] Sun Jung Kim, Soonchunhyang University, College of Medical Science, Department of Health Administration and Management, Asan, Republic of Korea, Email [email protected]
Objective: Atopic dermatitis (AD) is one of the common chronic diseases that occur in children and adolescents as a chronic relapsing pruritic inflammatory skin disease. This study investigated how AD is associated with stress and depressive symptoms in a large representative sample of adolescents in South Korea.
Methods: The Korea Youth Risk Behavior Web-based Survey 2019 was used in this study (n = 57,069, weighted national estimates = 2,672,170). Multivariate logistic regression was used to determine significant associations between AD and mental health, measured by stress and depressive symptoms. Sub-group analysis was also conducted using various socio-economic variables.
Results: Among the present sample, 6.5% of adolescents (n = 173,909) were diagnosed with AD in the past 12 months. After adjusting for other variables, AD diagnosis was associated with significantly higher odds of experiencing stress (OR = 1.43) and depressive symptoms (OR = 1.32) as compared to adolescents without AD. A similar trend is found in subgroup model analysis using socio-economic variables (ie, education levels, parent’s income levels, and residence area). Specifically, female adolescents with AD, adolescents of low socio-economic status, those reporting smoking and drinking experience, and who do not participate in regular physical activity are more vulnerable to stress and depressive symptoms.
Conclusion: This is a noteworthy finding because it denotes that AD may lead to negative outcomes, like depressive symptoms or stress, which could be prevented if suspected early.
Keywords: adolescent, atopic dermatitis, stress, depressive symptoms
Background
Atopic dermatitis (AD) is a chronic relapsing pruritic inflammatory skin disease characterized
by dysfunctions in skin barrier function, allergen sensitization, and recurrent skin infections that often have cycles of exacerbation and improvement of itchy eczema.1–3 AD commonly presents as itching and hypersensitivity to external stimuli and allergens, worsening in adolescence.4 The cause of AD is unclear, but current research investigates links between genetic, immunological, and environmental factors and skin barrier dysfunctions.5
AD is one of the most common chronic diseases for adolescents.2 Although AD may have differences between countries and ethnic groups,6 it affects more than 5–20% of children and adolescents worldwide, and previous studies have commonly observed that children and adolescents have a higher prevalence of AD than adults.7,8 Currently, in South Korea, the incidence of AD in children and adolescents is 13.5% and counting.3,9 Developing countries have also seen an increase in AD cases,9 but the cause of the increased AD prevalence is unclear. Some reports propose that environmental factors may be critical in the expression and aggravation of AD.10,11 Further, small family size, education, increased income, migration from rural to urban environments, and increased use of antibiotics may be associated with the increased prevalence of AD.12,13
The quality of life for patients with AD is severely affected by sleep disturbances, emotional
and mental health problems, and issues with social functioning.14 In addition, adolescents struggling with body image may develop low perceived happiness and increased stress as chronic and repeat inflammatory reactions cause esthetic and functional skin changes.15 The negative social and psychological changes experienced by adolescents can lead to depression and anxiety.16,17 Past research has found that adolescents with AD have increased suicidal ideation rates (44%) and suicide attempts (36%) compared to those without AD.18 A previous study using the Korea Youth Risk Behavior WebBased Survey (KYRBWS) VI reported that 46% of adolescents with high-stress levels and 21% with moderate stress levels suffer from AD.19 These past studies imply that stress may be an inducer and effect of AD.16 Therefore, managing stress levels in youths with AD may be essential to improve patients’ mental health.
Even though there have been concerns about adolescent mental health problems, very few
studies have attempted to examine the association between AD and psychiatric symptoms (stress and depression) in the adolescent population.16,17 Until now, few studies have documented the association between AD and psychiatric symptoms. Therefore, this study aimed to examine the association between psychiatric symptoms (stress and depressive symptoms) with the prevalence of AD in adolescents in South Korea using Korean Youth Risk Behavior Web-based Survey.
