Back to Journals » International Journal of Nanomedicine » Volume 17
Efficiency of Multifunctional Antibacterial Hydrogels for Chronic Wound Healing in Diabetes: A Comprehensive Review
Authors Ji JY, Ren DY, Weng YZ
Received 1 March 2022
Accepted for publication 16 June 2022
Published 22 July 2022 Volume 2022:17 Pages 3163—3176
DOI https://doi.org/10.2147/IJN.S363827
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Farooq A. Shiekh
Jia-Ying Ji,1,* Dan-Yang Ren,2,* Ying-Zheng Weng3
1Department of Plastic Surgery, The Affiliated People’s Hospital of Ningbo University, Ningbo, 315100, People’s Republic of China; 2Zhejiang University School of Medicine, Hangzhou, 310016, People’s Republic of China; 3Department of Cardiology, Zhejiang Hospital, Hangzhou, 310016, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jia-Ying Ji, Department of Plastic Surgery, The Affiliated People’s Hospital of Ningbo University, 251 East Baizhang Road, Ningbo, 315100, People’s Republic of China, Email [email protected]
Abstract: Diabetic chronic wounds or amputation, which are complications of diabetes mellitus (DM), are a cause of great suffering for diabetics. In addition to the lack of oxygen, elevated reactive oxygen species (ROS) and reduced vascularization, microbial invasion is also a critical factor that induces non-healing chronic diabetic wounds, ie, wounds still remaining in the stage of inflammation, after which the wound tissue begins to age and becomes necrotic. To clear up the infection, alleviate the inflammation in the wound and prevent necrosis, many kinds of hydrogel have been fabricated to eliminate infections with pathogens. The unique properties of hydrogels make them ideally suited to wound dressings because they provide a moist environment for wound healing and act as a barrier against bacteria. This review article will mainly cover the recent developments and innovations of antibacterial hydrogels for diabetic chronic wound healing.
Keywords: antibacterial, hydrogel, diabetic chronic wound, infection, inflammation, wound dressing
Graphical Abstract:
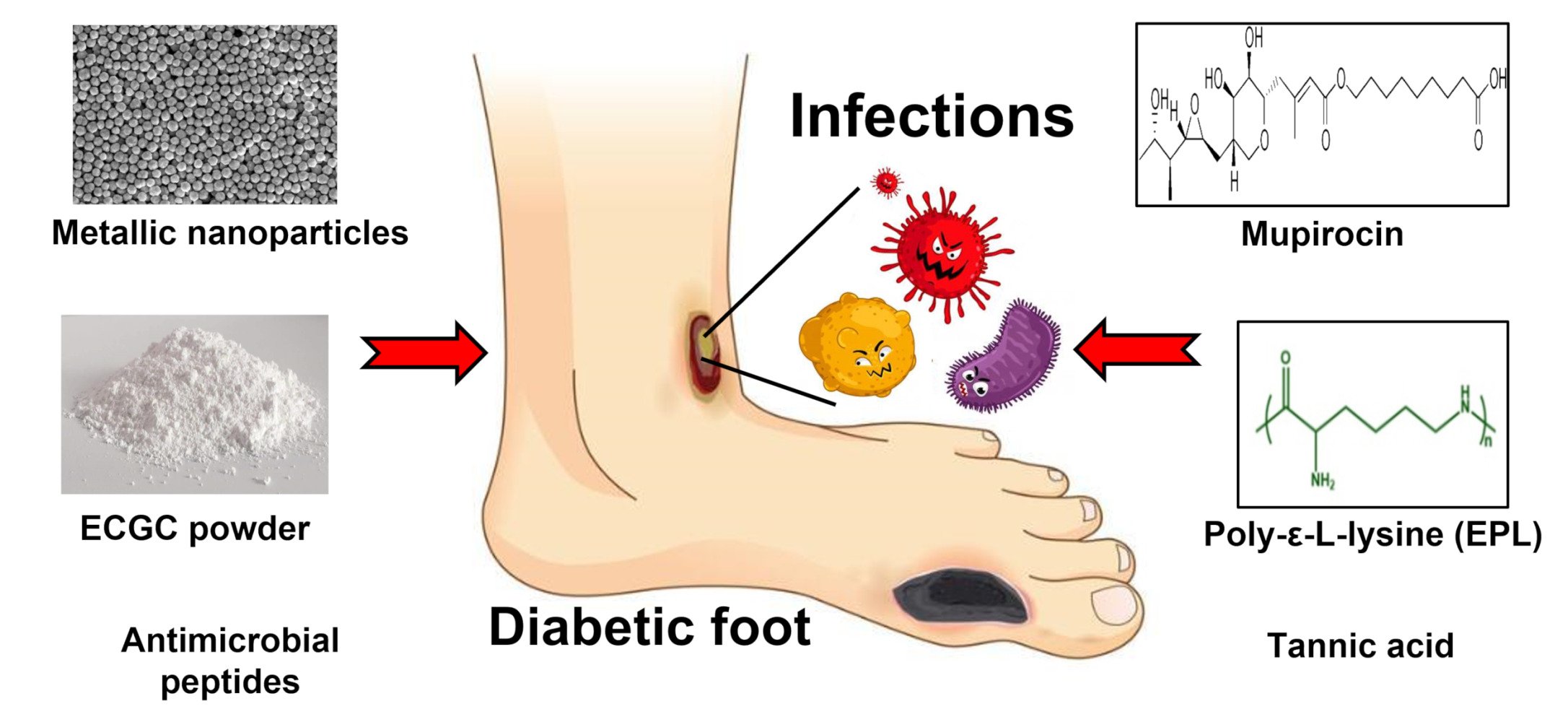
Introduction
Diabetes mellitus (DM), a common metabolic disease with over 400 million sufferers worldwide, puts a great burden on society, economies, and health care systems.1 Deaths among diabetics are mainly due to chronic complications caused by severe hyperglycemia.2 Non-healing diabetic wounds, especially those in the lower extremities, are common complications, and are called diabetic foot ulcer (DFU), which lead to 15–25% of diabetes patients needing amputation and suffering from disability during their lifetimes.3,4 Normal wound healing is a dynamic and complicated biological process involving four typical phases: hemostasis, inflammation, proliferation and remodeling, in which many types of cells, cytokines and the extracellular matrix (ECM) are involved (Figure 1).5–7 However, a wound with a micro-environment featuring high glycemic levels is more easily infected by bacteria, and macrophages produce more reactive oxygen species (ROS) to defend against foreign pathogens.8–10 Excessive ROS impairs normal cells and tissues resulting in lack of nutrients, impaired angiogenesis, hypoxia and neuropathy, which finally induce persistent inflammation and a long-time non-healing chronic wound (Figure 2).11–14 Therefore, clearing the infection and alleviating the inflammation are crucial for the management of diabetic chronic wound, and subsequently providing other growth factors (GFs) such as vascular endothelial growth factor (VEGF), stromal cell-derived factor-1a (SDF-1a) to promotes wound healing.15,16
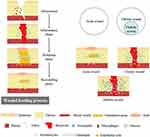 |
Figure 1 An illustration of how wounds heal and the major differences between an acute wound and diabetic chronic wound. Reprinted from Tan CT, Liang K, Ngo ZH, Dube CT, Lim CY. Application of 3D bioprinting technologies to the management and treatment of diabetic foot ulcers. Biomedicines. 2020;8:10. Creative Commons Attribution License.4 |
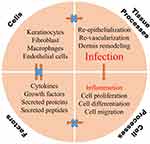 |
Figure 2 An illustration of major factors that contribute to the pathophysiology of diabetic chronic wounds. |
Using wound dressings is the most direct and convenient way to prevent the wound from being infected by microorganism and has been used for wound management for a very long time. A traditional form of wound dressing is a gauze made from cotton, which has been widely used in clinical treatment. However, it is unsuitable for the management of diabetic wound due to the secondary damage from each time of the dressing is exchanged, which is painful for the patients. Besides, traditional wound dressings have no bioactive promotion and moisturizing effects on wound healing.9,17,18 With the development of biomaterials, several kinds of biopolymers or synthetic polymers have been utilized in wound dressings, such as chitosan,19,20 collagen,21 gelatin,22 hyaluronic acid,23,24 cellulose,25,26 alginate,27 poly-(vinyl alcohol) (PVA),28 poly(lactic-co-glycolic acid) (PLGA)29 polylactide (PLA)30 and others. These polymers with properties such as non-toxicity, biodegradability and non-immunogenicity are conducive to wound management. Nevertheless, commercially available wound dressings for the treatment of diabetic wounds still have multitudinous limitations. For example, a kind of silver ion dressing, Biatain Alginate Ag® cannot be used for dry wounds due to the need for an external fixation dressing. Mepiform® is a kind of soft silicone dressing that should not be used for infected wounds, dry wounds or wounds with eschar, at the same time it is opaque and therefore is not convenient for viewing wounds.
