Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Downstaging and Conversation Strategy for Advanced Hepatocellular Carcinoma with Portal Vein Branch Tumor Thrombus: TACE, 125I Seed Implantation, and RFA for Hepatocellular Carcinoma with Portal Vein Branch Tumor Thrombus
Authors Zhao XH, Li HL , Guo CY, Yao QJ, Xia WL, Hu HT
Received 12 October 2022
Accepted for publication 25 December 2022
Published 14 February 2023 Volume 2023:10 Pages 231—240
DOI https://doi.org/10.2147/JHC.S392293
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr David Gerber
Xiao-Hui Zhao, Hai-Liang Li, Chen-Yang Guo, Quan-Jun Yao, Wei-Li Xia, Hong-Tao Hu
Department of Minimally-Invasive Intervention, The Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital, Zhengzhou City, People’s Republic of China
Correspondence: Hong-Tao Hu, Department of Minimally-Invasive Intervention, The Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital, Zhengzhou, Henan Province, 450008, People’s Republic of China, Tel +86-135-9264-6376, Email [email protected]
Background and Objectives: Trans-arterial chemoembolization (TACE) combined with 125I seed implantation is an effective treatment modality for hepatocellular carcinoma (HCC) with a portal vein tumor thrombus (PVTT). However, there are no reports on the effectiveness of radiofrequency ablation (RFA) after downstaging in such patients. This study aimed to investigate the efficacy and safety of TACE in combination with 125I seed implantation and RFA for the treatment of HCC complicated by PVTT.
Methods: 49 patients diagnosed with HCC with PVTT between February 2015 and December 2016 were included. All patients were clinically or pathologically diagnosed with advanced HCC, intrahepatic lesions ≤ 3, and a single tumor diameter ≤ 70 mm, total diameter ≤ 100 mm. PVTT was limited to the unilateral portal vein branches. All the patients were treated with TACE combined with PVTT 125I seed implantation. The size and activity of intrahepatic lesions and PVTT were evaluated using enhanced magnetic resonance imaging 3 months after treatment, and other indicators were combined to determine the success of downstaging.
Results: A total of 31 patients were successfully downstaged, while 18 patients did not achieve downstaging owing to the progression of intrahepatic lesions or PVTT activity/progression, the success rate of the downstaging was 63.27%. All 31 patients with successful downstaging underwent RFA for intrahepatic lesions. The 1-, 2-, and 3-year survival rates were 90.3%, 80.6%, and 48.4%, respectively. The median overall survival was 36 months (95% CI: 24.7– 47.3).
Conclusion: 125I seed implantation in combination with TACE can effectively inactivate PVTT and achieve downstaging. Furthermore, the addition of RFA can significantly improve patient survival.
Keywords: hepatocellular carcinoma, portal vein tumor thrombus, 125I seed, transcatheter arterial chemoembolization, downstaging treatment
Introduction
Approximately 44.0–62.2% of hepatocellular carcinomas have severe vascular invasion, mainly in the form of portal vein tumor thrombus.1,2 Once portal vein tumor thrombus (PVTT) occurs in hepatocellular carcinoma (HCC) patients, intrahepatic and extrahepatic metastases, portal hypertension, jaundice, and ascites can occur within a short period of time, and the median overall survival (OS) time is only 2.7 months.3 Systemic therapy is generally recommended for patients with HCC complicated by PVTT. However, some studies have shown that trans-arterial chemoembolization (TACE) combined with PVTT 125I seed implantation can improve the overall survival of such HCC patients.4,5 Downstaging therapy is defined as a treatment strategy for reducing the tumor load for radical treatment and risk reduction or tumor downstaging after resection/ablation so that the patient can have better survival benefits from other treatments.6 Multiple surgical resections are performed after successful downstaging of HCC. Local ablation is also a potential treatment, with the advantages of being cost-effective and minimally invasive.7,8
A previous study demonstrated that patients who underwent RFA after TACE downstaging had comparable long-term survival and major complication rates to those who initially met the Milan criteria.9 However, downstaging strategies for patients with HCC and PVTT have varied and include radiotherapy, systemic therapy, and multimodal therapies. Further, there are disadvantages such as a low downstaging rate and large side effects.10–12 125I seed implantation can effectively inactivate tumor thrombus in the portal vein branches and achieve downstaging.