Methods
Data Collection
This study was performed with data derived from the 2019 Korean Youth Risk Behavior Webbased Survey (KYRBWS). KYRBWS is a multi-year cross-sectional study conducted annually by the Korea Centers for Disease Control and Prevention (KCDC) since 2005. This government-approved statistical survey examines health behaviors, including AD/asthma diagnosis, tobacco/alcohol use, obesity, physical activity, sexual behaviors, substance use, Internet use, and mental health.20 The target population is nationally representative middle- and high-school students aged 12 to 18 years old in
Korea, sampled from 400 middle and 400 high schools every year. A total of 57,303 out of 60,100 (95.3%) participants completed the KYRBWS-15, and the final data of 57,069 participants were used in this study after deleting missing variables. During the data collection, KCDC delivered the full instructions of the purpose and methods of the study given to the students by trained teachers and written informed consent was obtained from the students. Students who agreed to participate completed the anonymous questionnaire presented on a computer. The KCDC’s Institutional Review Board has approved the protocols for KYRBWS. The raw data were gathered from the KCDC website after submitting an application outlining the purpose and plan of use and obtaining approval. KYRBWS is a government published and publicly available secondary dataset with de-identified information. Therefore, IRB approval was not required. Our original sample was used to validate the findings from the data.
Variables
The primary outcome of this study was to investigate if AD is associated with stress and
depressive symptoms. AD diagnosis was measured by asking, “Have you ever been diagnosed with AD by a physician?”. Adolescents who answered “No” to this question were categorized as having “No diagnosis”. An additional question was asked to those who answered “Yes” to the previous question, “Have you been diagnosed with AD by a physician during the past 12 months?”. Participants were then categorized into “Diagnosis within one year” and “Diagnosis during the lifetime”.
Stress was measured by asking, “How do you feel stress in your everyday life?”. Participants could respond “extremely high”, “high”, “some”, “low”, or “none”. From these response options, participants were then categorized as the “stress” group for those who answered “extremely high”, “high”, or “some” and “low stress” group for those who answered “low” or “none”. Depressive symptoms were measured by asking, “Have you felt any sadness or despair enough to suspend your daily life in the past 12 months?”. Responses were dichotomous for yes and no.
In this study, we adjusted various socio-economic and health behavior confounders measured
by questionnaire. Socio-economic variables included age, sex, grade (middle or high school), subjective parent’s economic status (high, middle, and low), and residence area (metropolitan, city, and rural). Health behavior variables included lifetime smoking (cigarette or electronic)/drinking and physical activities (More than 4 days a week, 1–3 days a week, and none).
Statistical Analysis
First, sampling weights were used for all statistical analyses to represent the entire national wide adolescents. We first examined the general characteristics of the sample dataset. Then we investigated sample characteristics by stress and depressive symptoms. The sample characteristics were presented as weighted frequency (percentage) or means (Standard Deviation). Finally, to examine group differences (whether having stress and/or depressive symptoms), Rao-Scott Chi-Square tests were used for categorical variables.
The odds ratios (ORs) and 95% confidence interval (95% CI) for having stress and depressive
symptoms were calculated using multivariate logistic regression analysis. We also tested sub-group analysis for socio-economic covariates. All analyses were conducted using SAS statistical software (version 9.4; SAS Institute Inc., Cary, NC, USA). All statistical tests were two-sided, and statistical significance was determined at p < 0.05.
Results
Characteristics and Descriptive Statistics of Sample
A total of 57,069 adolescents sample were identified in the 2019 KYRBWS (weighted n = 2,672,170). Among them, 173,909 (6.5%) had an AD diagnosis within one year, and 426,216 (16.0%) had a lifetime diagnosis. 80.9% and 28.1% of adolescents had experience stress and depressive symptoms during the past 12 months, respectively. See Table 1 for descriptive statistics for the full sample. Table 2 displays the descriptive statistics of the sample divided by mental health responses. Adolescents with AD diagnosis (with one year and lifetime diagnosis) showed more stress and depression experience compared to the no diagnosis group (p < 0.001). Stress was also more frequently present in participants who were female (87.4%), in high school (83.5%), low economic status (87.4%), history of smoking/drinking (83.2% and 83.8%), and reported none physical activity groups (82.6%). The frequency of depressive symptoms had a similar trend as stress, except there were no differences based on the frequency of physical activity.