Compared with other types of novel wound dressings, such as porous sponges, biocompatible membranes and electrospun nanofibers, hydrogels are superior candidates, due to their unique properties, for example, good flexibility, biocompatibility, moisturizing qualities and great sensitivity to physiological environments. Relative to other dressings, hydrogels can not only increase wound humidity, absorb wound exudate and reduce wound temperature, but are also comfortable, non-irritating, easy to change, and importantly, have analgesic effect for the injured tissue.31–33 With the development of biomaterial science, many functional hydrogels have been created by scientists to improve the ability of hydrogels for wound healing promotion.14,32,34 Different strategies exist for hydrogel preparation such as physical cross-linking, the radiation-induced gelation of polymer-water systems, chemical cross-linking, enzyme-catalyzed reactions and su-pramolecular interactions.14,32,34 Hydrogels are fabricated to work as intelligent drug-carriers and even adjust to the local wound microenvironment, but as a wound dressing, it is still highly important that they defend against microorganisms, especially in diabetic chronic wounds. Accordingly, to endow hydrogels with antibacterial activity, some antibacterial agents, metal nanoparticles, biopolymers and natural bioactive ingredients have been incorporated into the design of antibacterial multifunctional hydrogels.
In this review, we summarize and discuss the latest progress on multifunctional hydrogels with antibacterial ability, that decrease infection and promote diabetic chronic wound healing.
Loss of Wound Healing Ability in Diabetes Mellitus
The body responds to an injury in multiple ways to protect itself through restoring the integrity of damaged tissues, which involves a complex set of mechanisms. The wound healing process consists of four distinct phases: hemostasis, inflammation, proliferation, and remodeling.35 In healthy people, minor acute wounds heal within 2–3 weeks. However, when the physiological mechanism of healing is out of balance, diabetic wounds may become chronic and fail to heal within six to eight weeks.2,36,37
In the case of diabetes patients, the normal process of wound healing is interrupted, resulting in tissue damage, persistent infection and peripheral vascular problems. The imbalance between angiogenic factors such as TGF-β, FGF2, VEGF, angiogenin, angioinhibitory factors and abnormal apoptotic potential in diabetic patients may lead to disturbed angiogenesis.38 Diabetic wounds are in the stage of chronic inflammation and do not progress to the stage of proliferation and remodeling, thus obstructing the normal wound healing process.39 Due to normal phase interference, various parameters, including growth factors in the wound microenvironment, and immune cell circulation are interfered with, and the wound bed receives less energy, inhibiting the activation of caspase-3 and affecting metabolism here. Therefore, diabetic wounds are characterized by delayed wound healing, which is usually associated with infections caused by disrupted levels of microcirculating cell and decreased levels of endogenous growth factor, leading to the development of unhealing chronic ulcers. Further infection of the wound often leads to limb amputation.40
Antibacterial Hydrogels Based on Metal Ions
In the natural world, many kinds of metallics occur that possess antibacterial activity such as silver,41 copper42,43 and zinc,44 which also have excellent potential against multidrug-resistant bacteria. Metal elements act as antibacterial agents in their ionic form. In environments with relatively high concentrations of metal ions, the survival of microorganisms is affected in many aspects.45
Firstly, outside the membrane, the high concentration of metal cations alters the polarization state within and outside the biofilm, resulting in a new ion concentration difference, which blocks or disrupts the transport of small and large molecules required for cell maintenance, such as glucose and amino acid transport driven by the Na+/K+ pump.46 Some metal ions can also enter microbial cells. It has been demonstrated that heavy metals can inactivate most of the enzymes, although the mechanism of inactivation is still unclear.41,46 Some scholars consider that heavy metal ions with positive valence complexed with the N and O elements of protein destroy the spatial conformation of enzyme protein molecules.47 It is also possible that heavy metal ions react with -SH groups to replace protons, or even destroy or replace metal ions such as Mg2+, Fe3+ and Ca2+, which are necessary to maintain enzyme activity. Enzymes are the catalyst of all biological processes, also controlling microbial biochemical reactions.48 Once an enzyme is inactivated, it will cause the reduction of catalytic efficiency and performance, such that the biochemical reactions cannot be carried out normally, and affect related biochemical reactions, resulting in the blocked energy metabolism and material metabolism of microorganisms, to achieve the purpose of antibacterial effect. In addition, metal ions entering cells can combine with nucleic acids, destroying the ability of cells to divide and reproduce.48
Hydrogels Loaded with Silver Nanoparticles (AgNPs) for Diabetic Wound Management
Among the different metal nanoparticles, silver nanoparticles are the most dynamic nanoparticles for wound care management because of their antimicrobial activity, even against hospital strains of multidrug resistant microorganisms, thereby promoting wound healing.49,50 AgNPs have been mixed into many kinds of healthcare products, such as textiles,51 cosmetics,52 and wound dressings,53 as they have excellent antimicrobial properties and are electrically conductive. Recently, AgNPs were also applied in the fabrication of antibacterial hydrogel for the treatment of diabetic chronic wound. A composite hydrogel encapsulating silver nanoparticles and epidermal growth factor (EGF) co-loaded with chitosan was developed by Yu-Hsiang Lee et al and was named SNPECHG,54 The production process is shown in Figure 3A. They eventually found that the dosages of 24 mM Ag+ had an optimal antimicrobial effect, The antimicrobial activity of SNPECHG was demonstrated by the significant bactericidal effect of Ag+ on S. aureus and S. epidermidis, while CNP-coated EGF promoted the growth of NIH/3T3 cells, thus verifying its proliferation-promoting function. In addition, the optimized SNPECHG provides sustained release and excellent hydration of Ag+ and EGF in high ionic strength media, indicating that the developed composite hydrogel was highly suited to the exudate environment at the wound site.54 Nosheen Masood also observed that for AgNPs-loaded chitosan-polyethylene glycol hydrogels, 0.1g of AgNO3 was added into chitosan solution, which had superior antioxidant and antibacterial properties compared to bare chitosan-polyethylene glycol hydrogels, prompting researchers to use them to treat wounds in diabetic rabbits.55 They found that AgNP-loaded hydrogel, which released AgNPs slowly and continuously for at least seven days, had remarkable antibacterial ability against E. coli, P. aeruginosa, B. subtilis and S. aureus. Finally, the AgNP-loaded chitosan PEG hydrogel well promoted the healing of diabetic wound.55
