Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Implanting Iodine-125 Seed Strand Inside the Portal Vein Stent: An Improved Approach to Endovascular Brachytherapy for Treatment of Patients with Hepatocellular Carcinoma and Main Portal Vein Tumor Thrombus
Authors Zhang L, Wang J, Li Y, Hou L, Xia J, Shen J
Received 14 July 2023
Accepted for publication 9 November 2023
Published 6 December 2023 Volume 2023:10 Pages 2187—2196
DOI https://doi.org/10.2147/JHC.S430686
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Imam Waked
Liang Zhang,1,* Jun Wang,1,* Yang Li,2 Leina Hou,3 Jianguo Xia,4,* Jialin Shen1,*
1Department of Interventional Oncology, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 2Department of Radiology, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 3Department of Anesthesiology, Shaanxi Provincial Cancer Hospital, Xi’an, Shaanxi, People’s Republic of China; 4Department of Ultrasound, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianguo Xia, Department of Ultrasound, Renji Hospital, Shanghai Jiao Tong University School of Medicine, NO. 160 Pujian Road, Shanghai, People’s Republic of China, Tel +86-13671807078, Fax +86-21-34506514, Email [email protected] Jialin Shen, Department of Interventional Oncology, Renji Hospital, Shanghai Jiao Tong University School of Medicine, NO. 160 Pujian Road, Shanghai, People’s Republic of China, Tel +86-13501794493, Fax +86-21-34506368, Email [email protected]
Purpose: To investigate the feasibility and efficacy of implanting an iodine-125 (125I) seed strand inside a portal vein stent (PVS) in the treatment of patients with hepatocellular carcinoma (HCC) and main portal vein tumor thrombus (mPVTT).
Patients and Methods: Twenty-three patients who diagnosed with HCC and mPVTT and underwent endovascular implantation 125I seed strands and portal vein stenting were included in this study. Patients were divided into two groups. For patients in group A (n = 12), the 125I seed strand was placed outside the PVS, and for those in group B (n = 11), the strand was placed inside the PVS. Technical success, pain intensity during the procedure (numeric rating scale), procedure-related complications, changes in liver function, stent patency, and survival rates were recorded and analyzed.
Results: The procedures were successful in all patients, and no serious procedure-related complications occurred in either group. Pain intensity during the procedure was significantly lower in group B than in group A (2.64 ± 1.50 vs 4.08 ± 1.78, p = 0.048), and there were no significant differences between pre- and post-procedure liver function in either group. The median duration of stent patency was 9 months (95% CI 2.21– 15.79 months) in group A and 12 months (95% CI 3.63– 18.37 months) in group B (p = 0.670). Median survival was 12 months (95% CI 10.30– 13.70 months) in group A and 13 months (95% CI 10.03– 15.97 months) in group B (p = 0.822). The cumulative stent patency and survival rates at 3, 6, and 12 months were 75%, 50%, and 41.7%, and 83.3%, 75%, and 50% in group A and 72.7%, 62.3%, and 31.2%, and 90.9%, 80.8%, and 50.5%, respectively.
Conclusion: Implantation of 125I seed strand inside the PVS is effective and feasible for treating patients with HCC and mPVTT.
Keywords: portal vein tumor thrombus, 125I seed, portal vein stent, endovascular brachytherapy, hepatocellular carcinoma
Introduction
Portal vein tumor thrombus (PVTT) is a common complication of hepatocellular carcinoma (HCC) and is reported in 44–62% of HCC cases.1 PVTT can lead to portal hypertension, liver failure, tumor metastasis, and other complications, including ascites, jaundice, and gastrointestinal bleeding, resulting in poor prognosis for patients with HCC and PVTT.2–5 Thus, PVTT is an important prognostic factor for HCC and plays an essential role in the clinical staging system.6,7
Considerable progress has been made in the systemic treatment of HCC in recent years, especially since the introduction of immunotherapy.8,9 Encouraging results have been reported in use of immune checkpoint inhibitors (ICI) and ICI-based combinations in advance HCC.10–12 However, the prognosis of patients with HCC with main portal vein tumor thrombus (mPVTT) remains poor, and no standard treatment strategy has been recommended for these patients.13–16 As a local treatment strategy, the combination of endovascular brachytherapy and portal vein stent (PVS) placement has shown benefits for patients with HCC and mPVTT in some studies.17,18 This regimen can promptly restore blood flow in the obstructed main portal vein and effectively inhibit progression of the tumor thrombus, thus providing more opportunities for further treatment and improving survival.19,20
Iodine-125 (125I) seeds are the most commonly used radioactive source for endovascular brachytherapy and have demonstrated good safety and efficacy in treating PVTT.21,22 To prevent radioactive seed dislodgment and to ensure longitudinal radiation coverage of the target lesion, linear strands of 125I seeds are usually placed between the PVS and the PVTT.17–20 In this way, the 125I seed strand can be firmly fixed by the stent and tumor thrombus. However, implantation of the 125I seed strand outside the stent requires the introduction of two guidewires to allow two separate 6Fr delivery systems for simultaneous delivery of the stent and 125I seed strand through a single puncture track, which may increase the risk of bleeding of the puncture track and the patient’s pain intensity during the procedure.