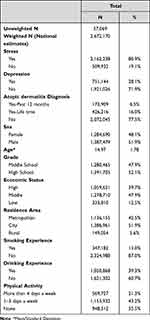 |
Table 1 General Characteristics of Patient Sample |
 |
Table 2 Descriptive Statistics of Sample by Stress and Depression |
Association of Atopic Dermatitis with Health-Related Behaviors and Psychological Status
The odds ratios of having stress and depressive symptoms from the multivariate logistic regression model are shown in Table 3. After controlling for all other variables, adolescents who have been diagnosed with AD within one year were significantly more likely to experience stress (OR =1.43) and depressive symptoms (OR = 1.33) when compared to adolescents without a diagnosis. Adolescents who were diagnosed over one year ago were also significantly more likely to experience stress (OR = 1.11) and depressive symptoms (OR = 1.08) compared to those with no diagnosis. For other variables, females (OR = 2.32 and OR = 2.19) with low economic status (OR = 1.81 and OR = 1.36), smoking (OR = 1.11 and OR = 1.47) and drinking groups (OR = 1.29 and OR = 1.67) were significantly more likely to report stress and depressive symptoms. Physical activity showed only a significant likelihood of stress (OR = 1.10).
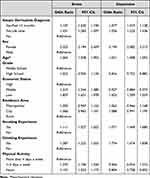 |
Table 3 Results of Survey Logistic Regression: Odds of Having Stress and Depression Among Adolescents |
Discussion
This study investigated if AD is related to stress and depressive symptoms in Korean adolescents. The data used in this study included a total of 57,069 adolescent samples, whose average age was 14.97 ± 1.78, showing 2,672,170 weighted samples. Among them, 173,909 adolescents (6.5%) were diagnosed with AD within a year, and 426,216 adolescents (16.0%) were diagnosed throughout their lives. The study found that female adolescent AD patients, patients with low socio-economic status, smoking and drinking experience, and who do not participate in regular physical activity are more vulnerable to stress and depressive symptoms.
AD was associated with gender, as female adolescents were more than twice as likely to report stress and depressive symptoms as male adolescents. A similar observation has been found in previous studies that women who were diagnosed with AD experienced high stress, anxiety, and depression more frequently than men.21–23 The predominance of these factors in females may be because females are more concerned about their physical appearance than males, which can lead to emotional disturbance.24 In addition, female adolescents experience new hormonal changes during the menstrual cycle in adolescence. One study found that AD symptoms were aggravated in female adolescents who experienced premenstrual or ovulatory phase25 and reported that female sex hormones affect the expression of AD symptoms.26 These differences in physiology, such as hormones between genders, may have reported significant differences in stress and depressive symptoms.
Results also demonstrated that AD adolescent patients with low socio-economic status were more vulnerable to high stress and depressive symptoms than adolescents from families of middle- and high socio-economic status. There are several studies that show low SES is linked to higher stress and increased cortisol.27–29 This is consistent with one study that the frequent occurrence of another itchy dermatitis, fungal infections, and urticaria was more common in the low socio-economic status group.30 Adolescents from families of low socio-economic status tend to have a low awareness of AD symptoms and delay reporting them; in particular, they have limited access to the medical care in which AD can be diagnosed and treated.28 Also, since they may be exposed to respiratory and dermatological symptoms frequently, abnormal skin symptoms such as AD may be taken these symptoms lightly. If dermatological symptoms are under-reported constantly, adolescents are likely to be exposed to high stress and depressive symptoms due to AD symptoms.
Furthermore, smoking and drinking experiences were closely related to stress and depressive symptoms in adolescents diagnosed with AD. Smoking stimulates the surface of immune cells in the body, increasing the total number of white blood cells,31 as well as increasing Th2, responsible for allergic immune responses, and lL-4, which plays an essential role in treating chronic inflammation.32 This leads to the development of major allergic diseases such as AD symptoms, as it makes the body sensitive to environmental antigens such as pollen, animal dander, and house dust mites. In addition, previous studies reported that environments easily exposed to smoking by family members and/or their friends who smoke and direct smoking increase the risk of AD.33,34 Given the positive association between AD and smoking, alcohol use is also associated with AD symptoms. Alcohol use excessively increases the production of IgE antibody corresponding to allergies in the body,35,36 and increased IgE antibody also stimulates Th2 cells.37 Because of these biological reactions, adolescent drinking experiences exacerbate AD and smoking experiences. Furthermore, alcohol use causes skin dryness and an acute inflammatory response due to the release of histamine from acetaldehyde.38,39 Since their smoking and drinking experiences in adolescence reduced their immune function, their AD symptoms would have been exacerbated and increased their stress and depressive symptoms.