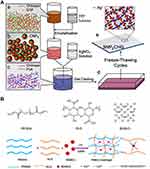 |
Figure 3 The process of making antibacterial hydrogel. (A) Schematic diagram of the SNPECHG fabrication procedures. Chitosan nanoparticles loaded with EGF were first prepared through a modified emulsification method (a-b), following the production of CNPE, AgNO3 was added to a chitosan-PVA solution, which was vigorously stirred afterward (c), an eight-cycle freezing/thawing procedure was performed on the polymeric mixture (d) to obtain SNPECHG (e); Reproduced from Lee YH, Hong YL, Wu TL. Novel silver and nanoparticle-encapsulated growth factor co-loaded chitosan composite hydrogel with sustained antimicrobility and promoted biological properties for diabetic wound healing. Mater Sci Eng C. 2021;118:111385. Copyright 2021, with permission from Elsevier.54 (B) Synthesis and potential wound healing application of PABC hydrogel: Main components of PABC hydrogel including PEGDA, ALG and BGN; Reproduced from Li Y, Xu T, Tu Z, et al. Bioactive antibacterial silica-based nanocomposites hydrogel scaffolds with high angiogenesis for promoting diabeticwound healing and skin repair. Theranostics. 2020;10(11):4929–4943. Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/).62 |
Zinc Oxide-Based Hydrogels for Diabetic Wound Management
Various wound dressings use zinc oxide nanoparticles as active ingredients, as they have antibacterial properties and promote fibroblast proliferation and angiogenesis.56–58 A study conducted by Rashid Ahmed et al discovered that chitosan /PVA/ZnO nanofibrous membranes had higher antibacterial potential against E. coli, Pseudomonas aeruginosa, Bacillus subtilis and Staphylococcus aureus compared with chitosan /PVA nanofiber mats. Furthermore, unlike chitosan/PVA/ZnO nanofibre mats, chitosan/PVA/ZnO nanofibrous membranes had higher antioxidant capacity and can facilitate diabetic wound healing in vivo.59
Recently a novel kind of ZnO particles, tetrapod-shaped ZnO particles, were integrated into 3D-printed GelMA hydrogels with the ability of light-controlled release of growth factors,60 tetrapod-shaped ZnO particles have better cytocompatibility than spherical ZnO nanoparticles. VEGF can be decorated onto the surface of t-ZnO, then treated with H2O2 and the surface turned into rough and porous.60 In vivo experiments have shown that the t-ZnO-laden composite hydrogels had a good effect, with lower immunogenicity and better wound healing Therefore, they can be also applied to diabetic wound healing due to their excellent antibacterial ability and controllable release of VEGF under ultraviolet/ visible light exposure.
Cu2+-Based Hydrogels for Diabetic Wound Management
Copper ions (Cu2+) with excellent antibacterial property can reduce wound infections and speed up wound healing.61 Recently, a novel bioactive, self-healing, antibacterial, dual-network nanocomposite hydrogel was developed, which significantly promoted diabetic wound healing/skin tissue formation by enhancing early angiogenesis without the addition of bioactive factors.62 Nanocomposite hydrogel consists of a network of polyethylene glycol diacrylate (PEGDA) and an auxiliary dynamic network between bioactive glass nanoparticles containing copper (BGNC) and sodium alginate (ALG), also named as PABC scaffolds, with the production process shown in Figure 3B.62 PABC scaffolds exhibit the mechanical properties of biomimetic elastomer with good injectability, self-repair, and strong broad-spectrum antibacterial activity. A significant increase in the proliferation and angiogenesis of endothelial progenitor cells (EPCs) was observed in vitro after the application of PABC hydrogels.62 In vivo, PABC hydrogels promoted wound healing and skin tissue regeneration in full-layer diabetic wounds by significantly increasing HIF-1*/VEGF expression and collagen matrix deposition.62 In addition, Sun et al developed a wound dressing from antibacterial nanocomposites based on chitosan, copper, and gallic acid61 and a novel hydrogel dressing (HKUST-Hs)63 containing copper metal organic framework nanoparticles can also be utilized in diabetic wound treatment, because both of them have dual effects as antibacterial and antioxidant.
Metal-Organic Framework (MOF)-Based Hydrogels for Diabetic Wound Management
When discussing the antibacterial effects of metal ions, a crucial aspect to cover is cytotoxicity. MOFs have emerged as a kind of porous, solid and adsorptive material, which are composed of metal ions and organic ligands. MOFs can carry drugs, enzymes, biological macromolecules and other substances to gain functionality while retaining low cytotoxicity, thus it is conducive to incorporated them into antibacterial wound dressings. Do Nam Lee et al conducted in-depth research in the field of MOFs. In 2020, they published a paper entitled “Novel Metal-Organic Frameword-based Photocrosslinked Hydrogel System for Efficient Antibacterial Applications” to present a hydrogel made of diacrylated polyethylene glycol (PEG), 4-arm-thiolated PEG, and MOFs. Their main contribution was to compare the structure and antibacterial performance of three kinds of MOF-based hydrogels: @Cu-MOF, @Co-MOF, and @Zn-MOF. The results showed that @Cu-MOF hydrogel the most stable 3D structure, low cytotoxicity, and high antibacterial activity, which could be attributed to Cu2+ and the excellent MOF system.64
Metal ions have favorable antibacterial ability, but when the concentration of metal ions in the body becomes toxic, their usage should be controlled at safe levels that still yield sufficient antibacterial activity.
