Back to Journals » Journal of Inflammation Research » Volume 17
Vitamin K: Infection, Inflammation, and Auto-Immunity
Authors Xie Y, Li S, Wu D, Wang Y, Chen J, Duan L, Li S, Li Y
Received 20 October 2023
Accepted for publication 23 January 2024
Published 20 February 2024 Volume 2024:17 Pages 1147—1160
DOI https://doi.org/10.2147/JIR.S445806
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Yuanyuan Xie,1,* Shifang Li,1,* Dinan Wu,2 Yining Wang,2 Jiepeng Chen,3 Lili Duan,3 Shuzhuang Li,4 Yuyuan Li2
1The First Affiliated Hospital of Dalian Medical University, Dalian, People’s Republic of China; 2Advanced Institute for Medical Sciences, Dalian Medical University, Dalian, People’s Republic of China; 3Sungen Bioscience Co., Ltd, Guangdong, People’s Republic of China; 4College of Basic Medical Science, Dalian Medical University, Dalian, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuyuan Li; Shuzhuang Li, Tel +86 411 86118983, Email [email protected]; [email protected]
Abstract: Vitamin K (VK) comprises a group of substances with chlorophyll quinone bioactivity and exists in nature in the form of VK1 and VK2. As its initial recognition originated from the ability to promote blood coagulation, it is known as the coagulation vitamin. However, based on extensive research, VK has shown potential for the prevention and treatment of various diseases. Studies demonstrating the beneficial effects of VK on immunity, antioxidant capacity, intestinal microbiota regulation, epithelial development, and bone protection have drawn growing interest in recent years. This review article focuses on the mechanism of action of VK and its potential preventive and therapeutic effects on infections (eg, asthma, COVID-19), inflammation (eg, in type 2 diabetes mellitus, Alzheimer’s disease, Parkinson’s disease, cancer, aging, atherosclerosis) and autoimmune disorders (eg, inflammatory bowel disease, type 1 diabetes mellitus, multiple sclerosis, rheumatoid arthritis). In addition, VK-dependent proteins (VKDPs) are another crucial mechanism by which VK exerts anti-inflammatory and immunomodulatory effects. This review explores the potential role of VK in preventing aging, combating neurological abnormalities, and treating diseases such as cancer and diabetes. Although current research appoints VK as a therapeutic tool for practical clinical applications in infections, inflammation, and autoimmune diseases, future research is necessary to elucidate the mechanism of action in more detail and overcome current limitations.
Keywords: vitamin K, VK-dependent proteins, infection, inflammation, auto-immune disease
Introduction
Infectious diseases pose a significant global health challenge.1 The human immune system responds to harmful foreign invaders and internal mutations, employing defense mechanisms to counteract such assaults.2 As we age, the immune system undergoes a process of development, maturation, and senescence, characterized by a progressive decline in immune function associated with an increased frequency of infections and chronic inflammation.3 Compared to healthy adults, children, the elderly, and patients with chronic disease and autoimmune disorders are more susceptible to pathogen invasion due to their weakened immune system. Therefore, further research is required to strengthen the development of effective drugs for immunity enhancement and pathogen infection resistance.
Vitamins are essential nutrients for a healthy body and the proper functioning of the immune system. Vitamins are subdivided into two groups: water-soluble (C and B-complex vitamins) and fat-soluble (vitamins A, D, E, and K). Vitamin K (VK), discovered by Danish biochemist Henrik Dam in the 1930s, is composed of phylloquinone and menaquinone and found in a wide variety of plant and animal products.4 In addition to the well-established biological function in blood coagulation,5 it has also shown some beneficial effects in immune response and anti-inflammation. However, to our knowledge, there is no review providing a comprehensive summary in this regard. Thus, we searched the online databases PubMed, Web of Sciences, Scopus, Google Scholar and Science Direct for literature on the effects of VK in infections (eg, asthma, COVID-19), inflammation (eg, in type 2 diabetes mellitus (T2DM), Alzheimer’s disease (AD), Parkinson’s disease (PD), cancer, aging, atherosclerosis) and autoimmune disorders (eg, inflammatory bowel disease (IBD), type 1 diabetes mellitus (T1DM), multiple sclerosis (MS), and rheumatoid arthritis (RA)). This review aims to summarize the current literature regarding the implications of VK supplementation in infections and the immune response, including evidence from both preclinical and clinical studies.
Chemical and Physiological Characteristics of Vitamin K
VK can be derived from natural sources and chemical synthesis. Two types of VK occur in nature: VK1 and VK2. VK1 (phylloquinone) is mainly present in leafy or flowering vegetables and vegetable oils.6 VK2 consists of a group of menaquinones (MK-n, wherein n represents the number of isoprenyl residues). It is found in meat, eggs, dairy products such as yoghurt or milk,7 and fermented foods such as soybeans (natto) and cheese.8,9 It can also be biosynthesized by gut bacteria, but these amounts are insufficient to meet physiological requirements.10 Among all menaquinones, MK4 and MK7 are the most well-studied in the human diet.11 VK3, also known as menadione, is a synthetic version of VK. It also originates from the intestine, as an intermediate product for the conversion of oral VK1 to VK2.12
Vitamin K and Infections
Asthma
Asthma, a complex disease with varied clinical features and physiological indicators, is categorized into multiple phenotypes. Among its causes, type 2 airway inflammation, linked to type 2 cytokines such as interleukin (IL)-4 and IL-13, plays a pivotal role.13 Clinical research has shown that VK2 supplementation gave effective rates of 90.9%, 86.7%, and 72.7% in mild, moderated, and severe patients, respectively.14 During infection, matrix gamma-carboxyglutamic acid protein (MGP) is a potent calcification inhibitor in the lung tissue which requires VK for its activation.15 The interplay between MGP, IL-6, and VK is a crucial determinant. When VK levels are high, MGP levels are high and IL-6 levels are low; when VK levels are low, the reverse occurs. Plasma levels of dephosphorylated-uncarboxylated MGP (dp-ucMGP) are a biomarker of VK deficit, which is associated with lower ventilatory capacity and higher risk of asthma.16
COVID-19
COVID-19 is a pervasive global infectious disease, which has had a profound impact on the well-being of individuals worldwide. Seeking effective treatment interventions is crucial, and VK has become a key research topic. It is worth noting that severe cases of COVID-19 are usually the result of excessive inflammation, in which the cytokine IL-6 plays a central role.17–19 Interestingly, VK can indirectly control the production of IL-6.20 Comparative analysis of patients with COVID-19 showed that patients with a better prognosis exhibited a decline in the level of IL-6. Importantly, dp-ucMGP is a key factor in the destructive inflammatory process of COVID-19. The increase in dp-ucMGP levels indicates a decrease in VK concentration, an increase in IL-6 levels, and exacerbation of inflammation.21
Vitamin K and Inflammation
Recently, the importance of low-grade inflammation in the progression of chronic inflammatory diseases has been confirmed through observations. Recent reports have linked the medicinal values of VK with its anti-inflammatory activities. Therefore, this section aims to discuss the therapeutic effects and potential mechanism of VK on inflammatory-related diseases based on existing in vitro and in vivo evidence.