Esthetic and functional skin changes caused by AD in adolescence lead to low perceived happiness, high stress, and depression.15 One previous study demonstrated that adolescents who suffer from AD could have problems establishing a healthy body image and making them experience negative social and psychological issues.16 However, one interesting observation in this study is that participation in physical activity helps adolescents with AD to decrease their psychological stress. It is consistent with outcomes from Kong et al, stating AD adolescent patients who participated in regular physical activity indicated a 30% lower risk of stress than those who did not participate in physical activity.40 This may be because physical activity not only helps the reabsorption process of cortisol, which is secreted in significant amounts in response to stress41 but also helps to produce and activate endorphins that directly affect the brain.42 Several previous studies have also shown that physical activity benefits the mental health of patients suffering from allergic diseases like AD, asthma, and allergic rhinitis.43,44 However, physical activity can delay recovery from the damaged skin of AD patients, and sweat induced by physical activity can cause itching, so it may cause AD symptoms to suffer more.45 Therefore, future studies need a guideline to promote physical activity so that AD symptoms are not exacerbated. The number of adolescents diagnosed with AD steadily increases yearly,3,9 but the specific cause and treatments are still unclear. Some scholars have reported that AD is a multidisciplinary disease.46,47 El Hachem et al indicated that medical specialists’ and even psychologists’ support should be provided to adolescent patients, and treatment education programs should also be provided to patients/families to treat AD.47 Although anti-inflammatory and antimicrobial treatments such as emollients, Fusidic acid, and calcineurin inhibitors can be one of the treatments for AD,48–50 the study found that health-related behaviors (drinking and smoking) and psychological factors in adolescents are related to AD, and it will be needed more multidisciplinary efforts to treat AD.
There are a few limitations to this study. First, this study utilized nationally representative data from an online survey; thus, we did not obtain information on AD treatment history and severity. Secondly, we conducted a cross-sectional study, so the causality between stress and depressive symptoms in adolescents with AD cannot be determined. Lastly, the prevalence of depressive symptoms in our population may have been overestimated because we used simplified diagnostic criteria. The prevalence of diagnosed depression is generally lower than that of depression symptoms. Despite these limitations, this study utilized a large sample size from a nationwide general adolescent population survey with good representativeness and generalizability. In addition, we used depressive feelings and stress feelings to identify the consistency of relationships with AD. This is a noteworthy finding because it denotes that AD may lead to destructive outcomes, like major depressive disorders or stress, which could be prevented if suspected early.
Conclusion
The present study found high levels of stress and depressive symptoms in adolescents with AD, especially for females, those from families of low socio-economic status, and those with a history of smoking and drinking and low physical activity. Pharmacological treatments like using drugs or steroids are the most common medications to alleviate AD, however, based on the results of this study, increased physical activity can be one of the effective treatments for AD adolescents. In addition, future studies need to be examined in a multilateral effort to decrease the high stress and depressive symptoms of female adolescents diagnosed with AD.
Funding
This work was supported by the Soonchunhyang University Research Fund and BK21 FOUR (Fostering Outstanding Universities for Research, No.: 5199990914048, Korean Ministry of Education).
Disclosure
The authors declare that they have no competing interests for this work.