In the present study, we report an improved approach to endovascular brachytherapy and stent placement for the treatment of patients with HCC and mPVTT, in which the PVS and 125I seed strand can be implanted sequentially over a single guidewire through a 6Fr sheath, with the seed strand placed inside the stent. To secure the 125I seed strand and block the puncture track, the distal end of the strand was retained in liver parenchyma. This study aimed to investigate the feasibility and efficacy of the improved approach by comparing it with a previously reported method.
Material and Methods
Patients
This study was approved by a local institutional review board, and informed consent was obtained from all eligible patients. Forty-nine HCC patients with PVTT were assessed at our institution between January 2020 and December 2021, and 23 patients were finally enrolled in the study. All HCC cases were diagnosed by biopsy or persistently elevated serum alpha-fetoprotein (AFP) levels (>400 ng/mL) with typical imaging findings and clinical history, and confirmed by contrast-enhanced abdominal computed tomography (CT) or magnetic resonance imaging (MRI). All cases were classified as advanced-stage and were unsuitable for resection, ablation, or liver transplantation according to the Barcelona Clinic Liver Cancer staging classification. Other inclusion criteria were as follows: 1) at least the second-order branch of the intrahepatic portal vein was patent in one lobe; 2) Child-Pugh classification grade A or B; and 3) Eastern Cooperative Oncology Group (ECOG) performance status ≤2. The exclusion criteria were as follows: 1) history of radiation therapy; 2) intrahepatic portal vein completely occluded; 3) superior mesenteric vein (SMV) or splenic vein (SV) invaded by tumor thrombus; and 4) uncorrectable coagulation disorders or hepatic/renal failure.
From January 1 to December 31, 2020, 12 patients were enrolled in Group A and underwent 125I seed strand implantation outside the PVS. From January 1 to December 31, 2021, 11 patients were enrolled in Group B and underwent 125I seed strand implantation inside the PVS (Figure 1).
 |
Figure 1 Study flowchart. Abbreviations: mPVTT, main portal vein tumor thrombus; ECOG PS, Eastern Cooperative Oncology Group performance status; SMV, superior mesenteric vein; SV, splenic vein. |
Stents and Iodine-125 Seed Strands
Self-expanding nitinol vascular stents (EPICTM, Boston Scientific, Natick, MA, USA; diameter 12 to 14 mm and length 60 to 100 mm) and model 6711 125I seeds (XinKe, Shanghai, China) with a half-life of 59.4 days and a half-value thickness of 17 mm were used in this study. The radioactivity of each 125I seed was 25.9 MBq and the incipient dose rate was 7 cGy/h. The principal photon emissions were 27.4 and 31.4-keV X-rays and 35.5-keV γ-rays.