Antibacterial Hydrogels Based on Natural Bioactive Ingredients
Antimicrobial compounds are emerging as a potential chemical alternative to conventional antibiotics, which consist an antibacterial strategy that is free from the problems of overuse and resistance associated with synthetic antibiotics.65
Epigallocatechin-3-Gallate (EGCG)-Based Hydrogels for Diabetic Wound Management
In recent years, EGCG has been the subject of extensive research,66,67 and its anticancer, anti-inflammatory, antioxidant, and anti-aging properties have led to its wide application in a number of fields.66 EGCG and its wound dressings play various roles in different wound healing stages, such as increasing hemadsorption, inhibiting neutrophil infiltration and monocyte migration and adhesion, promoting re-epithelialization, stimulating angiogenesis, altering collagen synthesis, and reducing ECM formation (Figure 4A).66 A team from Xi’an Jiao-tong University produced a smart hydrogel dressing,68 which can be conveniently obtained through copolymerization of the complex formed by EGCG and 3-acrylamido phenyl boronic acid (APBA) (resulting in the formation of boronate ester bond) with acrylamide. Scine E-A complexes are dynamic, the resulting hydrogels have good mechanical strength, moderate tissue adhesiveness and excellent self-regeneration capacity, which largely facilitates regeneration and self-healing. Besides its anti-oxidation and antibacterial properties, this functional hydrogel was shown to also anti-inflammatory, anti-inflammatory, and proangiogenic effects, as well as to modulate macrophage polarization. EGCG, however, also reduced the adhesive strength of tissue to facilitate dressing changes, all of which resulted in outstanding wound healing efficiency. in the chronic diabetic wound bed.68
 |
Figure 4 Four kinds of natural bioactive ingredients based antibacterial hydrogel. (A) Mechanism of EGCG promoting wound healing; Reproduced from Zhao X, Pei D, Yang Y, et al. Green tea derivative driven smart hydrogels with desired functions for chronic diabetic wound treatment. Adv Funct Mater. 2021;31(18):2009442. Copyright 2021, John Wiley and sons.68 (B) Several antimicrobial pathways of EPL; Reproduced from Wang L, Zhang C, Zhang J, et al. Epsilon-poly-L-lysine: recent advances in biomanufacturing and applications. Front Bioeng Biotechnol.2021;9:748976. Copyright © 2021 Wang, Zhang, Zhang, Rao, Xu, Mao and Chen. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY).72 (C) Composition and action mode of the Gelma-dopa-amp-Ceons dressing; Reproduced from Cheng H, Shi Z, Yue K, et al. Sprayable hydrogel dressing accelerates wound healing with combined reactive oxygen species-scavenging and antibacterial abilities. Acta Biomater. 2021;124:219–232. Copyright 2021, with permission from Elsevier.76 (D) Structure of TA@bilayer hydrogel and its interaction with wound; Reproduced from Li Y, Fu R, Zhu C, Fan D. An antibacterial bilayer hydrogel modified by tannic acid with oxidation resistance and adhesiveness to accelerate woundrepair. Colloids Surf B. 2021;205:111869. Copyright 2021, with permission from Elsevier.78 |
Polyε-L-Lysine (EPL)-Based Hydrogel for Diabetic Wound Management
EPL is biodegradable, antibacterial, and biocompatible naturally occurring cationic polypeptide produced by S. albus.69–71 EPL acts as a cationic surface-active compound that is known to inhibit the proliferation of microorganisms by effecting on the outer membrane of bacteria, Specifically, in EPL-treated cells, electrostatic adsorption of EPL and external membrane stripping, accompanied by abnormal cytoplasm distribution, resulted in physiological damage. (Figure 4B).72 A representative example of the antibacterial effect of EPL in vitro is shown in Figure 5. It was found to be active against both Gram-positive and Gram-negative bacteria.73 Two kinds of multifunctional antibacterial hydrogels have been developed that contain EPL. Firstly, an injectable, self-healing and antimicrobial peptide-based FHE hydrogel (F127/OHA-EPL) featuring the stimuli-responsive release of adipose derived mesenchymal stem cell exosomes (AMSCs-exo) was proposed by Chenggui Wang for the synergistic enhancement of chronic wound healing and relative tissue regeneration. FHE@exo hydrogel with 5% (wt/vol) of EPL and 10% (wt/vol) of EPL both had excellent antibacterial activity.72 Further in vivo studies confirmed that neo-vascular formation and cell proliferation were promoted in FHE@ exo hydrogel-treated wounds, leading to faster granulation tissue formation, re-epithelialization and collagen remodeling within the wound site, which accelerated the healing process of diabetic wounds.72 Secondly, a polyacrylamide, gelatin, and ε-polylysine dressing that is temperature tolerant (−20 to 60℃) was prepared and called G-PAGL, which displayed good heat resistance and anti-freezing properties. They established that the G-PAGL with 20% (wt/vol) EPL exerted the highest antibacterial activity against E. coli and S. aureus and the inherent and long-lasting antimicrobial properties conferred by ε-PL were considered essential for G-PAGL hydrogels used as DFU wound dressings.74
 |
Figure 5 Digital image of E. coli (A) and S. aureus (C) clones on an AGAR plate after exposure; reproduced from Xu Z, Xu Z, Feng X, Xu D, Liang J, Xu H. Recent advances in the biotechnological production of microbial poly(ɛ-L-lysine) and understanding of its biosynthetic mechanism. Appl Microbiol Biotechnol. 2016;100(15):6619–6630. Copyright 2016, Springer Nature.73 Red circles indicate bacterial clones killed by hydrogel. In the presence of blank LB medium, G-PAGL-0, G-PAGL-I, G-PAGL-II, G-PAGL-III and G-PAGL-IV, the growth curves of (B) Escherichia coli and (D) Staphylococcus aureus varied with culture time. |
Antimicrobial Peptides (AMPs)-Based Hydrogel for Diabetic Wound Management
AMPs are a type of natural bioactive ingredients with a wide range of antimicrobial and immunomodulatory activities to combat drug resistance, which inhibit the survival of bacteria through targeting the bacterial cell membrane by electrostatic interactions.75 To date, a number of antimicrobial bioactive hydrogel designs have been based on AMPs on account of their low resistance, high biocompatibility and antibacterial benefits.75
Wang et al developed a sprayable hydrogel containing GelMA functionalized by DOPA and encapsulating AMP HC-36 and CeONs, which possesses antibacterial, ROS scavenging and wound healing effects by (Figure 4C).76 By comparing the antibacterial performance of nano-silver, vancomycin and non-AMP loaded hydrogels, hydrogels loaded with AMP could ablate approximately 100% (and GT; 99%) of bacterias especially S. aureus and S. epidermidis.76 Antibacterial hydrogels based on AMPs have shown great antibacterial benefits, which attributes a certain instructive meaning to the investigation of infection wound healing.