References
1. Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396(10247):345–360. doi:10.1016/S0140-6736(20)31286-1
2. Lee JH, Do Han K, Min Kim K, Park YG, Lee JY, Park YM. Prevalence of atopic dermatitis in Korean children based on data from the 2008–2011 Korean National Health and Nutrition Examination Survey. Allergy Asthma Immunol Res. 2016;8(1):79–83. doi:10.4168/aair.2016.8.1.79
3. Boguniewicz M, Leung DY. Recent insights into atopic dermatitis and implications for management of infectious complications. J Allergy Clin Immunol. 2010;125(1):4–13. doi:10.1016/j.jaci.2009.11.027
4. Hon KL, Tsang Y-CK, Poon TCW, et al. Predicting eczema severity beyond childhood. World J Pediatr. 2016;12(1):44–48. doi:10.1007/s12519-015-0064-9
5. Lee MK, Seo J-H, Chu H, et al. Current status of patient education in the management of atopic dermatitis in Korea. Yonsei Med J. 2019;60(7):694–699. doi:10.3349/ymj.2019.60.7.694
6. Williams H, Robertson C, Stewart A, et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. J Allergy Clin Immunol. 1999;103(1):125–138. doi:10.1016/S0091-6749(99)70536-1
7. Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI. Global variations in prevalence of eczema symptoms in children from ISAAC Phase three. J Allergy Clin Immunol. 2009;124(6):1251–1258. e23. doi:10.1016/j.jaci.2009.10.009
8. Kishimoto M, Deshpande GA, Fukui S, Komagata Y, Ohyama M, Kaname S. Upadacitinib for moderate-to-severe atopic dermatitis, in adults and adolescents 12 years and older: review of international and Japanese populations. Expert Rev Clin Immunol. 2023;19(1):19–35. doi:10.1080/1744666X.2023.2149494
9. Buys LM. Treatment options for atopic dermatitis. Am Fam Physician. 2007;75(4):523–528.
10. Otsuka A, Nomura T, Rerknimitr P, Seidel JA, Honda T, Kabashima K. The interplay between genetic and environmental factors in the pathogenesis of atopic dermatitis. Immunol Rev. 2017;278(1):246–262. doi:10.1111/imr.12545
11. Kantor R, Silverberg JI. Environmental risk factors and their role in the management of atopic dermatitis. Expert Rev Clin Immunol. 2017;13(1):15–26. doi:10.1080/1744666X.2016.1212660
12. Hansen TE, Evjenth B, Holt J. Increasing prevalence of asthma, allergic rhinoconjunctivitis and eczema among schoolchildren: three surveys during the period 1985– 2008. Acta Paediatrica. 2013;102(1):47–52. doi:10.1111/apa.12030
13. Lee JH, Kim EH, Cho J, et al. Comparison of prevalence and risk factors of atopic dermatitis by physical examination and questionnaire survey in elementary school children. Pediatr Allergy Respir Dis. 2011;21(3):186–196. doi:10.7581/pard.2011.21.3.186
14. Koszorú K, Borza J, Gulácsi L, Sárdy M. Quality of life in patients with atopic dermatitis. Cutis. 2019;104(3):174–177.
15. Choi C. The factors affecting the life satisfaction of adolescents with atopic dermatitis. Stud Korean Youth. 2015;26:111–144. doi:10.14816/sky.2015.26.1.111
16. Schut C, Weik U, Tews N, Gieler U, Deinzer R, Kupfer J. Psychophysiological effects of stress management in patients with atopic dermatitis: a randomized controlled trial. Acta Derm Venereol. 2013;93(1):57–61. doi:10.2340/00015555-1415
17. Buske-Kirschbaum A, Geiben A, Hellhammer D. Psychobiological aspects of atopic dermatitis: an overview. Psychother Psychosom. 2001;70(1):6–16. doi:10.1159/000056219
18. Sandhu JK, Wu KK, Bui T-L, Armstrong AW. Association between atopic dermatitis and suicidality: a systematic review and meta-analysis. JAMA Dermatol. 2019;155(2):178187. doi:10.1001/jamadermatol.2018.4566
19. Kwon JA, Park E-C, Lee M, Yoo K-B, Park S. Does stress increase the risk of atopic dermatitis in adolescents? Results of the Korea Youth Risk Behavior Web-based Survey (KYRBWS-VI). PLoS One. 2013;8(8):e67890. doi:10.1371/journal.pone.0067890
20. Kim Y, Choi S, Chun C, Park S, Khang Y-H, Oh K. Data resource profile: the Korea youth risk behavior web-based survey (KYRBS). Int J Epidemiol. 2016;45(4):1076–1076e. doi:10.1093/ije/dyw070
21. Zachariae R, Zachariae C, Ibsen HHW, Mortensen JT, Wulf HC. Psychological symptoms and quality of life of dermatology outpatients and hospitalized dermatology patients. Acta Derm Venereol. 2004;84(3):205–212. doi:10.1080/00015550410023284
22. Amorim-Gaudêncio C, Roustan G, Sirgo A. Evaluation of anxiety in chronic dermatoses: differences between sexes. Revista Interamericana de Psicologia. 2004;38(1):54.