Stent Placement and Iodine-125 Seed Strand Implantation
For patients in group A, under ultrasound guidance, a 22G puncture needle (NAPs puncture kit, Cook, Inc., Bloomington, IN, USA) was used to puncture the branch of the portal vein free of tumor thrombus. After a successful puncture, a 6Fr sheath (APT Medical, Changsha, China) was introduced. A 4Fr pigtail catheter (APT Medical) was used to cross the obstructed or narrow part of the main portal vein and was placed in the SV or SMV. Portography was performed to measure the degree and extent of obstruction of the main portal vein. The stent size was determined according to the portography results. To ensure complete coverage of the tumor thrombus by the stent, the ends of the stent were extended at least 1 cm beyond the ends of the tumor thrombus. If gastroesophageal varices were observed, coil embolization (Cook) of the left gastric vein, the short gastric vein, or both was performed. 125I seeds were arranged linearly into a seed strand inside a 4Fr catheter. The number of seeds (N) was calculated using the following formula: N = length of the obstructed portal vein (mm) / 4.5 + 4. Subsequently, two 0.035-inch, 260-cm-long stiff wires (Terumo, Tokyo, Japan) were inserted into the SMV through a 6Fr sheath. Withdrawn the 6Fr sheath from the two wires and introduce two 6Fr sheaths over the two wires, respectively. A self-expandable stent with appropriate size was advanced to the obstructed segment of the portal vein over one of the stiff wires (without deployment); A 6Fr guiding catheter (ENVOY, Cordis, Miami Lakes, FL) was placed into the portal vein over another stiff wire, and the 125I seed strand was introduced to the target position through the 6Fr guiding catheter. After accurate positioning of the seed strand, the PVS was deployed over the obstruction, and the 125I seed strand was delivered between the stent and tumor thrombus by withdrawing the guiding catheter. Repeat portography was performed to evaluate the recovery of portal venous blood flow. Finally, the transhepatic puncture track was occluded by using a coil (Cook).
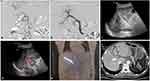
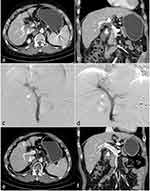
For patients in group B: After a successful puncture of the portal vein, a 6Fr sheath was introduced. As in group A, a 4Fr pigtail catheter was used to perform portography to measure the degree and extent of obstruction of the main portal vein. An appropriately sized PVS was then introduced over a stiff wire and deployed after accurate positioning under fluoroscopy, after which the pigtail catheter was reintroduced to evaluate the recovery of portal venous blood flow. Next, a 125I seed strand was prepared as described above. However, the number of seeds was determined according to the length of the stent under fluoroscopy, with the length of the 125I seed strand exceeding both ends of the stent by 2 cm. The 125I seed strand was then introduced through a 6Fr guiding catheter and was placed inside the PVS. The guiding catheter was withdrawn, and the 125I seed strand was released, ensuring that the proximal end of the 125I seed strand exceeded the stent by 1 cm and that the distal end of the 125I seed strand was in the liver parenchyma. The distal end of the 125I seed strand in the liver parenchyma blocked the puncture tract; therefore, no additional coil was necessary for occlusion of the puncture tract (Figures 2 and 3).
Post-Procedure Management
All patients received supportive liver protection and acid suppression therapy for 3–5 days, and anticoagulation treatment with low-molecular-weight heparin and warfarin. Analgesics and antipyretics were prescribed if necessary. Single-photon emission computed tomography combined with CT (SPECT/CT) was performed on the second day after surgery to evaluate the distribution of radiation by the 125I seed strand. Laboratory tests were performed on day 7 to evaluate hepatic and renal function, blood cell counts, and coagulation parameters. Antiviral drugs were administered to patients with hepatitis B, and transarterial chemoembolization (TACE) was performed if the residual tumor was visible or a new lesion was detected on follow-up liver contrast-enhanced CT, unless contraindicated. Sorafenib was administered until disease progression or unacceptable toxicity was observed.
Evaluation and Follow-Up
Patient follow-up was conducted at 1 and 3 months after the procedure and every 3 months thereafter until death or the last follow-up. Follow-up evaluations included physical examination, laboratory testing, and contrast-enhanced liver CT or MRI.
Technical success, pain intensity during the procedure, procedure-related adverse events, overall survival (OS), and duration of stent patency were noted and compared between the two groups. Technical success was defined as stent deployment and 125I seed strand placement at the target site. Pain intensity during the procedure was evaluated the day after the operation using a numeric rating scale (NRS). Patients were asked to describe the severity of the worst pain they felt during the procedure on a scale of 1 (no pain) to 10 (pain as bad as possible). OS was defined as the period from the day of the procedure to the time of death or last follow-up. Stent occlusion was present when no contrast medium was detected inside the stent in the portal phase of a contrast-enhanced CT scan, and the duration of stent patency was measured from the day of stent placement until stent occlusion or the day of the last follow-up. Procedure-related adverse events were recorded using the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 classification. The levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin (TB) were compared before and at 1 week after operation.