Materials and Methods
Study Design and Patients
This retrospective study was approved by the Research Ethics Committee of the Affiliated Cancer Hospital of Zhengzhou University (IRB number: 2016ct005) and complied with the ethical guidelines of the World Medical Association Declaration of Helsinki. Written informed consent was obtained from all patients before undergoing TACE combined with PVTT 125I seed implantation and RFA.
The subjects were patients who were diagnosed with HCC with PVTT and who received TACE combined with PVTT 125I seed implantation between February 2015 and December 2016. HCC was diagnosed according to the European Association for the Study of Liver Diseases/American Association for the Study of Liver Diseases guidelines. The inclusion criteria were as follows: (1) Child-Pugh grade A; (2) Eastern Cooperative Oncology Group physical status activity score ≤ 1 point; (3) ≤ 3 intrahepatic lesions, and the largest single tumor diameter ≤ 70 mm, total diameter ≤100 mm; (4) the portal vein tumor thrombus was limited to the left or right branch of the portal vein; (5) no history of anti-tumor therapy such as surgery, radiotherapy, and systemic therapy; and (6) unwillingness to use or could not afford targeted drug treatment. The patient selection flowchart is shown in Figure 1.
 |
Figure 1 Patient selection flowchart. |
Protocol for TACE Combined with 125I Seed Implantation
TACE was performed as we previously described.5,13 Briefly, femoral artery puncture was performed using the modified Seldinger method. A 5F RH catheter (Terumo, Japan) was inserted to the common hepatic artery under guidewire guidance for arteriography to determine the location of the tumor and the feeding artery. Superselective arterial cannulation was performed to enter the blood supply branch of the tumor. Doxorubicin (50–70 mg, Zhejiang Hisun Pharmaceutical Co., Ltd., China) and lipiodol (5–20 mL, Guerbet, France) were used according to the patient’s liver function, tumor size, and body surface area. The mixture was slowly injected for arterial chemoembolization. For donors with residual blood after lipiodol emulsion embolization, gelatin sponge particles (diameter 500–700 µm, Hangzhou Ailikang Pharmaceutical Technology Co., Ltd., China) were added to supplement the embolization. The endpoint of embolization was stagnation of blood flow in the arterial blood supply to the tumor.
PVTT 125I seed implantation was immediately performed 3–7 days after TACE if the patient’s liver function reached Child-Pugh grade A and there was no contraindication to the implantation. Planning was performed based on preoperative computed tomography (CT) imaging findings, using a treatment planning system (TPS) (Beijing Kelinzhong Institute of Medical Technology, China). To number of particles required was determined based on the total dose, location of the implanted particles, and the puncture route. Percutaneous PVTT puncture was performed using a special puncture needle for seeds, and radioactive 125I seeds were implanted individually according to the preoperative TPS plan. Post-seed implantation was assessed using TPS, and 125I seed reseeding was performed if the radiation dose distribution was insufficient.
Imaging Evaluation of HCC and PVTT
Follow-up imaging examinations were performed every 4–6 weeks after TACE, mainly with a multiphase plain scan of the upper abdomen and dynamic contrast-enhanced magnetic resonance imaging (MRI). Alpha-fetoprotein levels were measured and liver function tests were performed concurrently. TACE was repeated if contrast-enhanced MRI revealed recurrence or residual active disease in the liver. Reseeding with PVTT 125I seeds was performed if imaging studies within 3 months showed PVTT activity.