Type 2 Diabetes Mellitus (T2DM)
T2DM remains a major challenge for the global healthcare industry and is the most common form of diabetes. Commonly linked to lifestyle factors and genetic predisposition, the condition occurs as a result of insulin secretion dysfunction or when efficiency of insulin absorption and utilization of glucose decreases, causing persistent increases in blood glucose levels. In a hyperglycemic internal environment, the typical symptoms of diabetes, such as polyuria, polydipsia, polyphagia and weight loss, as well as serious complications such as cardiovascular disease, can appear.22 At present, the primary method for the prevention and treatment of T2DM is to control blood sugar. Although the most well-known function of VK is coagulation, it also plays an important role in stabilizing blood sugar, improving insulin sensitivity and controlling diabetes.
Studies have evaluated the effects of VK on insulin response and blood glucose status. A study of healthy young men controlled for VK intake found that, after administering glucose load plasma glucose (PG)was higher, while immunoreactive insulin (IRI) and insulin production index (incremental IRI/incremental PG, 0–30 minutes) were significantly lower in the lowVK intake group in comparison with the high VK intake group. These results indicate that VK may play an important role in acute insulin response.23 Regarding the impact of VK on the pancreas, Sakamoto et al24 compared the glucose tolerance of healthy young men before and after administering VK2 (menadione-4), and concluded that glucose tolerance after taking VK2 was significantly higher than before taking VK2, demonstrating that VK2 may play a positive role in reducing the blood sugar function of the pancreas. The association between VK intake and insulin sensitivity and blood glucose status was also studied through a set of data. The findings indicated that intake of VK1 and VK2 has a beneficial effect on glucose homeostasis.25 Supplementing VK1 at a dietary dose for 36 months can improve insulin in elderly men, but it is almost ineffective in women.26 Meanwhile, some studies have shown that supplementary nutrition can improve blood glucose status and insulin sensitivity of women with pre-diabetes. However, it does not affect insulin resistance.27,28 According to the available data, VK1 has a positive effect on improving insulin levels in both men and women, but it also shows relative limitations. Table 1 summarizes the studies of VK related to insulin sensitivity and blood glucose levels. The data shows that both VK1 and VK2 can improve blood glucose homeostasis and insulin sensitivity, and have positive effects on the treatment of T2DM. However, the differences in the form of VK have not been clearly defined for blood glucose control, and the optimal intake is still unclear. Urgent research on phylloquinone and menaquinone is warranted. In addition, the specific mechanism of VK in controlling blood glucose balance is still unclear, and more studies are needed to evaluate the effect of VK on glucose metabolism and its role in T2DM.
 |
Table 1 Summary of the Research Results of Vitamin K Regulating Blood Glucose and Insulin |
Alzheimer’s Disease (AD)
AD is a neurodegenerative disease that primarily damages brain neurons and is the most common form of dementia. However, treatment methods for AD require further exploration. The mainstream view on the pathogenesis of AD is that the gradual deposition of extracellular β-amyloid (Aβ) in the brain to form neuritic plaques produces neurotoxicity, leading to neuronal degeneration and cognitive impairment.29 It has been shown that VK participates in the synthesis of sphingolipids, which are involved in brain cell proliferation and neuronal myelin formation.30,31 VK also participates in the biological activation of a range of VK-dependent proteins (VKDPs) with complex activities, such as the dependent protein growth arrest-specific gene 6 (Gas6) and protein S.32 Gas-6 regulates neuroinflammation, reduces hippocampal neuronal cell death, promotes myelin formation, and exhibits regulatory effects on the sciatic nerve after nerve transection, indicating that Gas-6 may be a novel neurotrophic factor for hippocampal neurons.33,34 Gas-6 also hinders Aβ-induced calcium influx, thereby attenuating neuronal apoptosis and neurotoxicity.35 Protein S is another VKDP. Studies have demonstrated that protein S protects neurons during ischemic brain injury.36 Some studies have shown that ischemia may drive the development of AD,37 as such protein S may also have a positive effect on reducing the risk of AD. Overall, both VK1 and VK2 are beneficial for protecting the nervous system and maintaining brain homeostasis, thus playing a positive role in the treatment of AD. According to existing studies, the correlation between VK2 and AD is greater than that of VK1. The mechanism of VK action in nervous system development, the differences between VK1 and VK2, and the mechanism of the role of VKDPs have not been fully explored. Further in-depth studies are urgently needed.
Parkinson’s Disease (PD)
PD is a complex neurodegenerative disorder that affects the nervous system and neurologically-controlled body parts. Due to the unclear pathological mechanism, only symptomatic treatment is possible. Aging, genetics, and oxidative stress may be related to the development of the disease.38 Studies have shown that mitochondrial function and structural changes to the mitochondrial respiratory chain are associated with the loss of dopaminergic neurons in the substantia nigra of patients with PD.39 VK1 can be converted into VK2 in human mitochondria, which can serve as an electron carrier for bacterial mitochondrial membrane binding. It has been found that VK2 (MK-4), as an electron carrier, bypasses complexes I and II of ETC through the Q cycle, which can transfer electrons from the mitochondria of Drosophila melanogaster, repair mitochondrial defects, and maintain normal ATP production.40 Therefore, VK, as an electron carrier for mitochondrial membrane binding, has a positive effect on the treatment of mitochondrial function. There is data indicating that after one week of MK-7 treatment, the brain ATP levels in PD patients increased compared to healthy controls.41 Serum VK2 levels in PD patients have been found to be significantly lower than in healthy controls.42 Research has shown that VK2 levels may be related to the occurrence and development of PD. α-Synuclein (α-Syn) is a presynaptic neuronal protein that may participate in the pathogenesis of PD in multiple ways and disrupt cellular homeostasis, leading to neuronal death. Some studies have explored the interaction between VK and α-synapses, indicating that VK can slow down the fibrosis of α-Syn.43 Overall, VK2 has the potential to treat PD, mainly by repairing mitochondrial defects and improving the production efficiency of ATP as an electronic carrier. However, existing research has not yet confirmed the relationship between VK2 and PD, and more data are required to draw conclusions.
Cancer
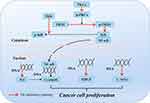
Cancer is considered the second leading cause of death from non-communicable diseases,44 and is a complex and variable process with many potential genetic causes.45 Although VK is mainly used for coagulation, studies have shown that it can induce cancer cell apoptosis and cell cycle arrest.46 Among the VK type, VK3 exhibits effective inhibitory effects on cancer cells,47 but has high toxicity so is generally not used for cancer treatment. VK2 can also inhibit the proliferation of cancer cells with a weaker effect compared to VK3, while VK1 has the weakest function.48,49 Therefore, the inhibitory effect of VK2 on tumor cells has become a focus of VK anticancer research. Studies have shown that VK2 prevents the phosphorylation of IκB by inhibiting IKK kinase activity, thereby inhibiting the expression of cell cycle protein D1 and suppressing the growth of cancer cells.50 In addition, it also inhibits NF-κB activation by inhibiting PKC kinase activity and PKD1 activation.51 When VK2 is used as a combination drug to block the differentiation and growth of monocytes in leukemia HL-60 cells, its therapeutic value in leukemia has been demonstrated.51,52 It has been found that VK2 inhibits the growth and invasion of hepatocellular carcinoma cells by activating protein kinase A.53 It also blocks the cell cycle, inhibits the proliferation of HepG2 cells by activating the transcription of the p21 gene,54 significantly inhibits the expression of hepatocellular carcinoma derived growth factor (HDGF) and mRNA in hepatocellular carcinoma cells (HCCs), inhibits the growth of HCC, and reduces the risk of hepatocellular carcinoma development. In summary, whether used alone or in combination, VK2 can inhibit tumor cell growth and induce cell cycle arrest through various pathways (Figure 1), and its therapeutic effect on cancer has been fully confirmed. However, further exploration is required to improve understanding of the mechanisms of action for different types of cancer.