Statistical Analysis
Statistical software (SPSS version 19.0; SPSS, Chicago, IL, USA) was used for analysis. Continuous variables are presented as mean ± standard deviation and were compared using independent or paired sample t-tests. Categorical variables are presented as frequencies and were compared using the chi-square test. The overall survival and stent patency periods were analyzed using Kaplan–Meier curves and Log rank tests. Statistical significance was set at p < 0.05.
Results
Patient Data
The characteristics of the study population are summarized in Table 1. There were no significant differences in patient characteristics between the 2 groups (all p > 0.05).
 |
Table 1 Characteristics of the Study Population |
Stent and Iodine-125 Seed Strand Placement
Endovascular PVS and 125I seed strand placement were successful in all the patients. The average number of 125I seeds was 16.67 ± 5.29 in group A and 23.45 ± 3.59 in group B (p = 0.002). Portal venous blood flow was restored immediately after the stent implantation in 21 patients. Portal venous flow was not restored after stent deployment in two patients (one in group A and one in group B) and did not recover during follow-up in either patient.
Procedure-Related Adverse Events
No serious procedure-related complications, such as intraperitoneal bleeding, acute hepatic failure, radiation hepatitis, or enteritis, were observed during the course of the study. The NRS score for pain intensity during the procedure in group B was 2.64 ±1.50, which was significantly lower than that in group A (4.08 ±1.78, p = 0.048). There was no significant difference between pre- and post-procedure liver function in either group (Table 2).
 |
Table 2 Pre- and Post-Procedure Liver Function |
Evaluation of Iodine-125 Seed Strands
SPECT/CT on post-procedure day 1 showed that all stents and 125I seed strands had been correctly placed in the obstructed mPVTT without displacement in all patients. The radiation emitted by the 125I seed strand was homogeneously distributed. It was cylindrical in shape and completely covered the target lesion. During follow-up, no displacement or drop-off of 125I seeds was observed.
Overall Survival and Stent Patency
In group A, the mean follow-up time was 11.75 ± 5.97 (2–24) months, and all patients died during follow-up. One patient died of tumor rupture and bleeding, 2 patients died of gastrointestinal bleeding, and 9 patients died of hepatic failure due to tumor progression. The mean follow-up time in group B was 10.18 ± 4.47 (2–15) months, and nine patients died during the follow-up period. One patient died of gastrointestinal bleeding, one of heart failure, and seven of hepatic failure due to tumor progression. No TACE- or sorafenib-related deaths were observed in either group during the follow-up. The median OS was 12 months (95% CI 10.30–13.70 months) in group A and 13 months (95% CI 10.03–5.97 months) in group B (p = 0.822). The cumulative survival rate of the patients at 3, 6, and 12 months were 83.3%, 75%, and 50% in group A and 90.9%, 80.8%, and 50.5% in group B, respectively (Figure 4a).
The median duration of stent patency was 9 months (95% CI 2.21–15.79 months) in group A compared to 12 months (95% CI 3.63–18.37 months) in group B (p = 0.670). No thrombosis-related stent obstructions were observed. The cumulative stent patency rate of the patients at 3, 6, and 12 months were 75%, 50%, and 41.7% in group A and 72.7%, 62.3%, and 31.2% in group B, respectively (Figure 4b).