Downstaging Standard
The criteria for successful downstaging included: (1) liver function and general condition of the patient, defined as Child-Pugh grade A and Eastern Cooperative Oncology Group Performance Status score ≤ 1, (2) PVTT downstaging, defined as loss of PVTT activity on enhanced MRI on follow-up; and (3) post treatment reduction of intrahepatic lesions. Persistent PVTT activity or progression on 3-month post treatment imaging was judged as downstaging failure.
Radiofrequency Ablation
RFA was immediately performed after downstaging was confirmed. The tumor ablation criteria were set as follows according to the modified Response Evaluation Criteria in Solid Tumors: a single lesion (active part) with a diameter of ≤5 cm or 2–3 lesions with the largest diameter (active part) ≤3 cm. Treatment plans were generated based on CT images obtained after general anesthesia and preoperative MR images. For lesions with diameters <40 mm and 40–50 mm, a single needle and overlapping needles were used for treatment, respectively. A RITA cluster needle (100–150 mm in length, 20–50 mm in diameter at the ablation end) was used to puncture the target. RFA was performed after confirming that the sub needle was not in a visible venous vessel or a dilated bile duct. The temperature was 85–105°C; rated power, 150–200 W; and duration, 5–15 min.14
The temperature of the sub-needle adjacent to the blood vessel was observed. If the temperature cannot be raised, it was considered as that the sub-needle part entered the blood vessel or bile duct. The needle insertion depth and angle was accordingly closed and adjusted. Needle tract ablation was performed prior to needle extraction to prevent needle tract implantation. The ablation scope covered at least 10 mm of the paracancerous tissue to obtain a “safe edge” and to completely eliminate the lesion. For tumors with unclear boundaries and irregular shapes, the ablation range was appropriately expanded if the adjacent liver tissue and structural conditions permitted. If a residual contrast-enhancing tumor appeared on follow-up CT imaging, additional RFA was performed immediately.
Follow-Up and Post-Ablation Evaluation
Dynamic contrast-enhanced MRI was performed 1 month after the first ablation to evaluate the effect of ablation. RFA was then repeated in patients with residual target lesions after the first RFA. If there was still a tumor (target lesion) after two ablations, it was defined as incomplete ablation and treated according to routine practice. For patients with multiple target lesions, the lesion with the worst therapeutic effect was used for the final evaluation. Patients were followed-up every 3 months after the first follow-up visit and every 6 months thereafter following institutional guidelines. The effects of target lesion ablation were divided into complete and incomplete ablations.11 Complete ablation referred to a dynamic contrast-enhanced MRI scan, with no enhancement in the arterial phase of the intrahepatic ablation lesion, indicating complete necrosis of the tumor. Incomplete ablation referred to local enhancement of intrahepatic ablation lesions in the arterial phase, suggesting a residual tumor. The complete ablation rate of target lesions was determined as the number of target lesions completely ablated/total number of target lesions × 100%. OS was defined as the interval between the date of initiation of combination therapy and the date of death. Adverse events were defined as complications that occurred within 30 days postoperatively as well as other potentially treatment-related complications observed during follow-up.
Statistical Analysis
Categorical variables were expressed as percentages and analyzed using the chi-square test, while continuous variables were expressed as the mean ± standard deviation and analyzed using the Student’s t-test. Kaplan-Meier analysis was used to determine the survival rate at each time point, and the 1-, 2-, and 3-year survival rates and median OS were calculated. All statistical analyses were performed using the SPSS software (version 13.0; SPSS. Inc., Chicago, IL, USA). P<0.05 was considered statistically significant.