Aging
Aging is a multifactorial process that leads to gradual aging of human tissues and structures, organ dysfunction, and weakened body resistance, including in the brain, musculoskeletal, and cardiovascular systems. With the extension of human life expectancy, age-related diseases are increasing.55,56 Research has confirmed that VK has antioxidant and anti-inflammatory activities, which can improve quality of life and combat age-related diseases.57 The intake of VK is beneficial for reducing the risk of age-related bone diseases. It has been established that VK deficiency leads to the development of fractures. The biological aging associated with VK deficiency further exacerbates abnormalities in bone structure and reduces bone strength. VK plays a cofactor role in enzymatic conversion of glutamic acid (Glu) into γ-Carboxyglutamic acid (Gla). VK deficiency leads to insufficient γ-carboxylation of VKDPs.58 VKDPs, including mineralization inhibitor MGP and osteocalcin (OC), are present in joint tissues. Some studies have shown that VK is beneficial in reducing the risk of developing osteoarthritis.59 VK enhances serum OC gamma-carboxylation in a dose-dependent manner and participates in bone metabolism to improve bone health.60 Undercarboxylated OC (ucOC) accounts for 40–60% of total OC and is a risk factor for fractures in the elderly.61,62 Increased VK intake is effective in reducing ucOC levels, favoring bone renewal and enhancing bone density,63 and maintaining VK intake reduces the prevalence of hip fractures.64 In a study linking VK1 to bone health in postmenopausal women, VK1 can reduced the risk of fracture in postmenopausal osteoporosis and enhanced hip joint strength.65 Long-term stable intake of VK1 can reduce bone loss and help reduce the incidence of fractures, indicating that VK may be beneficial for inhibiting bone resorption and maintaining bone formation.66 VK2 inhibits NF-κB activation, which is associated with osteoclast formation as well as osteoblast differentiation. Therefore, VK can inhibit osteoclast synthesis and stimulate osteoblast differentiation by inhibiting NF-κB activation. In addition, VK2 prevents the inhibition of transforming growth factor β (TGF-β) or bone morphogenetic protein-2 (BMP-2)-induced small mother against decapentaplegic (SMAD) signaling by tumor necrosis factor α (TNF-α), which further stimulates osteoblast formation, ameliorates bone loss and promotes bone health.67 VK2 combines with vitamin D (VD) and calcium to improve bone mineral density and bone quality and reduce bone loss. VD can improve osteoporosis and reduce the risk of fracture by enhancing the carboxylation of OC, which facilitates the entry of calcium into the bone matrix and its participation in bone metabolism; VK2 shows positive synergistic effects in this regard.68
Atherosclerosis
Increasing VK intake is beneficial for combating cardiovascular disease (CVD). Aging leads to degenerative changes in arterial vessel walls, such as vascular calcification (VC), atherosclerosis, and structural and functional abnormalities of the vessel wall. Medial VC accelerated vascular aging and increased CVD morbidity and mortality in a population with chronic kidney disease.69 Studies have shown that the anti-inflammatory and antioxidant effects of VK may be conducive to reducing cardiovascular risk, morbidity and mortality, and ameliorating the effects of atherosclerosis and atherosclerosis.70 VK carboxylation effectively activates MGP, which highly binds to calcium after activation, thereby inhibiting VC and preventing CVD.71 dp-ucMGP is associated with cardiac function and mortality.72 Elevated dp-ucMGP levels aggravates renal dysfunction, atherosclerosis, and vascular calcification.73 Studies have shown that plasma dp-ucMGP levels decrease in a dose-dependent manner after increasing VK2 intake.74 In addition, VC is a chronic inflammatory process mediated mainly through the NF-κB signaling pathway, producing pro-inflammatory cytokines (eg, IL-1β, IL-6, TNF-α, etc.), which are closely associated with CVD. VK can exert anti-inflammatory effects and prevent VC by blocking NF-κB signaling.75 It has been shown that nuclear factor red factor 2-related factor 2 (NRF2) signaling and VK are beneficial for blocking reactive oxygen species production, slowing down aging, reducing DNA damage, and resisting the onset of inflammatory responses.69
Vitamin K and Auto-Immune Disease
Inflammatory Bowel Disease (IBD)
IBD, comprising the conditions ulcerative colitis (UC) and Crohn’s disease (CD), is characterized by chronic relapsing intestinal inflammation.76 IBD increases the risk of colorectal cancer, especially in patients with intestinal microbiota disorders.77 Studies have shown that approximately half of IBD patients exhibit micronutrient deficiencies, including VK and VD.78 Micronutrient supplementation emerges as a promising strategy to mitigate IBD risks. VK plays a role in regulating intestinal microbiota, antioxidant function, and reducing inflammation by modulating the interaction between gut microbiota and the immune system. Although VK is synthesized by gut bacteria, dietary intake is the main source of VK2 due to its low bioavailability.11 IBD patients often face malabsorption due to intestinal damage, leading to a deficiency of VK2 and exacerbating the condition. The effect of VK on the diversity of intestinal microbiota is significant. The reduced diversity of patients with VK deficiency highlights the role of vitamins in intestinal health and their potential as effective IBD treatments.79
Type 1 Diabetes Mellitus (T1DM)
T1DM originates from autoimmune abnormality, which is characterized by autoimmune destruction of the insulin-producing beta cells. It has been demonstrated that VK1 treatment reduces oxidative stress, enhances antioxidants, and inhibits aldose reductase in T1DM. In addition, it also protects pancreatic endocrine cells, promotes increased insulin secretion and normal glucose levels, and improves glycated hemoglobin levels.80 Iwamoto et al81 reported that administration of VK2 to rats with STZ-induced T1DM prevented the development of hyperglycemia and cancellous osteopenia by inhibiting a decrease in bone formation, suggesting that VK2 has beneficial effects on glucose concentration and cancellous bone quality in T1DM.
Multiple Sclerosis (MS)
MS is a chronic autoimmune disease caused by abnormalities of the central nervous system (CNS) and is a major cause of neurological disorders in young people. MS is mainly caused by an immune complex response induced by oligodendrocytes, which leads to CNS plaques or lesions, progressive destruction of myelin sheaths, and interruption of nerve impulse transmission.82 The production of new myelin sheaths around the CNS can restore its function. A clinical study has shown VK2 levels in MS patients are significantly lower than in healthy controls.83 In an animal model of MS, VK2 has been proven to be a good promoter of myelin regeneration and is now one of the candidate drugs for MS treatment.84 The oxidative mechanism of injury plays an important role in neurological diseases, and VK1 and VK2 (MK-4) protect oligodendrocytes from oxidative damage.85 Carrié et al86 reported that the concentration of MK-4 in the brain was significantly higher in myelinated regions than in nonmyelinated regions, suggesting that it was strongly associated with sphingomyelin. Taken together, VK1 and VK2 (MK-4) can effectively prevent MS or MS progression, and can be effectively used for the treatment of MS (Figure 2).