Discussion
The optimal treatment for HCC and mPVTT remains controversial. Liver transplantation is not recommended for patients with mPVTT because of the high tumor recurrence rate.23 Only a minority of patients with mPVTT are amenable to radical surgical resection at diagnosis,24 and even where radical surgical resection is possible, the median survival time is still less than 11 months.25 mPVTT was once considered a relative contraindication to TACE because of the potential risk of liver failure,26 and while sorafenib can be recommended for patients with PVTT,7 it is not recommended for patients with portal invasion at the main portal branch because of the risk of hepatic failure.27 In recent years, it has been reported that atezolizumab plus bevacizumab can provide better survival than sorafenib for patients with advanced unresectable HCC,9 but the subgroup data showed that the median survival was only 7.6 months for patients with mPVTT.28 Other research have shown the efficacy of both external beam radiation therapy29 and hepatic arterial infusion chemotherapy30 in treating HCC with PVTT, but blood flow in the obstructed main portal vein cannot be restored immediately. Transarterial radioembolization with yttrium-90 microspheres has also been used for the treatment of HCC with PVTT,31 but this technology is not widely available. The combination of endovascular brachytherapy and stent placement is a promising treatment for patients with HCC and mPVTT.32 The reported median OS after combination 125I seed strand and PVS placement for HCC patients with mPVTT is 9.2 to 12 months.17,18 In the present study, the combination of endovascular brachytherapy and PVS placement was chosen as the treatment of HCC patients with mPVTT. The median OS was 12 and 13 months in groups A and B, respectively, which is in line with the results of previous studies.
To fix the 125I seed strand, it is usually placed between the PVS and tumor thrombus,17–20 which requires simultaneous deployment of the stent and release of the 125I seed strand in the portal vein via two separate 6Fr delivery systems. This implantation approach is associated with an increased risk of bleeding in the puncture track and greater pain in patients during the procedure. The complexity of the surgery and corresponding risks limit the clinical application of this technology to some extent. More HCC patients with mPVTT may benefit from simplifying the surgical procedures and reducing surgical risks.
In the present study, we report an improved approach to endovascular brachytherapy and stent placement for the treatment of patients with HCC and mPVTT, in which a 125I seed strand was placed inside the stent over a single guidewire through a single 6Fr sheath. The 125I seed strand was fixed by the liver parenchyma instead of by the stent. Compared with the traditional method, the improved approach avoids the simultaneous introduction of two delivery systems, which simplifies the procedure, decreases the risk of bleeding of the puncture track, and improves tolerability for the patient while providing similar survival and stent patency rates in HCC patients with mPVTT.
An important point of the improved approach is the placement of the 125I seed strand such that the proximal end of the strand exceeds the stent, and the distal end of the strand is in the liver parenchyma. The former can prevent a tumor thrombus growing into the stent from the end of the stent more effectively, prolonging the patency time of the stent. The latter can prevent the displacement of the seed strand and block the puncture tract. In the present study, the puncture tract was not occluded by a coil in group B patients, and there was no bleeding of the puncture tract in any patient. In addition, the 125I seed strand was suspended in the stent, which made the distribution of irradiation on the tumor thrombus more homogeneous.
Although implanting the 125I seed strand inside the stent may increase the risk of thrombosis, no stent occlusion caused by thrombosis was found in any patient during the follow-up in this study. This may be because the diameter of the 125I seed strand was only 1.3 mm (4Fr), which is considerably narrower than that of the PVS. Therefore, the effect of the strand on the risk of portal vein thrombosis may be limited. Standardized anticoagulant therapy after the procedure also reduces the probability of stent thrombosis.
Most patients in this study received further treatment after implantation of the 125I seed strand and PVS including TACE, sorafenib, or both. There were no TACE- or sorafenib-related deaths, possibly indicating that placement of the 125I seed strand/PVS combination can increase the safety of subsequent treatment in HCC patients with mPVTT. Therefore, patients may benefit from additional opportunities for further treatment.
The present study had some limitations. First, the sample size is relatively small. Prospective randomized controlled studies with larger numbers of patients are needed to provide higher-level evidence-based medicine. In addition, because portal vein pressure was not measured in this study, the variation in portal vein pressure caused by stent implantation could not be quantified or compared. Thirdly, immunotherapy was not administrated in the present study. Previous study has reported that radiotherapy can enhance the release of tumor antigens and has a proven synergism with immunotherapy.33 Whether endovascular brachytherapy of 125I seed could also enhance tumor responses with immunotherapy is worthy to be further investigated.
Fourthly, in the present study, an improvement of technique rather than a major advance was reported. In the future, the procedure of this surgery could be further simplified, for example, a novel portal vein stent with radioactive source on it may be a feasible solution. Finally, this was a single-center study, which may have affected the generalization of our results.