Result
Baseline Characteristics
A total of 31 patients (63.27%) were successfully downstaged, while 18 patients (36.73%) did not achieve downstaging. The baseline patient characteristics are shown in Table 1. Compared with failed downstaging group, the successful downstaging group had fewer intrahepatic lesions (P=0.008) and lower alpha-fetoprotein levels (P=0.007). The other independent variables were not significantly different between the two groups.
 |
Table 1 Comparison of Baseline Characteristics Between the Success and Failed Downstaging Groups |
Downstaging
The mean number of TACE treatments for intrahepatic lesions was 2.48 (range, 1–6). The average number of seed implants was 1.10 (range, 1–2). The mean interval between the first TACE treatment and RFA was 3.8 months (range, 3.0–6.8 months). Before TACE, the mean number of lesions was 1.26 (range, 1–3), the mean largest tumor diameter was 5.63 cm (range, 3.5–6.8 cm), and the mean total tumor diameter was 6.21 cm (range, 3.5–9.8 cm). After downstaging, the mean number of lesions was 1.23 (range, 1–3 lesions), the mean largest tumor diameter was 2.76 cm (range, 1.0–4.9 cm), and the mean total tumor diameter was 3.06 cm (range, 1.0–6.0 cm). There was no significant difference in the number of intrahepatic tumors before and after downstaging (P=0.325), but the mean maximum and total diameters were significantly different (P<0.10). After downstaging treatment, none of the 31 patients in the successful downstaging group showed PVTT activity. Among the 18 patients in failed downstaging group, 3 patients had enlarged intrahepatic lesions, 15 patients had PVTT progression or activity, and most patients (12/15) had intrahepatic sub-foci.
Technical Success Rate and Curative Effect of RFA
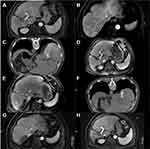
There were 38 hepatic lesions in the successful downstaging group. All 31 patients underwent CT-guided RFA, and the technical success rate was 100%. The 1- and 6-month complete ablation rate of intrahepatic tumors was 94.74% (36/38) and 84.21% (32/38), respectively. Six patients did not achieve complete ablation; 2 patients did not achieve complete ablation at the first RFA, 1 patient underwent secondary ablation, and 3 patients underwent TACE (Table 2). Based on the results of ablation treatment within 6 months, patients were divided into an complete ablation and incomplete ablation group. Baseline information of patients in both groups is shown in Table 3. Compared with patients in the complete ablation group, most of the lesions in the incomplete ablation group were adjacent to large vessels/gallbladder (P=0.013), and the proportion of APF≥400ng/m was higher (P=0.026). During the follow-up period, 10 patients (32.26%) developed intrahepatic recurrences. 4 patients continued conventional TACE therapy for a large intrahepatic recurrence or due to refusal of ablation therapy. In total, 6 patients met the ablation criteria and underwent a total of 10 ablation treatments. Contrast-enhanced MRI showed that 4 patients had PVTT activity again, and a total of 4 seed implantations were performed. Figure 2 shows the imaging during treatment of a representative case patient.
 |
Table 2 Treatment of RFA Within 6 Months in Patients in the Successful Downstaging Group |
 |
Table 3 Baseline Information on Different Ablation Outcomes in Patients with the Successful Downstaging Group |
Survival Analysis
The follow-up period ranged from 8 months to 69 months and was terminated on May 2020. In the successful downstaging group, the 1-, 2-, and 3-year survival rates were 90.3% (28/31), 80.6% (25/31), and 48.4% (15/31), respectively. And the median OS was 36.0 months (95% CI: 24.7–47.3) (Figure 3A). The median recurrence free survival (RFS) was 17.4 months (95% CI:12.41–22.40) (Figure 3B). The mOS of patients in the complete ablation and incomplete ablation groups was 37.20 months (95% CI: 20.60–53.80) and 18.00 months (95% CI: 10.32–25.68), respectively (Figure 3C), with a statistically significant difference (P=0.042). The main cause of death was intrahepatic progression, accounting for 67.7% (21/31) of all deaths, followed by gastrointestinal bleeding in 19.35% (6/31) and liver failure in 6.45% (2/31).