TA-Based Hydrogel for Diabetic Wound Management
An inevitable issue in developing antibacterial hydrogels based on natural active ingredients is biocompatibility. Some researchers have found that a phenolic non-cytotoxic natural plant extract, tannic acid (TA), works by attaching to bacteria, inhibiting the uptake of sugars and amino acids, and interfering with their metabolism.77 A research group led by Chenhui Zhu, from Northwest University, changed the structure of the hydrogel by TA and proposed the TA@bilayer hydrogel, which showed excellent properties including adhesion, self-healing, antibacterial and antioxidant, which made the product become a multifunctional antibacterial hydrogel with great advantages (Figure 4D).78
Special Antibiotic Based Antibacterial Hydrogel
Mupirocin antibiotics have been used to treat secondary skin infections caused by S. aureus and S. pyogenes.79–81 However, recent reports indicated a rise in Staphylococci with mupirocin resistance.82,83 Therefore, it is necessary to develop alternative anti-microbial drugs or to improve the efficacy of mupirocin.
Golmohammadi et al created the Selenium-chitosan-Mupirocin (M-SeNPs-CCH) complex, which is a nanohybrid system, prepared using chitosan-cetyltrimethylammonium bromide (CTAB)-based hydrogel (CCH) with mupirocin (M) and selenium nanoparticles (SeNPs) entrapped.84 Its antibacterial activity and toxicity were evaluated on the L929 mouse fibroblast cell line. The concentration of M 20 mg/mL had the best antibacterial ability against S. aureus (MRSA), and the wounds were subsequently treated by M-SeNPs-CCH nanohybrid system with concentrations of M; 20 mg/mL, CCH; 2 mg/mL and SeNPs; 512 μg/mL in two times/day for 21 days.84 It was discovered that this system could play a crucial role in the formation and contraction of wounds, angiogenesis, fibroblastosis, and collagenesis, as well as the proliferation of hair follicles and epidermis.84
An ROS-scavenging hydrogel to promote the healing of infected diabetic wounds was put forward by Jian Wang and his team which was fabricated by using polyvinyl alcohol (PVA) cross-linked by a ROS-responsive linker. This hydrogel could allow the release of GM-CSF and therapeutics, including mupirocin to kill bacteria.84 The doses of M and GM-CSF were 100 μg/wound and 0.5 μg/wound, after treatment with PBS, Hydrogel, M@Hydrogel, G@Hydrogel or M+G@Hydrogel under infection with S. aureus, and the M@Hydrogel and M+G@Hydrogel groups showed more powerful antibacterial activity.84 This work provided an antibacterial ROS-scavenging hydrogel with different therapeutic ingredients such as mupiroxacin, which is expected to be used to treat chronic wounds including infected diabetic wounds.14
However, it is undeniable that the use of antibacterial drugs will bring the serious consequence of antibiotic resistance, therefore, when during developing antibiotic based antibacterial hydrogels, one needs to pay special attention to the limitation of dosage and indications.
Biopolymer-Based Antibacterial Hydrogels
In addition to adding antibacterial substances to hydrogels, some of the biopolymers used to make hydrogels also have antibacterial properties.
Using the dynamic Schiff-based reaction, Qian Xu and Wenxin Wang’s team developed a self-healing hydrogel system made of chitosan (CTS) and dialdehyde chitosan (CTS-CHO), which prevents infection during wound healing. The results showed that the antibacterial properties of chitosan and aldehyde chitosan exhibited in the hydrogel system significantly impaired bacterial growth upon contact.85 However, the antibacterial mechanism of chitosan and its derivatives is still not clear. There is a generally accepted theory that the large number of positively charged amino groups in their molecular structure plays a vital role, they can also absorb bacteria and enter bacterial cells to inhibit bacterial growth by interfering with the transcription of bacterial DNA and block the absorption of trace elements and nutrients necessary for cell growth. These kind of hydrogels with antibacterial action based on biopolymers has the advantages of convenient preparation, high economic benefit and great development prospect.
The Future Perspectives
Chronic wounds fall into different categories,86 such as diabetic foot ulcers (DFU), venous leg ulcers (VLU) and pressure ulcers (PU), surgical site infections (SSI), abscesses, or traumatic ulcers, in which colonization by pathogenic bacteria at the wound site results in wound chronicity.87 Therefore, it is important to perform antibacterial treatment in the process of chronic wound therapy. As we have previously reviewed, many kinds of antibacterial hydrogel have been created and applied for diabetic chronic wound treatment, owing to the special properties of hydrogel (Table 1). With the development of biopolymers, hydrogel synthetic procedures are becoming more mature, along with the diversification of synthetic pathways and products, which is conducive to the further industrialization of hydrogel application and faster translation into clinical treatments. Meanwhile, combining antibacterial metal ions and natural antibacterial substances into hydrogel for antibacterial treatment can help to reduce the use of antibiotics and prevent the generation of bacterial resistance, ultimately reducing the risk of superbug emergence. Photodynamic and photothermal are also widely used in wound antibacterial applications by combining with hydrogels.88,89 Effective antibacterial hydrogels can help to heal patients’ wounds and reduce the pain from chronic diabetic wounds as well as reduce the healthcare burden.
 |
Table 1 The Roles of Antibiotic Substance and Their Wound Dressings |
Currently, Hydrosorb®, 3M™Tegaderm™, AQUACELAg®, TenderWet® and Comfeel® are the main hydrogel dressings available on the market (Table 2), whose components include PU, CHG, CMC, PEA, or other biological large molecules, and Ag+. The antibacterial activity of these hydrogels is mainly attributed to the action of Ag+ and the adsorption of bacteria. However, most of the commercially available products are not multifunctional, lack biological activity, are difficult to cut, are costly, etc. Although the scientific achievements on multi-functional antibacterial hydrogels are increasing, there are too many ideas to be selected from and some of the scholars lack a business aptitude, which leads to few products being commercialized. However, there is still a potential market and extensive prospects of antibacterial hydrogel dressings. Therefore, the development of antibacterial hydrogel dressings needs to rely on the joint efforts of researchers, doctors, patients, and medical device manufacturers to transform emerging technological achievements into products, in order to help more patients that suffer from chronic wounds and realize a win-win situation.
 |
Table 2 The Commercially Available Hydrogels Used for Wound Dressings |
Acknowledgment
This work was supported by grants from Scientific Research Fund of Zhejiang Hospital (No. Z210057).
Disclosure
There are no conflicts of interest to declare.