Rheumatoid Arthritis (RA)
RA is one of the most common chronic inflammatory autoimmune diseases, mainly manifested as progressive synovial inflammation of the joints, which symmetrically affects the small joints of the hands and feet.87 In severe cases, it can lead to joint deformities and even disability. Patients with RA experience a degree of damage to the blood system and multiple organs throughout the body,88 including systemic osteoporosis, neutropenia, and anaemia.89 RA is mainly caused by the infiltration of various inflammatory factors into the joints, leading to synovial hyperplasia and bone destruction. Research has shown that the proliferation of synovial cells in RA patients is as intense as that of tumor cells.87 Some studies have found that RA patients have lower levels of VK in serum and stool, which is negatively correlated with the clinical severity of the disease. In addition, it has been shown that VK plays a positive role in reducing RA activity and delaying RA’s onset and progression when taken orally.90 Moreover, RA also leads to a deficiency of VK-dependent coagulation factors, leading to acquired coagulation dysfunction. In this case, supplementing with VK1 has a significant impact on coagulation dysfunction.91 An in vitro study reported that VK2 inhibited the proliferation of mitogen-activated peripheral blood mononuclear cells in RA patients, suggesting that VK2 supplementation may prevent and treat RA via its immunosuppressive function.92
Discussions
This article reviews the latest scientific evidence indicating that VK has a positive role in infections, inflammation, and autoimmune diseases, and it may become a therapeutic tool for practical clinical applications. Table 2 shows the clinical application of VK. The main mechanisms of action of VK in disease are anti-inflammatory and antioxidant. This review summarizes the positive effects of VK on prevalent diseases, including asthma, neurodegenerative diseases, aging, CVD and cancer, as well as metabolic disorders, T1DM and T2DM (Figure 3).
 |
Table 2 Summary of the Clinical Application of VK |
VK2 attenuates the inflammatory response and relieves asthma symptoms by inhibiting the release of inflammatory cytokines (IL-4, IL-13 and TGF-β, etc.). VK is associated with COVID-19 recovery, in which it effectively activates MGP activity, inhibits uc-duGMP levels, attenuates lung elastic fiber damage, and activates protein S. It has been proven to block the production of inflammatory cytokines and cytokine storms found in COVID-19 patients. In addition, VK may regulate pancreatic β-cells and improve insulin sensitivity by blocking the signaling pathway of NF-κB, thereby maintaining glucose homeostasis and reducing the risk of diabetes. This review also illustrates the role of VK2 in the prevention and treatment of neurodegenerative diseases (AD and PD). VK2 regulates neuroinflammation by activating Gas-6 and protein S, protects neuronal cells in the hippocampus, and promotes myelin sheath formation, thereby maintaining normal brain function and alleviating cognitive deficits in the brain. VK2 also serves as an electron carrier and a signaling pathway for mitochondrial defects to protect the nervous system. Studies have shown that VK can inhibit NF-κB activation and induce cancer cell apoptosis and cell cycle arrest. As a combination medication, it can also effectively inhibit cell proliferation, promote cancer cell apoptosis, and reduce cancer risk. Increasing VK intake can effectively reduce ucOC levels, promote bone renewal, enhance bone density, inhibit NF-κB activation and osteoclast synthesis, and stimulate osteoblast differentiation. Specifically, when combined with VD and calcium, VK2 inhibits SMAD signaling, further stimulating osteoblast formation and reducing bone loss. In addition, VK prevents vascular calcification by activating GMP, inhibits uc-duGMP production, reduces cardiovascular risk, morbidity and mortality, and improves atherosclerosis and arteriosclerosis. It also participates in the regeneration of myelin sheath and improves MS. VK alleviates IBD by regulating the interaction between the microbiota and the immune system, modulating the intestinal microbiota, and antioxidant and anti-inflammatory activity.
Conclusions and Future Perspective
Maintaining VK intake is critical for maintaining health during the disease management process. The potential effectiveness of VK, especially VK2, in the treatment of infections, inflammation, and autoimmune diseases has been fully confirmed. However, the specific mechanisms of many diseases are still unclear, and elucidating the pathological mechanisms is the basis for exploring effective therapies. In addition, the recommended intake of VK still needs to be explored, and, as the bioavailability and biological activity of VK1, VK2, and VK3 are different, their role in disease prevention and treatment needs to be further refined. Future research is therefore warranted to further explore this issue, and it is hoped that the positive roles of VK in disease prevention and treatment could be applied to practical clinical treatment as soon as possible.
Abbreviations
AD, alzheimer’s disease; ATP, adenosine-triphosphate; AVC, aortic valve calcification; Aβ, β-amyloid; BMC, bone mineral content; BMD, bone mineral density; BMP-2, bone morphogenetic protein-2; CAC, coronary artery calcification; CD, Crohn’s disease; CKD, chronic kidney disease; CNS, central nervous system; cOC, carboxylated osteocalcin; COVID-19, Corona Virus Disease 2019; CVD, cardiovascular diseases; DNA, Deoxyribonucleic acid; dp-ucMGP, dp-undercarboxylated matrix Gla protein; ETC, electron transport chain; EVA, accelerates vascular aging; FPG, fasting plasma glucose; Gas-6, Growth arrest-specific protein 6; Gla, γ-Carboxyglutamic acid; Glu, glutamic acid; HbA1c, glycosylated hemoglobin; HCC, hepatocellular-cancer; HDGF, hepatocellular carcinoma derived growth factor; HepG2, human hepatocellular carcinomas; HOMA-IR, homeostatic model assessment of insulin resistance; HR, hazard ratio; IBD, Inflammatory bowel disease; IKK, nuclear factor kappa-B; IL-13, interleukin-13
IL-4, interleukin-4; IL-6, interleukin-6; IRI, immunoreactive insulin; MGP, matrix gamma-carboxyglutamic acid protein; MK, menaquinone; MS, Multiple sclerosis; NF-κB, nuclear factor kappa-B; NRF2, nuclear factor red factor 2-related factor 2; OC, osteocalcin; P21, protein 21; PD, parkinson’s disease; PG, plasma glucose; PKC, protein kinase C; PKCε, recombinant Protein Kinase C Epsilon; PKD1, protein kinase D1; PMO, postmenopausal osteoporosis; PMOa, postmenopausal osteopenia; PMW, postmenopausal women; p-PKCε, phospho-recombinant Protein Kinase C Epsilon; p-PKD1, phospho-protein kinase D1; RA, Rheumatoid arthritis; RCT, randomized controlled trial; ROS, reactive oxygen species; SMAD, Recombinant Mothers Against Decapentaplegic Homolog; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; TGF-β, transforming growth factor β; TNF-α, tumor necrosis factor α; UC, ulcerative colitis; ucOC, undercarboxylated osteocalcin; VC, vascular calcification; VD, vitamin D; VK, vitamin K; VK1, vitamin K1; VK2, vitamin K2; VK3, vitamin K3; VKDP, VK dependent protein; W, women; α-Syn, α-Synuclein; ↑, increase; ↓, decrease.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the General Project of Liaoning Provincial Department of Education (JYTMS20230566 to Yuyuan Li) and the China Health Promotion Association (Z093001 to Shuzhuang Li).