Adverse Reactions
Most patients (40/49) had mild TACE-related adverse reactions, including a transient increase in postoperative bilirubin levels, and the symptoms were relieved after symptomatic treatment. The main adverse reaction related to 125I seed implantation was particle displacement. After immediate postoperative TPS verification, there were 3 patients who had insufficient radiation dose distribution due to particle displacement, and seed re-implantation was performed later. Five patients had slight subcapsular liver hemorrhage due to puncture, and the bleeding stopped after drug hemostasis was achieved. Ablation-related adverse reactions included fever and subcapsular hemorrhage, which were cured after symptomatic and supportive treatment. No serious adverse reactions, such as surgery-related death, occurred in any patient within 30 days postoperatively (Table 4).
 |
Table 4 Treatment Complications |
 |
Figure 3 Kaplan-Meier curves for OS (A) and RFS (B) in patients with successful downstaging. Kaplan-Meier curves for OS (C) in patients with complete ablation and incomplete ablation. |
Discussion
TACE combined with PVTT 125I seed implantation can downstage HCC patients with portal vein branch invasion. In addition, RFA after successful downstaging enabled radical cure or longer survival, with a median OS of 36.0 months.
In a study of the long-term results of RFA after TACE downstaging,9 the estimated OS rates for patients in the downstaging group were 99%, 80%, and 66% at 1, 3, and 5 years, respectively. These results were better than those of the current study. However, the study did not include patients with PVTT, and thus, the risk of portal hypertension and intrahepatic spread would be lower, which would affect the long-term survival. Meanwhile, the 1- and 2-year survival rates in this study were comparable (90.3% and 80.6%, respectively), while, the 3-year survival rate was significantly lower (48.4%). Further, the HCC patients with PVTT had poor long-term survival. In another case series combining sorafenib, camrelizumab, TACE, and stereotactic radiation therapy as a downstaging strategy for advanced HCC with PVTT,15 the 1-year OS rate was 83.3%, lower than the 90.3% in this study. This may be related to the inclusion of only unilateral branch portal vein tumor thrombus in the current study. However, the median OS in this case series was not reached. Previous downstaging studies for unresectable HCC patients are all based on TACE therapy, but TACE alone has limited benefits in HCC patients with PVTT.16 125I seed implantation, as an internal brachytherapy method,17 has a good effect on PVTT and is a potential downstaging treatment method.
According to literature reports,18 the treatment of liver cancer can be considered to maximize the function of the remaining liver while fully treating the tumor lesions, which achieves a balance between the two. The appearance of PVTT is an additional burden for patients with HCC. Blockage of the portal vein reduces the blood supply to the liver. In the current study, the patients who were successfully downstaged may have had liver function damage from the repeated TACE treatment. However, PVTT 125I seed implantation led to PVTT shrinkage, and the portal vein blocked by the tumor thrombus was recanalized or partially recanalized. This increased blood perfusion in the liver and effectively recovered liver function. A prospective study in China reported that effective treatment of PVTT can restore liver function to a certain extent,5 thus providing patients with more opportunities for treatment and reducing mortality from liver decompensation.
Effective PVTT control can enable early RFA treatment, ultimately leading to long-term survival. This strategy can be described as sequential TACE combined with RFA. TACE treatment can effectively reduce the blood supply to the tumor. RFA enhances the efficacy of ablation by reducing the loss of drugs or heat due to the blood flow during treatment. RFA also causes apoptosis of the remaining tumor cells in or around the lipiodol deposition area. Thus, these two modalities have a synergistic effect to significantly increase the complete necrosis rate of the targeted lesions, thereby further improving the therapeutic effect in liver cancer.19 Previous studies have suggested that TACE combined with RFA is superior to RFA alone, especially for the treatment of HCC patients with medium and large liver tumors.20 Therefore, TACE followed by RFA has the potential to expand indications for ablation.