References
1. Patty Y, Nita Y, Nita Y. Cost of illness of diabetes mellitus in Indonesia: a systematic review. J Basic Clin Physiol Pharmacol. 2021;32(4):285–295. doi:10.1515/jbcpp-2020-0502
2. Clements JM, West BT, Yaker Z, et al. Disparities in diabetes-related multiple chronic conditions and mortality: the influence of race. Diabetes Res Clin Pract. 2020;159:107984. doi:10.1016/j.diabres.2019.107984
3. Al Wahbi A. Operative versus non-operative treatment in diabetic dry toe gangrene. Diabetes Metab Syndr. 2019;13(2):959–963. doi:10.1016/j.dsx.2018.12.021
4. Tan CT, Liang K, Ngo ZH, Dube CT, Lim CY. Application of 3D bioprinting technologies to the management and treatment of diabetic foot ulcers. Biomedicines. 2020;8:10. doi:10.3390/biomedicines8100441
5. Broughton G, Janis JE, Attinger CE. Wound healing: an overview. Plast Reconstr Surg. 2006;117(7Suppl):
6. Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther. 2017;34(3):599–610. doi:10.1007/s12325-017-0478-y
7. Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37(5):1528–1542. doi:10.1177/147323000903700531
8. Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366(9498):1736–1743. doi:10.1016/S0140-6736(05)67700-8
9. Lim JZ, Ng NS, Thomas C. Prevention and treatment of diabetic foot ulcers. J R Soc Med. 2017;110(3):104–109. doi:10.1177/0141076816688346
10. Nouvong A, Ambrus AM, Zhang ER, Hultman L, Coller HA. Reactive oxygen species and bacterial biofilms in diabetic wound healing. Physiol Genomics. 2016;48(12):889–896. doi:10.1152/physiolgenomics.00066.2016
11. Thangarajah H, Vial IN, Grogan RH, et al. HIF-1alpha dysfunction in diabetes. Cell Cycle. 2010;9(1):75–79. doi:10.4161/cc.9.1.10371
12. Tandara AA, Mustoe TA. Oxygen in wound healing–more than a nutrient. World J Surg. 2004;28(3):294–300. doi:10.1007/s00268-003-7400-2
13. Thangarajah H, Yao D, Chang EI, et al. The molecular basis for impaired hypoxia-induced VEGF expression in diabetic tissues. Proc Natl Acad Sci U S A. 2009;106(32):13505–13510. doi:10.1073/pnas.0906670106
14. Zhao H, Huang J, Li Y, et al. ROS-scavenging hydrogel to promote healing of bacteria infected diabetic wounds. Biomaterials. 2020;258:120286. doi:10.1016/j.biomaterials.2020.120286
15. Hong HS, Kim S, Jin Y, Son Y. Substance P enhances the therapeutic effect of MSCs by modulating their angiogenic potential. J Cell Mol Med. 2020;24(21):12560–12571. doi:10.1111/jcmm.15804
16. Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83(3):835–870. doi:10.1152/physrev.2003.83.3.835
17. Fang QQ, Wang XF, Zhao WY, et al. Development of a chitosan-vaseline gauze dressing with wound-healing properties in murine models. Am J Trop Med Hyg. 2020;102(2):468–475. doi:10.4269/ajtmh.19-0387
18. Rekha PD, Rao SS, Sahana TG, Prabhu A. Diabetic wound management. Br J Community Nurs. 2018;23(Sup9):S16–S22. doi:10.12968/bjcn.2018.23.Sup9.S16
19. Matica MA, Aachmann FL, Tøndervik A, Sletta H, Ostafe V. Chitosan as a wound dressing starting material: antimicrobial properties and mode of action. Int J Mol Sci. 2019;20:23. doi:10.3390/ijms20235889
20. Park JU, Song EH, Jeong SH, Song J, Kim HE, Kim S. Chitosan-based dressing materials for problematic wound management. Adv Exp Med Biol. 2018;1077:527–537.
21. Chattopadhyay S, Raines RT, Glick GD. Review collagen-based biomaterials for wound healing. Biopolymers. 2014;101(8):821–833. doi:10.1002/bip.22486
22. Hubner P, Donati N, Quines LKM, Tessaro IC, Marcilio NR. Gelatin-based films containing clinoptilolite-Ag for application as wound dressing. Mater Sci Eng C Mater Biol Appl. 2020;107:110215. doi:10.1016/j.msec.2019.110215
23. Graça MFP, Miguel SP, Cabral CSD, Correia IJ. Hyaluronic acid-Based wound dressings: a review. Carbohydr Polym. 2020;241:116364. doi:10.1016/j.carbpol.2020.116364
24. Cortes H, Caballero-Florán IH, Mendoza-Muñoz N, et al. Hyaluronic acid in wound dressings. Cell Mol Biol. 2020;66(4):191–198. doi:10.14715/cmb/2020.66.4.23
25. Portela R, Leal CR, Almeida PL, Sobral RG. Bacterial cellulose: a versatile biopolymer for wound dressing applications. Microb Biotechnol. 2019;12(4):586–610. doi:10.1111/1751-7915.13392
26. Alven S, Aderibigbe BA. Chitosan and cellulose-based hydrogels for wound management. Int J Mol Sci. 2020;21:24. doi:10.3390/ijms21249656
27. Varaprasad K, Jayaramudu T, Kanikireddy V, Toro C, Sadiku ER. Alginate-based composite materials for wound dressing application: a mini review. Carbohydr Polym. 2020;236:116025. doi:10.1016/j.carbpol.2020.116025
28. Zheng C, Liu C, Chen H, et al. Effective wound dressing based on Poly (vinyl alcohol)/Dextran-aldehyde composite hydrogel. Int J Biol Macromol. 2019;132:1098–1105. doi:10.1016/j.ijbiomac.2019.04.038
29. Tang KC, Yang KC, Lin CW, et al. Human adipose-derived stem cell secreted extracellular matrix incorporated into electrospun poly(lactic-co-glycolic acid) nanofibrous dressing for enhancing wound healing. Polymers. 2019;11:10. doi:10.3390/polym11101609
30. Giammona G, Craparo EF. Biomedical Applications of Polylactide (PLA) and its copolymers. Molecules. 2018;23(4). doi:10.3390/molecules23040980
31. Francesko A, Petkova P, Tzanov T. Hydrogel dressings for advanced wound management. Curr Med Chem. 2018;25(41):5782–5797. doi:10.2174/0929867324666170920161246
32. Li W, Wang S, Zhong D, Du Z, Zhou M. A bioactive living hydrogel: photosynthetic bacteria mediated hypoxia elimination and bacteria‐killing to promote infected wound healing. Adv Ther. 2020;4(1):2000107.