Disclosure
Jiepeng Chen and Lili Duan are affiliated with Sungen Bioscience Co., Ltd. The authors declare no other conflicts of interest in this work.
References
1. Wong F, de la Fuente-Nunez C, Collins JJ. Leveraging artificial intelligence in the fight against infectious diseases. Science. 2023;381(6654):164–170. doi:10.1126/science.adh1114
2. Sangeetha Vijayan P, Xavier J, Valappil MP. A review of immune modulators and immunotherapy in infectious diseases. Mol Cell Biochem. 2023. doi:10.1007/s11010-023-04825-w
3. Zheng Y, Liu Q, Goronzy JJ, Weyand CM. Immune aging - A mechanism in autoimmune disease. Semin Immunol. 2023;69:101814. doi:10.1016/j.smim.2023.101814
4. Merra G, Dominici F, Gualtieri P, et al. Role of vitamin K2 in bone-vascular crosstalk. Int J Vitam Nutr Res. 2022. doi:10.1024/0300-9831/a000761
5. Swanson JC, Suttie JW. Vitamin K dependent in vitro production of prothrombin. Biochemistry. 1982;21(23):6011–6018. doi:10.1021/bi00266a044
6. Schurgers LJ, Vermeer C. Determination of phylloquinone and menaquinones in food. Effect of food matrix on circulating vitamin K concentrations. Haemostasis. 2000;30(6):298–307. doi:10.1159/000054147
7. Dunlop E, Jakobsen J, Jensen MB, et al. Vitamin K content of cheese, yoghurt and meat products in Australia. Food Chem. 2022;397:133772. doi:10.1016/j.foodchem.2022.133772
8. Kamao M, Suhara Y, Tsugawa N, et al. Vitamin K content of foods and dietary vitamin K intake in Japanese young women. J Nutr Sci Vitaminol. 2007;53(6):464–470. doi:10.3177/jnsv.53.464
9. Stafford DW. The vitamin K cycle. J Thromb Haemost. 2005;3(8):1873–1878. doi:10.1111/j.1538-7836.2005.01419.x
10. Bentley R, Meganathan R. Biosynthesis of vitamin K (menaquinone) in bacteria. Microbiol Rev. 1982;46(3):241–280. doi:10.1128/mr.46.3.241-280.1982
11. Beulens JWJ, Booth SL, van den Heuvel EG, Stoecklin E, Baka A, Vermeer C. The role of menaquinones (vitamin K₂) in human health. Br J Nutr. 2013;110(8):1357–1368. doi:10.1017/S0007114513001013
12. Shearer MJ, Okano T. Key pathways and regulators of vitamin K function and intermediary metabolism. Annu Rev Nutr. 2018;38:127–151. doi:10.1146/annurev-nutr-082117-051741
13. Saglani S, Yates L, Lloyd CM. Immunoregulation of asthma by type 2 cytokine therapies: treatments for all ages? Eur J Immunol. 2023;53(8):e2249919. doi:10.1002/eji.202249919
14. Kimur I, Tanizaki Y, Sato S, Saito K, Takahashi K. Menaquinone (vitamin K2) therapy for bronchial asthma. II. Clinical effect of menaquinone on bronchial asthma. Acta Med Okayama. 1975;29(2):127–135.
15. Janssen R, Vermeer C. Vitamin K deficit and elastolysis theory in pulmonary elasto-degenerative diseases. Med Hypotheses. 2017;108:38–41. doi:10.1016/j.mehy.2017.07.029
16. Jespersen T, Kampmann FB, Dantoft TM, et al. The association of vitamin K status with lung function and disease in a general population. ERJ Open Res. 2023;9:5.
17. Aziz M, Fatima R, Assaly R. Elevated interleukin-6 and severe COVID-19: a meta-analysis. J Med Virol. 2020;92(11):2283–2285. doi:10.1002/jmv.25948
18. McElvaney OJ, Curley GF, Rose-John S, McElvaney NG. Interleukin-6: obstacles to targeting a complex cytokine in critical illness. Lancet Respir Med. 2021;9(6):643–654. doi:10.1016/S2213-2600(21)00103-X
19. Liu B, Li M, Zhou Z, Guan X, Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun. 2020;111:102452. doi:10.1016/j.jaut.2020.102452
20. Visser MPJ, Dofferhoff ASM, van den Ouweland JMW, et al. Effects of Vitamin D and K on Interleukin-6 in COVID-19. Front Nutr. 2021;8:761191. doi:10.3389/fnut.2021.761191
21. Mangge H, Prueller F, Dawczynski C, et al. Dramatic decrease of vitamin K2 subtype menaquinone-7 in COVID-19 patients. Antioxidants. 2022;11:7.
22. Su J, Luo Y, Hu S, Tang L, Ouyang S. Advances in research on type 2 diabetes mellitus targets and therapeutic agents. Int J Mol Sci. 2023;24:17. doi:10.3390/ijms241713381
23. Sakamoto N, Nishiike T, Iguchi H, Sakamoto K. Relationship between acute insulin response and vitamin K intake in healthy young male volunteers. Diabetes Nutr Metab. 1999;12(1):37–41.
24. Sakamoto N, Nishiike T, Iguchi H, Sakamoto K. Possible effects of one week vitamin K (menaquinone-4) tablets intake on glucose tolerance in healthy young male volunteers with different descarboxy prothrombin levels. Clin Nutr. 2000;19(4):259–263. doi:10.1054/clnu.2000.0102
25. Yoshida M, Booth SL, Meigs JB, Saltzman E, Jacques PF. Phylloquinone intake, insulin sensitivity, and glycemic status in men and women12. Am J Clin Nutr. 2008;88(1):210–215. doi:10.1093/ajcn/88.1.210
26. Yoshida M, Jacques PF, Meigs JB, et al. Effect of vitamin K supplementation on insulin resistance in older men and women. Diabetes Care. 2008;31(11):2092–2096. doi:10.2337/dc08-1204
27. Rasekhi H, Karandish M, Jalali MT, et al. Phylloquinone supplementation improves glycemic status independent of the effects of adiponectin levels in premonopause women with prediabetes: a double-blind randomized controlled clinical trial. J Diabetes Metab Disord. 2015;14(1):1. doi:10.1186/s40200-014-0127-9
28. Rasekhi H, Karandish M, Jalali MT, et al. The effect of vitamin K1 supplementation on sensitivity and insulin resistance via osteocalcin in prediabetic women: a double-blind randomized controlled clinical trial. Eur J Clin Nutr. 2015;69(8):891–895. doi:10.1038/ejcn.2015.17
29. Zhang Q, Yan Y. The role of natural flavonoids on neuroinflammation as a therapeutic target for Alzheimer’s disease: a narrative review. Neural Regen Res. 2023;18(12):2582–2591. doi:10.4103/1673-5374.373680
30. Alisi L, Cao R, De Angelis C, et al. The relationships between vitamin K and cognition: a review of current evidence. Front Neurol. 2019;10:239. doi:10.3389/fneur.2019.00239
31. Wattenberg BW. Intra- and intercellular trafficking in sphingolipid metabolism in myelination. advances in Biological Regulation. 2019;71:97–103. doi:10.1016/j.jbior.2018.11.002
32. Manfioletti G, Brancolini C, Avanzi GC, Schneider C. The protein encoded by a growth arrest-specific gene (gas6) is a new member of the vitamin K-dependent proteins related to protein S, a negative coregulator in the blood coagulation cascade. Mol Cell Biol. 1993;13:4976–4985. doi:10.1128/mcb.13.8.4976-4985.1993