However, even after PVTT was effectively controlled, the 3-year survival rate of patients in this study was still low. The main causes of death were tumor recurrence and metastasis. The criterion for successful downstaging in the current study was essentially the radiographic response, which has certain limitations. Even if the PVTT is radiographically inactive, it does not guarantee the absence of residual cancer cells. Once portal blood flow is restored in the presence of activity, residual cancer cells may enter the liver, resulting in new sub-foci in the liver. In this study, the 3-year survival rate was low, and new sub-foci continued to appear in the liver. This may be related to the aforementioned reasons. In addition, liver metastasis of colorectal cancer has been reported.21 More than 50% of lesions recur on follow-up, even in those that disappear on imaging after chemotherapy (complete radiographic response). Therefore, combination systemic therapy (eg, targeted therapy or immunotherapy) is recommended22,23 to not only improve the success of downstaging, but also to inhibit or delay postoperative recurrence. This will be the focus of our next research.
Our study had some limitations. First, this was a retrospective study with a small sample size, and patient selection depended on physician experience. Thus, data bias could not be avoided. The results should be verified in prospective randomized controlled trials with larger sample sizes. Second, imaging assessments alone have limitations. However, PET/CT could not be performed in all patients owing to economic constraints. As such, it was difficult to accurately assess the efficacy of PVTT.
In conclusion, TACE combined with PVTT 125I seed implantation can significantly prolong patient survival in HCC patients with PVTT. Downstaging can be achieved in some patients. In addition, RFA after successful downstaging can further improve the survival rate, achieving tumor-free survival.
Data Sharing Statement
The Excel format data used to support the findings of this study are available from the corresponding author at [[email protected]] upon request.
Ethical Statement
This retrospective study was approved by the Research Ethics Committee of the Cancer Hospital affiliated with Zhengzhou University and complied with the ethical guidelines of the World Medical Association Declaration of Helsinki.
Acknowledgments
We thank all our authors listed in this manuscript, and also thank all the patients participated in the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Henan Province Natural Science Foundation (212300410403). Medical Education Research Project of Henan Province (Wjlx2021334). Technology Major Project of the Ministry of Science and Technology of China (2018ZX10303502).
Disclosure
The authors have no conflict of interest related to this publication.
References
1. Wei X, Jiang Y, Zhang X, et al. Neoadjuvant three-dimensional conformal radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: a randomized, open-label, multicenter controlled study. J Clin Oncol. 2019;37(24):2141–2151. doi:10.1200/JCO.18.02184
2. Liu PH, Huo TI, Miksad RA. Hepatocellular carcinoma with portal vein tumor involvement: best management strategies. Semin Liver Dis. 2018;38(3):242–251. doi:10.1055/s-0038-1666805
3. Pawarode A, Voravud N, Sriuranpong V, Kullavanijaya P, Patt YZ. Natural history of untreated primary hepatocellular carcinoma: a retrospective study of 157 patients. Am J Clin Oncol. 1998;21(4):386–391. doi:10.1097/00000421-199808000-00014
4. Sun H, Zhang M, Liu R, Liu Y, Hou Y, Wu C. Endovascular implantation of 125I seed combined with transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma. Future Oncol. 2018;14(12):1165–1176. doi:10.2217/fon-2017-0354
5. Hu HT, Luo JP, Cao GS, et al. Hepatocellular carcinoma with portal vein tumor thrombus treated with transarterial chemoembolization and Sorafenib vs.125Iodine implantation. Front Oncol. 2021;11:806907. doi:10.3389/fonc.2021.806907