33. Sepantafar M, Maheronnaghsh R, Mohammadi H, et al. Stem cells and injectable hydrogels: synergistic therapeutics in myocardial repair. Biotechnol Adv. 2016;34(4):62–379. doi:10.1016/j.biotechadv.2016.03.003
34. Lee PY, Li Z, Huang L. Thermosensitive hydrogel as a Tgf-β1 gene delivery vehicle enhances diabetic wound healing. Pharm Res. 2003;20(12):1995–2000. doi:10.1023/B:PHAM.0000008048.58777.da
35. Piperigkou Z, Götte M, Theocharis AD, Karamanos NK. Insights into the key roles of epigenetics in matrix macromolecules-associated wound healing. Adv Drug Deliv Rev. 2018;129:16–36. doi:10.1016/j.addr.2017.10.008
36. Taub A, Bucay V, Keller G, Williams J, Mehregan D. Multi-center, double-blind, vehicle-controlled clinical trial of an alpha and beta defensin-containing anti-aging skin care regimen with clinical, histopathologic, immunohistochemical, photographic, and ultrasound evaluation. J Drugs Dermatol. 2018;17(4):426–441.
37. Peppa M, Raptis SA. Glycoxidation and wound healing in diabetes: an interesting relationship. Curr Diabetes Rev. 2011;7(6):416–425. doi:10.2174/157339911797579188
38. Rana S, Burke SD, Karumanchi SA. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am J Obstet Gynecol. 2020;226:S1019–S1034.
39. Vagesjo E, Ohnstedt E, Mortier A, et al. Accelerated wound healing in mice by on-site production and delivery of CXCL12 by transformed lactic acid bacteria. Proc Natl Acad Sci U S A. 2018;115(8):1895–1900. doi:10.1073/pnas.1716580115
40. Shankhdhar K. Diabetic foot amputation prevention during COVID-19. Adv Skin Wound Care. 2021;34(5):1–4. doi:10.1097/01.ASW.0000741532.29113.78
41. Yin IX, Zhang J, Zhao IS, Mei ML, Li Q, Chu CH. The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int J Nanomed. 2020;15:2555–2562. doi:10.2147/IJN.S246764
42. Zong M, Bai L, Liu Y, et al. Antibacterial ability and angiogenic activity of Cu-Ti-O nanotube arrays. Mater Sci Eng C. 2017;71:93–99. doi:10.1016/j.msec.2016.09.077
43. Yang L, Chen L, Chen YC, et al. Homogeneously alloyed nanoparticles of immiscible Ag-Cu with ultrahigh antibacterial activity. Colloids Surf B. 2019;180:466–472. doi:10.1016/j.colsurfb.2019.05.018
44. Prado-Prone G, Silva-Bermudez P, Bazzar M, et al. Antibacterial composite membranes of polycaprolactone/gelatin loaded with zinc oxide nanoparticles for guided tissue regeneration. Biomed Mater. 2020;15(3):035006. doi:10.1088/1748-605X/ab70ef
45. Wyszogrodzka G, Marszałek B, Gil B, Dorożyński P. Metal-organic frameworks: mechanisms of antibacterial action and potential applications. Drug Discov Today. 2016;21(6):1009–1018. doi:10.1016/j.drudis.2016.04.009
46. Wang X, Liu S, Li M, et al. The synergistic antibacterial activity and mechanism of multicomponent metal ions-containing aqueous solutions against Staphylococcus aureus. J Inorg Biochem. 2016;163:214–220. doi:10.1016/j.jinorgbio.2016.07.019
47. Sharma SK, Goloubinoff P, Christen P. Heavy metal ions are potent inhibitors of protein folding. Biochem Biophys Res Commun. 2008;372(2):341–345. doi:10.1016/j.bbrc.2008.05.052
48. Kulakovskaya T. Inorganic polyphosphates and heavy metal resistance in microorganisms. World J Microbiol Biotechnol. 2018;34(9):139. doi:10.1007/s11274-018-2523-7
49. Kalantari K, Mostafavi E, Afifi AM, et al. Wound dressings functionalized with silver nanoparticles: promises and pitfalls. Nanoscale. 2020;12(4):2268–2291. doi:10.1039/C9NR08234D
50. Pangli H, Vatanpour S, Hortamani S, Jalili R, Ghahary A. Incorporation of silver nanoparticles in hydrogel matrices for controlling wound infection. J Burn Care Res. 2021;42(4):785–793. doi:10.1093/jbcr/iraa205
51. Gauger A. Silver-coated textiles in the therapy of atopic eczema. Curr Probl Dermatol. 2006;33:152–164.
52. Arroyo GV, Madrid AT, Gavilanes AF, et al. Green synthesis of silver nanoparticles for application in cosmetics. J Environ Sci Health A. 2020;55(11):1304–1320. doi:10.1080/10934529.2020.1790953
53. Probst S, Saini C, Rosset C, Skinner MB. Superabsorbent charcoal dressing versus silver foam dressing in wound area reduction: a randomised controlled trial. J Wound Care. 2022;31(2):140–146. doi:10.12968/jowc.2022.31.2.140
54. Lee YH, Hong YL, Wu TL. Novel silver and nanoparticle-encapsulated growth factor co-loaded chitosan composite hydrogel with sustained antimicrobility and promoted biological properties for diabetic wound healing. Mater Sci Eng C. 2021;118:111385. doi:10.1016/j.msec.2020.111385
55. Masood N, Ahmed R, Tariq M, et al. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int J Pharm. 2019;559:23–36. doi:10.1016/j.ijpharm.2019.01.019
56. Vijayakumar V, Samal SK, Mohanty S, Nayak SK. Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management. Int J Biol Macromol. 2019;122:137–148. doi:10.1016/j.ijbiomac.2018.10.120
57. Gharpure S, Ankamwar B. Synthesis and antimicrobial properties of zinc oxide nanoparticles. J Nanosci Nanotechnol. 2020;20(10):5977–5996. doi:10.1166/jnn.2020.18707
58. Mishra PK, Mishra H, Ekielski A, Talegaonkar S, Vaidya B. Zinc oxide nanoparticles: a promising nanomaterial for biomedical applications. Drug Discov Today. 2017;22(12):1825–1834. doi:10.1016/j.drudis.2017.08.006
59. Ahmed R, Tariq M, Ali I, et al. Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing. Int J Biol Macromol. 2018;120(Pt A):385–393. doi:10.1016/j.ijbiomac.2018.08.057
60. Siebert L, Luna‐Cerón E, García‐Rivera LE, et al. Light‐controlled growth factors release on tetrapodal ZnO‐incorporated 3D‐printed hydrogels for developing smart wound scaffold. Adv Funct Mater. 2021;31(22):2007555.