33. Bellido-Martín L, de Frutos PG. Vitamin K-dependent actions of Gas6. Vitam Horm. 2008;78:185–209.
34. Funakoshi H, Yonemasu T, Nakano T, Matumoto K, Nakamura T. Identification of Gas6, a putative ligand for Sky and Axl receptor tyrosine kinases, as a novel neurotrophic factor for hippocampal neurons. J Neurosci Res. 2002;68(2):150–160. doi:10.1002/jnr.10211
35. Yagami T, Ueda K, Asakura K, et al. Gas6 rescues cortical neurons from amyloid beta protein-induced apoptosis. Neuropharmacology. 2002;43(8):1289–1296. doi:10.1016/S0028-3908(02)00333-7
36. Liu D, Guo H, Griffin JH, Fernández JA, Zlokovic BV. Protein S confers neuronal protection during ischemic/hypoxic injury in mice. Circulation. 2003;107(13):1791–1796. doi:10.1161/01.CIR.0000058460.34453.5A
37. Pluta R, Januszewski S, Czuczwar SJ. Brain ischemia as a prelude to alzheimer’s disease. Front Aging Neurosci. 2021;13:636653. doi:10.3389/fnagi.2021.636653
38. Dexter DT, Jenner P. Parkinson disease: from pathology to molecular disease mechanisms. Free Radic Biol Med. 2013;62:132–144. doi:10.1016/j.freeradbiomed.2013.01.018
39. Lopez-Fabuel I, Martin-Martin L, Resch-Beusher M, Azkona G, Sanchez-Pernaute R, Bolaños JP. Mitochondrial respiratory chain disorganization in Parkinson’s disease-relevant PINK1 and DJ1 mutants. Neurochem Int. 2017;109:101–105. doi:10.1016/j.neuint.2017.03.023
40. Vos M, Esposito G, Edirisinghe JN, et al. Vitamin K2 is a mitochondrial electron carrier that rescues pink1 deficiency. Science. 2012;336(6086):1306–1310. doi:10.1126/science.1218632
41. Prasuhn J, Kasten M, Vos M, et al. The use of vitamin K2 in patients with parkinson’s disease and mitochondrial dysfunction (PD-K2): a theranostic pilot study in a placebo-controlled parallel group design. Front Neurol. 2020;11:592104. doi:10.3389/fneur.2020.592104
42. Yu Y-X, Yu X-D, Cheng Q-Z, Tang L, Shen M-Q. The association of serum vitamin K2 levels with Parkinson’s disease: from basic case-control study to big data mining analysis. Aging. 2020;12(16):16410–16419. doi:10.18632/aging.103691
43. da Silva FL, Coelho Cerqueira E, de Freitas MS, Gonçalves DL, Costa LT, Follmer C. Vitamins K interact with N-terminus α-synuclein and modulate the protein fibrillization in vitro. Exploring the interaction between quinones and α-synuclein. Neurochem Int. 2013;62(1):103–112. doi:10.1016/j.neuint.2012.10.001
44. Zafar E, Maqbool MF, Iqbal A, et al. A comprehensive review on anticancer mechanism of bazedoxifene. Biotechnol Appl Biochem. 2022;69(2):767–782. doi:10.1002/bab.2150
45. Fernald K, Kurokawa M. Evading apoptosis in cancer. Trends Cell Biol. 2013;23(12):620–633. doi:10.1016/j.tcb.2013.07.006
46. Bouzahzah B, Nishikawa Y, Simon D, Carr BI. Growth control and gene expression in a new hepatocellular carcinoma cell line, Hep40: inhibitory actions of vitamin K. J Cell Physiol. 1995;165(3):459–467. doi:10.1002/jcp.1041650303
47. Chlebowski RT, Dietrich M, Akman S, Block JB. Vitamin K3 inhibition of malignant murine cell growth and human tumor colony formation. Cancer Treat Rep. 1985;69(5):527–532.
48. Wu FY, Liao WC, Chang HM. Comparison of antitumor activity of vitamins K1, K2 and K3 on human tumor cells by two (MTT and SRB) cell viability assays. Life Sci. 1993;52(22):1797–1804. doi:10.1016/0024-3205(93)90469-J
49. Hitomi M, Yokoyama F, Kita Y, et al. Antitumor effects of vitamins K1, K2 and K3 on hepatocellular carcinoma in vitro and in vivo. Int J Oncol. 2005;26(3):713–720.
50. Ozaki I, Zhang H, Mizuta T, et al. Menatetrenone, a vitamin K2 analogue, inhibits hepatocellular carcinoma cell growth by suppressing cyclin D1 expression through inhibition of nuclear factor kappaB activation. Clin Cancer Res. 2007;13(7):2236–2245. doi:10.1158/1078-0432.CCR-06-2308
51. Xia J, Matsuhashi S, Hamajima H, et al. The role of PKC isoforms in the inhibition of NF-κB activation by vitamin K2 in human hepatocellular carcinoma cells. J Nutr Biochem. 2012;23(12):1668–1675. doi:10.1016/j.jnutbio.2011.11.010
52. Maniwa Y, Kasukabe T, Kumakura S. Vitamin K2 and cotylenin A synergistically induce monocytic differentiation and growth arrest along with the suppression of c-MYC expression and induction of cyclin G2 expression in human leukemia HL-60 cells. Int J Oncol. 2015;47(2):473–480. doi:10.3892/ijo.2015.3028
53. Otsuka M, Kato N, Shao RX, et al. Vitamin K2 inhibits the growth and invasiveness of hepatocellular carcinoma cells via protein kinase A activation. Hepatology. 2004;40(1):243–251. doi:10.1002/hep.20260
54. Liu W, Nakamura H, Yamamoto T, et al. Vitamin K2 inhibits the proliferation of HepG2 cells by up-regulating the transcription of p21 gene. Hepatol Res. 2007;37(5):360–365. doi:10.1111/j.1872-034X.2007.00058.x
55. Maurya PK, Kumar P, Chandra P. Biomarkers of oxidative stress in erythrocytes as a function of human age. World J Methodol. 2015;5(4):216–222. doi:10.5662/wjm.v5.i4.216
56. Harshman SG, Shea MK. The role of vitamin K in chronic aging diseases: inflammation, cardiovascular disease, and osteoarthritis. Curr Nutr Rep. 2016;5(2):90–98. doi:10.1007/s13668-016-0162-x
57. Popa DS, Bigman G, Rusu ME. The role of vitamin K in humans: implication in aging and age-associated diseases. Antioxidants. 2021;10:4.