23. Khan S, Shaikh M, Ali M. Depression in common dermatological patients attending outpatient department. KYAMC J. 2012;2(2):164–171. doi:10.3329/kyamcj.v2i2.13260
24. Mina S, Jabeen M, Singh S, Verma R. Gender differences in depression and anxiety among atopic dermatitis patients. Indian J Dermatol. 2015;60(2):211. doi:10.4103/0019-5154.152564
25. Eliasson O, Scherzer HH, Degraffjr AC. Morbidity in asthma in relation to the menstrual cycle. J Allergy Clin Immunol. 1986;77(1):87–94. doi:10.1016/0091-6749(86)90328-3
26. Osman M. Therapeutic implications of sex differences in asthma and atopy. Arch Dis Child. 2003;88(7):587–590. doi:10.1136/adc.88.7.587
27. Schmitz R, Atzpodien K, Schlaud M. Prevalence and risk factors of atopic diseases in German children and adolescents. Pediatr Allergy Immunol. 2012;23(8):716–723. doi:10.1111/j.1399-3038.2012.01342.x
28. Mercer M, Joubert G, Ehrlich R, et al. Socioeconomic status and prevalence of allergic rhinitis and atopic eczema symptoms in young adolescents. Pediatr Allergy Immunol. 2004;15(3):234–241. doi:10.1111/j.1399-3038.2004.00125.x
29. Lee K-S, Rha Y-H, Oh I-H, Choi Y-S, Choi S-H. Socioeconomic and sociodemographic factors related to allergic diseases in Korean adolescents based on the Seventh Korea Youth Risk Behavior Web-based Survey: a cross-sectional study. BMC Pediatr. 2016;16(1):1–9. doi:10.1186/s12887-016-0549-2
30. Ogunbiyi A, Owoaje E, Ndahi A. Prevalence of skin disorders in school children in Ibadan, Nigeria. Pediatr Dermatol. 2005;22(1):6–10. doi:10.1111/j.1525-1470.2005.22101.x
31. Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EF. Systemic effects of smoking. Chest. 2007;131(5):1557–1566. doi:10.1378/chest.06-2179
32. Byron K, Varigos G, Wootton A. Il‐4 production is increased in cigarette smokers. Clin Exp Immunol. 1994;95(2):333–336. doi:10.1111/j.1365-2249.1994.tb06533.x
33. Krämer U, Lemmen C, Behrendt H, et al. The effect of environmental tobacco smoke on eczema and allergic sensitization in children. Br J Dermatol. 2004;150(1):111–118. doi:10.1111/j.1365-2133.2004.05710.x
34. Schäfer T, Dirschedlb P, Kunz B, Ring J, Überla K. Maternal smoking during pregnancy and lactation increases the risk for atopic eczema in the offspring. J Am Acad Dermatol. 1997;36(4):550–556. doi:10.1016/S0190-9622(97)70242-1
35. Linneberg A, Petersen J, Nielsen N, et al. The relationship of alcohol consumption to total immunoglobulin E and the development of immunoglobulin E sensitization: the Copenhagen Allergy Study. Clin Exp Allergy. 2003;33(2):192–198. doi:10.1046/j.1365-2222.2003.01515.x
36. Gonzalez‐Quintela A, Gude F, Boquete O, et al. Association of alcohol consumption with total serum immunoglobulin E levels and allergic sensitization in an adult population based survey. Clin Exp Allergy. 2003;33(2):199–205. doi:10.1046/j.1365-2222.2003.01582.x
37. McFadden J, Thyssen J, Basketter D, Puangpet P, Kimber I. T helper cell 2 immune skewing in pregnancy/early life: chemical exposure and the development of atopic disease and allergy. Br J Dermatol. 2015;172(3):584–591. doi:10.1111/bjd.13497
38. Nakashima C, Ishida Y, Kitoh A, Otsuka A, Kabashima K. Interaction of peripheral nerves and mast cells, eosinophils, and basophils in the development of pruritus. Exp Dermatol. 2019;28(12):1405–1411. doi:10.1111/exd.14014
39. Zhu Y-B, Xu L, Wang Y, et al. Posterior thalamic nucleus mediates facial histaminergic itch. Neuroscience. 2020;444:54–63. doi:10.1016/j.neuroscience.2020.07.048