Conclusion
Our preliminary results suggest that a simplified approach of implanting the 125I seed strand inside the PVS is effective and feasible for treating patients with HCC and mPVTT. The procedure was less painful and had acceptable stent patency rates and satisfactory patient survival.
Ethics and Consent Statements
This study was approved by the Ethic Committee of Renji Hospital, Shanghai Jiao Tong University School of Medicine, and all patients provided written informed consent. The present study complies with the declaration of Helsinki.
Funding
This study was funded by Shanghai Health Committee (Grant Number 20204Y0041) and Shanghai Jiao Tong University (Grant Number YG2022QN024).
Disclosure
Liang Zhang and Jun Wang are co-first authors for this study. Jianguo Xia and Jialin Shen are co-correspondence authors for this study. The authors declare that they have no conflicts of interest in this work.
References
1. Zhang ZM, Lai ECH, Zhang C, et al. The strategies for treating primary hepatocellular carcinoma with portal vein tumor thrombus. Int J Surgery. 2015;20:8–16. doi:10.1016/j.ijsu.2015.05.009
2. Minagawa M, Makuuchi M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol. 2006;12(47):7561–7567. doi:10.3748/wjg.v12.i47.7561
3. Poddar N, Avezbakiyev B, He Z, et al. Hepatocellular carcinoma presenting as an incidental isolated malignant Portal vein thrombosis. J Gastrointestinal Cancer. 2012;43(3):486–489. doi:10.1007/s12029-010-9235-z
4. Zhang XP, Gao YZ, Chen ZH, et al. An Eastern Hepatobiliary Surgery Hospital/Portal Vein Tumor Thrombus Scoring System as an Aid to Decision Making on Hepatectomy for Hepatocellular Carcinoma Patients With Portal Vein Tumor Thrombus: a Multicenter Study. Hepatology. 2019;69:2076–2090. doi:10.1002/hep.30490
5. Katagiri S, Yamamoto M. Multidisciplinary treatments for hepatocellular carcinoma with major portal vein tumor thrombus. Surgery Today. 2014;44(2):219–226. doi:10.1007/s00595-013-0585-6
6. Li SH, Wei W, Guo RP, et al. Long-term outcomes after curative resection for patients with macroscopically solitary hepatocellular carcinoma without macrovascular invasion and an analysis of prognostic factors. Med Oncol. 2013:30. doi:10.1007/s12032-013-0696-3
7. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
8. Bruix J, Chan SL, Galle PR, et al. Systemic treatment of hepatocellular carcinoma: an EASL position paper. J Hepatol. 2021;75(4):960–974. doi:10.1016/j.jhep.2021.07.004
9. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/nejmoa1915745
10. Rizzo A, Ricci AD, Brandi G. Systemic adjuvant treatment in hepatocellular carcinoma: tempted to do something rather than nothing. Future Oncol. 2020;16(32):2587–2589. doi:10.2217/fon-2020-0669
11. Santoni M, Rizzo A, Kucharz J, et al. Complete remissions following immunotherapy or immuno-oncology combinations in cancer patients: the MOUSEION-03 meta-analysis. Cancer Immunol Immunother. 2023;72(6):1365–1379. doi:10.1007/s00262-022-03349-4
12. Rizzo A, Ricci AD, Brandi G. Atezolizumab in advanced hepatocellular carcinoma: good things come to those who wait. Immunotherapy. 2021;13(8):637–644. doi:10.2217/imt-2021-0026