58. Tsugawa N, Shiraki M. Vitamin K nutrition and bone health. Nutrients. 2020;12(7). doi:10.3390/nu12071909
59. Shea MK, Kritchevsky SB, Hsu FC, et al. The association between vitamin K status and knee osteoarthritis features in older adults: the health, aging and body composition study. Osteoarthritis Cartilage. 2015;23(3):370–378. doi:10.1016/j.joca.2014.12.008
60. Binkley NC, Krueger DC, Kawahara TN, Engelke JA, Chappell RJ, Suttie JW. A high phylloquinone intake is required to achieve maximal osteocalcin gamma-carboxylation. Am J Clin Nutr. 2002;76(5):1055–1060. doi:10.1093/ajcn/76.5.1055
61. Lin X, Brennan-Speranza TC, Levinger I, Yeap BB. Undercarboxylated osteocalcin: experimental and human evidence for a role in glucose homeostasis and muscle regulation of insulin sensitivity. Nutrients. 2018;10(7):847. doi:10.3390/nu10070847
62. Luukinen H, Käkönen SM, Pettersson K, et al. Strong prediction of fractures among older adults by the ratio of carboxylated to total serum osteocalcin. J Bone Miner Res. 2000;15(12):2473–2478. doi:10.1359/jbmr.2000.15.12.2473
63. Sim M, Lewis JR, Prince RL, et al. The effects of vitamin K-rich green leafy vegetables on bone metabolism: a 4-week randomised controlled trial in middle-aged and older individuals. Bone Rep. 2020;12:100274. doi:10.1016/j.bonr.2020.100274
64. Bultynck C, Munim N, Harrington DJ, et al. Prevalence of vitamin K deficiency in older people with Hip fracture. Acta Clin Belg. 2020;75(2):136–140. doi:10.1080/17843286.2018.1564174
65. Moore AE, Kim E, Dulnoan D, et al. Serum vitamin K(1) (phylloquinone) is associated with fracture risk and Hip strength in post-menopausal osteoporosis: a cross-sectional study. Bone. 2020;141:115630. doi:10.1016/j.bone.2020.115630
66. Hooshmand S, Kern M, Metti D, et al. The effect of two doses of dried plum on bone density and bone biomarkers in osteopenic postmenopausal women: a randomized, controlled trial. Osteoporos Int. 2016;27(7):2271–2279. doi:10.1007/s00198-016-3524-8
67. Yamaguchi M, Weitzmann MN. Vitamin K2 stimulates osteoblastogenesis and suppresses osteoclastogenesis by suppressing NF-κB activation. Int J Mol Med. 2011;27(1):3–14. doi:10.3892/ijmm.2010.562
68. Capozzi A, Scambia G, Lello S. Calcium, vitamin D, vitamin K2, and magnesium supplementation and skeletal health. Maturitas. 2020;140:55–63. doi:10.1016/j.maturitas.2020.05.020
69. Dai L, Schurgers LJ, Shiels PG, Stenvinkel P. Early vascular ageing in chronic kidney disease: impact of inflammation, vitamin K, senescence and genomic damage. Nephrol Dial Transplant. 2020;35(Suppl 2):ii31–ii7. doi:10.1093/ndt/gfaa006
70. Mozos I, Stoian D, Luca CT. Crosstalk between vitamins A, B12, D, K, C, and E status and arterial stiffness. Dis Markers. 2017;2017:8784971. doi:10.1155/2017/8784971
71. Roumeliotis S, Dounousi E, Salmas M, Eleftheriadis T, Liakopoulos V. vascular calcification in chronic kidney disease: the role of vitamin K- dependent matrix gla protein. Front Med Lausanne. 2020;7:154. doi:10.3389/fmed.2020.00154
72. Ueland T, Dahl CP, Gullestad L, et al. Circulating levels of non-phosphorylated undercarboxylated matrix Gla protein are associated with disease severity in patients with chronic heart failure. Clin Sci (Lond). 2011;121(3):119–127. doi:10.1042/CS20100589
73. Puzantian H, Akers SR, Oldland G, et al. Circulating dephospho-uncarboxylated matrix gla-protein is associated with kidney dysfunction and arterial stiffness. Am J Hypertens. 2018;31(9):988–994. doi:10.1093/ajh/hpy079
74. Caluwé R, Vandecasteele S, Van Vlem B, Vermeer C, De Vriese AS. Vitamin K2 supplementation in haemodialysis patients: a randomized dose-finding study. Nephrol Dial Transplant. 2014;29(7):1385–1390. doi:10.1093/ndt/gft464
75. Shioi A, Morioka T, Shoji T, Emoto M. The inhibitory roles of vitamin K in progression of vascular calcification. Nutrients. 2020;12(2). doi:10.3390/nu12020583
76. Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology. 2014;146(6):1489–1499. doi:10.1053/j.gastro.2014.02.009
77. Nadeem MS, Kumar V, Al-Abbasi FA, Kamal MA, Anwar F. Risk of colorectal cancer in inflammatory bowel diseases. Semin Cancer Biol. 2020;64:51–60. doi:10.1016/j.semcancer.2019.05.001
78. Yoon SM. Micronutrient deficiencies in inflammatory bowel disease: trivial or crucial? Intest Res. 2016;14(2):109–110. doi:10.5217/ir.2016.14.2.109
79. Lai Y, Masatoshi H, Ma Y, Guo Y, Zhang B. Role of vitamin K in intestinal health. Front Immunol. 2021;12:791565. doi:10.3389/fimmu.2021.791565
80. Varsha MK, Thiagarajan R, Manikandan R, Dhanasekaran G. Vitamin K1 alleviates streptozotocin-induced type 1 diabetes by mitigating free radical stress, as well as inhibiting NF-κB activation and iNOS expression in rat pancreas. Nutrition. 2015;31(1):214–222. doi:10.1016/j.nut.2014.05.012
81. Iwamoto J, Seki A, Sato Y, Matsumoto H, Takeda T, Yeh JK. Vitamin K₂ prevents hyperglycemia and cancellous osteopenia in rats with streptozotocin-induced type 1 diabetes. Calcif Tissue Int. 2011;88(2):162–168. doi:10.1007/s00223-010-9441-5
82. Dulamea AO. Role of oligodendrocyte dysfunction in demyelination, remyelination and neurodegeneration in multiple sclerosis. Adv Exp Med Biol. 2017;958:91–127. doi:10.1007/978-3-319-47861-6_7
83. Lasemi R, Kundi M, Moghadam NB, Moshammer H, Hainfellner JA. Vitamin K2 in multiple sclerosis patients. Wien Klin Wochenschr. 2018;130(9–10):307–313. doi:10.1007/s00508-018-1328-x
84. Moriya M, Nakatsuji Y, Okuno T, Hamasaki T, Sawada M, Sakoda S. Vitamin K2 ameliorates experimental autoimmune encephalomyelitis in Lewis rats. J Neuroimmunol. 2005;170(1–2):11–20. doi:10.1016/j.jneuroim.2005.08.001
85. Li J, Wang H, Rosenberg PA. Vitamin K prevents oxidative cell death by inhibiting activation of 12-lipoxygenase in developing oligodendrocytes. J Neurosci Res. 2009;87(9):1997–2005. doi:10.1002/jnr.22029
86. Carrié I, Portoukalian J, Vicaretti R, Rochford J, Potvin S, Ferland G. Menaquinone-4 concentration is correlated with sphingolipid concentrations in rat brain. J Nutr. 2004;134(1):167–172. doi:10.1093/jn/134.1.167
87. Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:6937):356–61. doi:10.1038/nature01661