61. Sun X, Dong M, Guo Z, et al. Multifunctional chitosan-copper-gallic acid based antibacterial nanocomposite wound dressing. Int J Biol Macromol. 2021;167:10–22. doi:10.1016/j.ijbiomac.2020.11.153
62. Li Y, Xu T, Tu Z, et al. Bioactive antibacterial silica-based nanocomposites hydrogel scaffolds with high angiogenesis for promoting diabetic wound healing and skin repair. Theranostics. 2020;10(11):4929–4943. doi:10.7150/thno.41839
63. Wang M, Huang H, Ma X, Huang C, Peng X. Copper metal-organic framework embedded carboxymethyl chitosan-g-glutathione/polyacrylamide hydrogels for killing bacteria and promoting wound healing. Int J Biol Macromol. 2021;187:699–709. doi:10.1016/j.ijbiomac.2021.07.139
64. Gwon K, Han I, Lee S, Kim Y, Lee DN. Novel metal-organic framework-based photocrosslinked hydrogel system for efficient antibacterial applications. ACS Appl Mater Interfaces. 2020;12(18):20234–20242. doi:10.1021/acsami.0c03187
65. Wang Y, Yang Y, Shi Y, Song H, Yu C. Antibiotic-free antibacterial strategies enabled by nanomaterials: progress and perspectives. Adv Mater. 2020;32(18):e1904106. doi:10.1002/adma.201904106
66. Xu FW, Lv YL, Zhong YF, et al. Beneficial effects of green tea EGCG on skin wound healing: a comprehensive review. Molecules. 2021;26:20. doi:10.3390/molecules26206123
67. Steinmann J, Buer J, Pietschmann T, Steinmann E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br J Pharmacol. 2013;168(5):1059–1073. doi:10.1111/bph.12009
68. Zhao X, Pei D, Yang Y, et al. Green tea derivative driven smart hydrogels with desired functions for chronic diabetic wound treatment. Adv Funct Mater. 2021;31(18):2009442. doi:10.1002/adfm.202009442
69. Chen S, Huang S, Li Y, Zhou C. Recent advances in epsilon-Poly-L-lysine and L-lysine-based dendrimer synthesis, modification, and biomedical applications. Front Chem. 2021;9:659304. doi:10.3389/fchem.2021.659304
70. Nishikawa M, Ogawa K. Inhibition of epsilon-poly-L-lysine biosynthesis in Streptomycetaceae bacteria by short-chain polyols. Appl Environ Microbiol. 2006;72(4):2306–2312. doi:10.1128/AEM.72.4.2306-2312.2006
71. Wang L, Zhang C, Zhang J, et al. Epsilon-poly-L-lysine: recent advances in biomanufacturing and applications. Front Bioeng Biotechnol. 2021;9:748976. doi:10.3389/fbioe.2021.748976
72. Wang C, Wang M, Xu T, et al. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics. 2019;9(1):65–76. doi:10.7150/thno.29766
73. Xu Z, Xu Z, Feng X, Xu D, Liang J, Xu H. Recent advances in the biotechnological production of microbial poly(ɛ-L-lysine) and understanding of its biosynthetic mechanism. Appl Microbiol Biotechnol. 2016;100(15):6619–6630. doi:10.1007/s00253-016-7677-3
74. Liu H, Li Z, Zhao Y, et al. Novel diabetic foot wound dressing based on multifunctional hydrogels with extensive temperature-tolerant, durable, adhesive, and intrinsic antibacterial properties. ACS Appl Mater Interfaces. 2021;13(23):26770–26781. doi:10.1021/acsami.1c05514
75. Boparai JK, Sharma PK. Mini review on antimicrobial peptides, sources, mechanism and recent applications. Protein Pept Lett. 2020;27(1):4–16. doi:10.2174/0929866526666190822165812
76. Cheng H, Shi Z, Yue K, et al. Sprayable hydrogel dressing accelerates wound healing with combined reactive oxygen species-scavenging and antibacterial abilities. Acta Biomater. 2021;124:219–232. doi:10.1016/j.actbio.2021.02.002
77. Kaczmarek B. Tannic acid with antiviral and antibacterial activity as a promising component of Biomaterials-A minireview. Materials. 2020;13:14. doi:10.3390/ma13143224
78. Li Y, Fu R, Zhu C, Fan D. An antibacterial bilayer hydrogel modified by tannic acid with oxidation resistance and adhesiveness to accelerate wound repair. Colloids Surf B. 2021;205:111869. doi:10.1016/j.colsurfb.2021.111869
79. Tucaliuc A, Blaga AC, Galaction AI, Cascaval D. Mupirocin: applications and production. Biotechnol Lett. 2019;41(4–5):495–502. doi:10.1007/s10529-019-02670-w
80. Sritharadol R, Hamada M, Kimura S, Ishii Y, Srichana T, Tateda K. Mupirocin at subinhibitory concentrations induces biofilm formation in Staphylococcus aureus. Microb Drug Resist. 2018;24(9):1249–1258. doi:10.1089/mdr.2017.0290
81. Kotloff KL, Shirley DT, Creech CB, et al. Mupirocin for Staphylococcus aureus decolonization of infants in neonatal intensive care units. Pediatrics. 2019;143(1). doi:10.1542/peds.2018-1565
82. Thomas CM, Hothersall J, Willis CL, Simpson TJ. Resistance to and synthesis of the antibiotic mupirocin. Nat Rev Microbiol. 2010;8(4):281–289. doi:10.1038/nrmicro2278
83. Hetem DJ, Bonten MJ. Clinical relevance of mupirocin resistance in Staphylococcus aureus. J Hosp Infect. 2013;85(4):249–256. doi:10.1016/j.jhin.2013.09.006
84. Golmohammadi R, Najar-Peerayeh S, Tohidi Moghadam T, Hosseini SMJ. Synergistic antibacterial activity and wound healing properties of selenium-chitosan-mupirocin nanohybrid system: an in vivo study on rat diabetic Staphylococcus aureus wound infection model. Sci Rep. 2020;10(1):2854. doi:10.1038/s41598-020-59510-5
85. Wang X, Song R, Johnson M, He C, Milne X, Wang I. An injectable chitosan-based self-healable hydrogel system as an antibacterial wound dressing. Materials. 2021;14:20.
86. Dissemond J, Kröger K, Storck M, Risse A, Engels P. Topical oxygen wound therapies for chronic wounds: a review. J Wound Care. 2015;24(2):
87. Rahim K, Saleha S, Zhu X, Huo L, Basit A, Franco OL. Bacterial contribution in chronicity of wounds. Microb Ecol. 2017;73(3):710–721. doi:10.1007/s00248-016-0867-9
88. Guo N, Xia Y, Zeng W, et al. Alginate-based aerogels as wound dressings for efficient bacterial capture and enhanced antibacterial photodynamic therapy. Drug Deliv. 2022;29(1):1086–1099. doi:10.1080/10717544.2022.2058650
89. Li Y, Fu R, Duan Z, Zhu C, Fan D. Artificial nonenzymatic antioxidant MXene nanosheet-anchored injectable hydrogel as a mild photothermal-controlled oxygen release platform for diabetic wound healing. ACS Nano. 2022;16(5):7486–7502. doi:10.1021/acsnano.1c10575
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