13. Galle PR, Forner A, Llovet JM, et al. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236.
14. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–750. doi:10.1002/hep.29913
15. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi:10.1016/S0140-6736(18)30010-2
16. Omata M, Cheng AL, Kokudo N, et al. Asia–Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317–370. doi:10.1007/s12072-017-9799-9
17. Luo J, Yan Z, Liu Q, et al. Endovascular placement of iodine-125 seed strand and stent combined with chemoembolization for treatment of hepatocellular carcinoma with tumor thrombus in main portal vein. J Vascular Int Radiol. 2011;22(4):479–489. doi:10.1016/j.jvir.2010.11.029
18. Luo JJ, Zhang ZH, Liu QX, et al. Endovascular brachytherapy combined with stent placement and TACE for treatment of HCC with main portal vein tumor thrombus. Hepatol Int. 2016;10:185–195. doi:10.1007/s12072-015-9663-8
19. Li S, Li L, Li B, et al. Safety and efficacy of endovascular implantation of a portal vein stent combined with iodine-125 seed-strips followed by transcatheter arterial chemoembolization with sorafenib for the treatment of hepatocellular carcinoma with portal vein tumor thrombosis. Br J Radiol. 2020;93(1112):20190279. doi:10.1259/bjr.20190279
20. Sun JH, Zhou T, Zhu T, et al. Portal Vein Stenting Combined with Iodine-125 Seeds Endovascular Implantation Followed by Transcatheter Arterial Chemoembolization for Treatment of Hepatocellular Carcinoma Patients with Portal Vein Tumor Thrombus. Biomed Res. Int. 2016;2016:1–7. doi:10.1155/2016/3048261
21. Hu HT, Luo JP, Cao GS, et al. Hepatocellular Carcinoma With Portal Vein Tumor Thrombus Treated With Transarterial Chemoembolization and Sorafenib vs. 125Iodine Implantation. Front Oncol. 2021;11. doi:10.3389/fonc.2021.806907
22. Yang M, Fang Z, Yan Z, et al. Transarterial chemoembolisation (TACE) combined with endovascular implantation of an iodine-125 seed strand for the treatment of hepatocellular carcinoma with portal vein tumour thrombosis vs. TACE alone: a two-arm, randomised clinical trial. J Cancer Res Clin Oncol. 2014;140(2):211–219. doi:10.1007/s00432-013-1568-0
23. Bruix J, Sherman M. AASLD PRACTICE GUIDELINE Management of Hepatocellular Carcinoma: an Update. Hepatology. 2010. doi:10.1002/hep.20933
24. Lau WY, Lai ECH. Hepatocellular carcinoma: current management and recent advances. Hepatobiliary Pancreatic Dis Int. 2008;7(3):237–257.
25. Kokudo T, Hasegawa K, Matsuyama Y, et al. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol. 2016;65(5):938–943. doi:10.1016/j.jhep.2016.05.044
26. Xiang X, Lau WY, Wu ZY, et al. Transarterial chemoembolization versus best supportive care for patients with hepatocellular carcinoma with portal vein tumor thrombus: a multicenter study. Eur J Surgical Oncol. 2019;45(8):1460–1467. doi:10.1016/j.ejso.2019.03.042
27. Kudo M, Matsui O, Izumi N, et al. JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 Update by the liver cancer study group of Japan. Liver Cancer. 2014;3(3–4):458–468. doi:10.1159/000343875
28. Breder VV, Vogel A, Merle P, et al. IMbrave150: exploratory efficacy and safety results of hepatocellular carcinoma (HCC) patients (pts) with main trunk and/or contralateral portal vein invasion (Vp4) treated with atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in a global Ph III study. J Clin Oncol. 2021;39(36):4073. doi:10.1200/JCO.21.01440
29. Lee DS, Seong J. Radiotherapeutic options for hepatocellular carcinoma with portal vein tumor thrombosis. Liver Cancer. 2014;3(1):18–30. doi:10.1159/000343855
30. He M, Li Q, Zou R, et al. Sorafenib Plus Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin vs Sorafenib Alone for Hepatocellular Carcinoma with Portal Vein Invasion: a Randomized Clinical Trial. JAMA Oncol. 2019. doi:10.1001/jamaoncol.2019.0250
31. la Torre MA D, Buades-Mateu J, de la Rosa PA, et al. A comparison of survival in patients with hepatocellular carcinoma and portal vein invasion treated by radioembolization or sorafenib. Liver Int. 2016;36(8):1206–1212. doi:10.1111/liv.13098
32. Li S, Guo JH, Lu J, et al. I125 irradiation stent for treatment of hepatocellular carcinoma with portal vein thrombosis: a meta-analysis. Cancer/Radiotherapie. 2021;25(4):340–349. doi:10.1016/j.canrad.2020.12.003
33. Di Federico A, Rizzo A, Carloni R, et al. Atezolizumab-bevacizumab plus Y-90 TARE for the treatment of hepatocellular carcinoma: preclinical rationale and ongoing clinical trials. Expert Opin Investig Drugs. 2022;31(4):361–369. doi:10.1080/13543784.2022.2009455
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.