88. Smolen JS, Aletaha D, Barton A, et al. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18002. doi:10.1038/nrdp.2018.2
89. Bowman SJ. Hematological manifestations of rheumatoid arthritis. Scand J Rheumatol. 2002;31(5):251–259. doi:10.1080/030097402760375124
90. Pereira L, Monteiro R. Tailoring gut microbiota with a combination of Vitamin K and probiotics as a possible adjuvant in the treatment of rheumatic arthritis: a systematic review. Clin Nutr ESPEN. 2022;51:37–49. doi:10.1016/j.clnesp.2022.08.002
91. Huang YJ, Han L, Li J, Chen C. Acquired coagulation dysfunction resulting from vitamin K-dependent coagulation factor deficiency associated with rheumatoid arthritis: a case report. World J Clin Cases. 2022;10(1):236–241. doi:10.12998/wjcc.v10.i1.236
92. Xu W, Chen S, Wang X, et al. Suppressive effect of vitamin K2 against mitogen-activated peripheral blood mononuclear cells of rheumatoid arthritis patients. Int J Clin Pharmacol Ther. 2021;59(1):55–62. doi:10.5414/CP203827
93. Shiraki M, Shiraki Y, Aoki C, Miura M. Vitamin K2 (menatetrenone) effectively prevents fractures and sustains lumbar bone mineral density in osteoporosis. J Bone Miner Res. 2000;15(3):515–521. doi:10.1359/jbmr.2000.15.3.515
94. Iwamoto J, Takeda T, Ichimura S. Effect of menatetrenone on bone mineral density and incidence of vertebral fractures in postmenopausal women with osteoporosis: a comparison with the effect of etidronate. J Orthop Sci. 2001;6(6):487–492. doi:10.1007/s007760100002
95. Purwosunu Y, Rachman IA, Reksoprodjo S, Sekizawa A. Vitamin K2 treatment for postmenopausal osteoporosis in Indonesia. J Obstetrics Gynaecol Res. 2006;32(2):230–234. doi:10.1111/j.1447-0756.2006.00386.x
96. Bolton-Smith C, McMurdo MET, Paterson CR, et al. Two-year randomized controlled trial of vitamin K1 (Phylloquinone) and Vitamin D3 plus calcium on the bone health of older women. J Bone Miner Res. 2007;22(4):509–519. doi:10.1359/jbmr.070116
97. Knapen MH, Schurgers LJ, Vermeer C. Vitamin K2 supplementation improves Hip bone geometry and bone strength indices in postmenopausal women. Osteoporos Int. 2007;18(7):963–972. doi:10.1007/s00198-007-0337-9
98. Cheung AM, Tile L, Lee Y, et al. Vitamin K supplementation in postmenopausal women with osteopenia (ECKO Trial): a randomized controlled trial. PLoS Med. 2008;5(1):- 12. doi:10.1371/journal.pmed.0050196
99. Hirao M, Hashimoto J, Ando W, Ono T, Yoshikawa H. Response of serum carboxylated and undercarboxylated osteocalcin to alendronate monotherapy and combined therapy with vitamin K2 in postmenopausal women. J Bone Miner Metab. 2008;26(3):260–264. doi:10.1007/s00774-007-0823-3
100. Tsugawa N, Shiraki M, Suhara Y, et al. Low plasma phylloquinone concentration is associated with high incidence of vertebral fracture in Japanese women. J Bone Miner Metab. 2008;26(1):79–85. doi:10.1007/s00774-007-0790-8
101. Je SH, Joo NS, Choi BH, et al. Vitamin K supplement along with vitamin D and calcium reduced serum concentration of undercarboxylated osteocalcin while increasing bone mineral density in Korean postmenopausal women over sixty-years-old. J Korean Med Sci. 2011;26(8):1093–1098. doi:10.3346/jkms.2011.26.8.1093
102. Knapen MH, Drummen NE, Smit E, Vermeer C, Theuwissen E. Three-year low-dose menaquinone-7 supplementation helps decrease bone loss in healthy postmenopausal women. Osteoporos Int. 2013;24(9):2499–2507. doi:10.1007/s00198-013-2325-6
103. Jiang Y, Zhang ZL, Zhang ZL, et al. Menatetrenone versus alfacalcidol in the treatment of Chinese postmenopausal women with osteoporosis: a multicenter, randomized, double-blinded, double-dummy, positive drug-controlled clinical trial. Clin Interv Aging. 2014;9:121–127. doi:10.2147/CIA.S54107
104. Rønn SH, Harsløf T, Pedersen SB, Langdahl BL. Vitamin K2 (menaquinone-7) prevents age-related deterioration of trabecular bone microarchitecture at the tibia in postmenopausal women. Europ J Endocrinol. 2016;175(6):541–549. doi:10.1530/EJE-16-0498
105. Shea MK, O’Donnell CJ, Hoffmann U, et al. Vitamin K supplementation and progression of coronary artery calcium in older men and women23. Am J Clin Nutr. 2009;89(6):1799–1807. doi:10.3945/ajcn.2008.27338
106. Cheung CL, Sahni S, Cheung BM, Sing CW, Wong IC. Vitamin K intake and mortality in people with chronic kidney disease from NHANES III. Clin Nutr. 2015;34(2):235–240. doi:10.1016/j.clnu.2014.03.011
107. Kurnatowska I, Grzelak P, Masajtis-Zagajewska A, et al. Effect of vitamin K2 on progression of atherosclerosis and vascular calcification in nondialyzed patients with chronic kidney disease stages 3–5. Pol Arch Med Wewn. 2015;125(9):631–640. doi:10.20452/pamw.3041
108. Brandenburg VM, Reinartz S, Kaesler N, et al. Slower progress of aortic valve calcification with vitamin K supplementation. Circulation. 2017;135(21):2081–2083. doi:10.1161/CIRCULATIONAHA.116.027011
109. Shea MK, Barger K, Booth SL, et al. Vitamin K status, cardiovascular disease, and all-cause mortality: a participant-level meta-analysis of 3 US cohorts. Am J Clin Nutr. 2020;111(6):1170–1177. doi:10.1093/ajcn/nqaa082
110. Shea MK, Dawson-Hughes B, Gundberg CM, Booth SL. Reducing undercarboxylated osteocalcin with vitamin K supplementation does not promote lean tissue loss or fat gain over 3 years in older women and men: a randomized controlled trial. J Bone Miner Res. 2017;32(2):243–249. doi:10.1002/jbmr.2989
111. Knapen MHJ, Jardon KM, Vermeer C. Vitamin K-induced effects on body fat and weight: results from a 3-year vitamin K2 intervention study. Eur J Clin Nutr. 2018;72(1):136–141. doi:10.1038/ejcn.2017.146
112. Aguayo-Ruiz JI, García-Cobián TA, Pascoe-González S, et al. Effect of supplementation with vitamins D3 and K2 on undercarboxylated osteocalcin and insulin serum levels in patients with type 2 diabetes mellitus: a randomized, double-blind, clinical trial. Diabetol Metab Syndr. 2020;12(1):73. doi:10.1186/s13098-020-00580-w
113. Rahimi Sakak F, Moslehi N, Niroomand M, Mirmiran P. Glycemic control improvement in individuals with type 2 diabetes with vitamin K(2) supplementation: a randomized controlled trial. Eur J Nutr. 2021;60(5):2495–2506. doi:10.1007/s00394-020-02419-6
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.