40. Kong S, Koo J, Lim SK. Associations between stress and physical activity in Korean adolescents with atopic dermatitis based on the 2018–2019 Korea Youth Risk Behavior WebBased Survey. Int J Environ Res Public Health. 2020;17(21):8175. doi:10.3390/ijerph17218175
41. Mücke M, Ludyga S, Colledge F, Gerber M. Influence of regular physical activity and fitness on stress reactivity as measured with the Trier Social Stress Test protocol: a systematic review. Sports Med. 2018;48(11):2607–2622. doi:10.1007/s40279-018-0979-0
42. Paluska SA, Schwenk TL. Physical activity and mental health. Sports Med. 2000;29(3):167–180. doi:10.2165/00007256-200029030-00003
43. O’Dougherty M, Hearst MO, Syed M, Kurzer MS, Schmitz KH. Life events, perceived stress and depressive symptoms in a physical activity intervention with young adult women. Ment Health Phys Act. 2012;5(2):148–154. doi:10.1016/j.mhpa.2012.05.001
44. Norris R, Carroll D, Cochrane R. The effects of physical activity and exercise training on psychological stress and well-being in an adolescent population. J Psychosom Res. 1992;36(1):55–65. doi:10.1016/0022-3999(92)90114-H
45. Murota H, Yamaga K, Ono E, Murayama N, Yokozeki H, Katayama I. Why does sweat lead to the development of itch in atopic dermatitis? Exp Dermatol. 2019;28(12):1416–1421. doi:10.1111/exd.13981
46. Russo F, Santi F, Cioppa V, et al. Meeting the needs of patients with atopic dermatitis: a multidisciplinary approach. Dermatitis. 2022;33(6S1):S141–S143. doi:10.1097/DER.0000000000000907
47. El Hachem M, Di Mauro G, Rotunno R, et al. Pruritus in pediatric patients with atopic dermatitis: a multidisciplinary approach-summary document from an Italian expert group. Ital J Pediatr. 2020;46:1–9. doi:10.1186/s13052-020-0777-9
48. Bonamonte D, Belloni Fortina A, Neri L, Patrizi A. Fusidic acid in skin infections and infected atopic eczema. G Ital Dermatol Venereol. 2014;149(4):453–459.
49. Wollenberg A, Barbarot S, Bieber T, et al. Consensus‐based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32(5):657–682. doi:10.1111/jdv.14891
50. Galli E, Neri I, Ricci G, et al. Consensus conference on clinical management of 372 pediatric atopic dermatitis. Ital J Pediatr. 2016;42(1):1–25. doi:10.1186/s13052-015-0208-5
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
The Psychological Impact of Quarantine During the COVID-19 Pandemic on Quarantined Non-Healthcare Workers, Quarantined Healthcare Workers, and Medical Staff at the Quarantine Facility in Saudi Arabia
Alfaifi A, Darraj A, El-Setouhy M
Psychology Research and Behavior Management 2022, 15:1259-1270
Published Date: 17 May 2022
Alleviating Excessive Worries Improves Co-Occurring Depression and Pain in Adolescent and Young Adult Cancer Patients: A Network Approach
Li W, Xu Y, Luo X, Wen Y, Ding K, Xu W, Garg S, Yang Y, Sun H
Neuropsychiatric Disease and Treatment 2022, 18:1843-1854
Published Date: 25 August 2022
Spiritual Well-Being, Depression, Anxiety, and Stress in Indonesian Muslim Communities During COVID-19
Hamka, Suen MW, Ramadhan YA, Yusuf M, Wang JH
Psychology Research and Behavior Management 2022, 15:3013-3025
Published Date: 17 October 2022
Dietary Supplement Consumption and Mental Health in Indonesian Adults During Second Wave of COVID-19 Pandemic
Yusof J, d'Arqom A, Andriani AP, Nasution MZ, Fatimah N, Mustika A, Handayani S, Syed Abdul Kadir SZ
Patient Preference and Adherence 2023, 17:1799-1811
Published Date: 24 July 2023
The Association Between Cognitive Functions and Psychological Factors in Patients with Severe COPD
Hansen KK, Hilberg O, Jensen HI, Løkke A, Farver-Vestergaard I
International Journal of Chronic Obstructive Pulmonary Disease 2023, 18:2065-2078
Published Date: 19 September 2023