6. Sun HC, Zhou J, Wang Z, et al. Chinese expert consensus on conversion therapy for hepatocellular carcinoma (2021 edition). Hepatobiliary Surg Nutr. 2022;11(2):227–252. doi:10.21037/hbsn-21-328
7. European Association for the Study of the Liver. Electronic address: [email protected]; European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
8. Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150(4):835–853. doi:10.1053/j.gastro.2015.12.041
9. Shi F, Wu M, Lian SS, et al. Radiofrequency ablation following downstaging of hepatocellular carcinoma by using transarterial chemoembolization: long-term outcomes. Radiology. 2019;293(3):707–715. doi:10.1148/radiol.2019181991
10. Zeng ZC, Tang ZY, Fan J, et al. A comparison of chemoembolization combination with and without radiotherapy for unresectable hepatocellular carcinoma. Cancer J. 2004;10(5):307–316. doi:10.1097/00130404-200409000-00008
11. Zhu XD, Huang C, Shen YH, et al. Downstaging and resection of initially unresectable hepatocellular carcinoma with tyrosine kinase inhibitor and Anti-PD-1 antibody combinations. Liver Cancer. 2021;10(4):320–329. doi:10.1159/000514313
12. Lee IJ, Kim JW, Han KH, et al. Concurrent chemoradiotherapy shows long-term survival after conversion from locally advanced to resectable hepatocellular carcinoma. Yonsei Med J. 2014;55(6):1489–1497. doi:10.3349/ymj.2014.55.6.1489
13. Yuan H, Cao P, Li HL, et al. Transarterial chemoembolization with radiofrequency ablation versus hepatectomy in hepatocellular carcinoma beyond the Milan criteria: a retrospective study. Cancer Manag Res. 2018;10:5545–5552. doi:10.2147/CMAR.S182914
14. Chen ML, Li HL, Guo CY, et al. Correction to: radiofrequency ablation combined with transarterial chemoembolization in treatment of hepatocellular carcinoma adjacent to the second hepatic hilus. Abdom Radiol. 2022;47(4):1509. doi:10.1007/s00261-022-03424-5
15. Huang Y, Zhang Z, Liao W, Hu K, Wang Z. Combination of sorafenib, camrelizumab, transcatheter arterial chemoembolization, and stereotactic body radiation therapy as a novel downstaging strategy in advanced hepatocellular carcinoma with portal vein tumor thrombus: a case series study. Front Oncol. 2021;11:650394. doi:10.3389/fonc.2021.650394
16. Zhang X, Wang K, Wang M, et al. Transarterial chemoembolization (TACE) combined with sorafenib versus TACE for hepatocellular carcinoma with portal vein tumor thrombus: a systematic review and meta-analysis. Oncotarget. 2017;8(17):29416–29427. doi:10.18632/oncotarget.15075
17. Li S, Guo JH, Lu J, et al. I 125 irradiation stent for treatment of hepatocellular carcinoma with portal vein thrombosis: a meta-analysis. Cancer Radiother. 2021;25(4):340–349. doi:10.1016/j.canrad.2020.12.003
18. Lucà MG, Nani R, Schranz M, et al. Treatment of hepatocellular carcinoma: a cost analysis of yttrium-90 transarterial radioembolization versus sorafenib. Future Oncol. 2018;14(8):727–735. doi:10.2217/fon-2017-0566
19. Ni JY, Liu SS, Xu LF, Sun HL, Chen YT. Meta-analysis of radiofrequency ablation in combination with transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2013;19(24):3872–3882. doi:10.3748/wjg.v19.i24.3872
20. Liu W, Xu H, Ying X, et al. Radiofrequency Ablation (RFA) combined with Transcatheter Arterial Chemoembolization (TACE) for patients with medium-to-large hepatocellular carcinoma: a retrospective analysis of long-term outcome. Med Sci Monit. 2020;26:e923263. doi:10.12659/MSM.923263
21. Dhir M, Sasson AR. Surgical management of liver metastases from colorectal cancer. J Oncol Pract. 2016;12(1):33–39. doi:10.1200/JOP.2015.009
22. Huang SL, Wang YM, Wang QY, et al. Mechanisms and Clinical Trials of Hepatocellular Carcinoma Immunotherapy. Front Genet. 2021;12:691391. doi:10.3389/fgene
23. Finn RS, Qin S, Ikeda M, et al. IMbrave150 investigators. atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.