Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Development and Validation of a Vascular Endothelial Growth Factor A-associated Prognostic Model for Unresectable Hepatocellular Carcinoma
Received 21 December 2022
Accepted for publication 26 January 2023
Published 5 February 2023 Volume 2023:10 Pages 139—156
DOI https://doi.org/10.2147/JHC.S399299
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr David Gerber
Kun He,* Xinyu Liu,* Zelong Yang
Department of Hepatobiliary Surgery, Xijing Hospital, Fourth Military Medical University, Xi’an, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zelong Yang, Department of Hepatobiliary Surgery, Xijing Hospital, Fourth Military Medical University, No. 15, Changle West Road, Xi’an, People’s Republic of China, Tel +86 17795714179, Email [email protected]
Purpose: High serum vascular endothelial growth factor (VEGF) levels have been identified as an independent risk factor for hepatocellular carcinoma (HCC). We aimed to construct a VEGF-included prognostic model to accurately perform individualized predictions of survival probability for patients with unresectable HCC.
Patients and Methods: From October 2018 to March 2021, 182 consecutive newly diagnosed patients with unresectable HCC were retrospectively enrolled. Baseline serum VEGF-A and other characteristics were collected for all patients. Univariate Cox regression analysis and LASSO regression model were applied to develop the prognostic model, enhanced bootstrap method with 100 replicates was performed to validate its discrimination and calibration. We compared the final model with China Liver Cancer (CNLC) stage, American Joint Committee on Cancer (AJCC) stage, Barcelona Clinic Liver Cancer (BCLC) stage, and the model without the “VEGF”. Finally, the established model was stratified by age.
Results: The VEGF-associated prognostic model we established has high accuracy with an overall C-index of 0.7892 after correction for optimistic estimates. The area under the curve (AUC) of the time-dependent receiver operating characteristic (ROC) curves at 6-month, 1-year, and 2-year after correction were 0.843, 0.860, 0.833, respectively, and the calibration of the model was 0.1153, 0.1514, and 0.1711, respectively. The final model showed significant improvement in predicting OS when compared to the other models according to Harrell’s C-index, The AUC of the time-dependent ROC, area under the decision curve analysis (AUDC), integrated discrimination improvement (IDI), and continuous net reclassification index (NRI).
Conclusion: The VEGF-associated prognostic model may help to predict the survival probabilities of HCC patients with favorable performance and discrimination. However, further validation is required since we only verified this model using internal but not external data.
Keywords: prognostic model, hepatocellular carcinoma, vascular endothelial growth factor, transcatheter arterial chemoembolization, sorafenib
Introduction
Liver cancer is the second leading cause of cancer-related mortality worldwide, and the most common type is hepatocellular carcinoma (HCC).1,2 The 5-year survival rate for HCC patients is below 50% because of delayed diagnosis, postoperative recurrence, and distant metastasis.3 Most patients with HCC are initially diagnosed at the advanced stage, leading to an inability to perform surgical resection.4,5
Transcatheter arterial chemoembolization (TACE) was recommended as the standard therapy for patients with intermediate-stage HCC according to the guidelines of the European Association for the Study of Liver (EASL) and the American Association for the Study of Liver Disease (AASLD).6,7 Regrettably, the median overall survival (OS) for these patients varies widely and ranges from 13 to 43 months,8 at the same time, patients with poor local control after receiving TACE had a significant decrease in OS, so satisfactory therapeutic results cannot be achieved.9 Hence, investigators have tried to combine TACE with other treatment modalities to increase the survival benefit for patients with advanced HCC. Sorafenib, an oral multikinase inhibitor, which targets multiple receptor tyrosine kinases including platelet-derived growth factor receptor (PDGFR) and vascular endothelial growth factor receptor (VEGFR), has been proven to improve the survival of patients with advanced HCC significantly.10,11 However, sorafenib-related adverse events and resistance diminish its survival benefits.12 A similar situation also occurred when patients received regorafenib.13
Previous studies found that serum VEGF levels increased in HCC patients after the TACE procedure.14,15 In addition, elevations in circulating VEGF were related to poor prognosis in patients with HCC.16 Thus, the researchers compared the survival benefit of TACE plus sorafenib and TACE monotherapy in patients with advanced HCC. Some studies have suggested that the combination therapy of TACE-Sorafenib significantly prolongs the OS of patients compared with TACE alone.17–21 Yet two trials demonstrated that the TACE plus sorafenib treatment only improved time to progression (TTP) and progression-free survival (PFS) but did not significantly prolong OS.22,23 Interestingly, the SPACE study revealed that TACE plus sorafenib failed to improve OS and TTP significantly compared to TACE alone.24 In recent years, immune checkpoint inhibitors (ICIs) have played a critical role in treating advanced HCC. Despite the disappointing monotherapy efficacy of ICIs in HCC, promising results have been obtained with the combination treatment of the antiangiogenic agent and the PD-L1 inhibitor. The IMbrave150 trial showed that atezolizumab-bevacizumab achieved significant survival benefits in OS and PFS compared to sorafenib. Even so, such therapeutic strategy could be beneficial only in a proportion of patients with HCC.25,26 It can be concluded that the survival benefit for advanced HCC patients is highly heterogeneous whether receiving TACE or TACE-Sorafenib. Therefore, accurately estimating the survival of HCC patients receiving these two treatment modalities remains a challenge.
An ideal prognostic model requires an individualized and accurate prediction of the patient’s probability of survival for some time to come. However, the current risk scores and models widely applied in clinical work, such as Barcelona Clinical Liver Cancer (BCLC) stage B,27 hepatoma arterial embolization prognostic (HAP) score,28 and “four-and-seven” criteria,29 which are used for risk stratification in HCC patients but unable to provide detailed information on individualized estimation of survival. In addition, to our knowledge, few studies have integrated two treatment modalities (TACE and TACE-Sorafenib) to establish a prognostic model to estimate the survival of patients with advanced HCC.
Thus, we performed this retrospective study to (i) integrate VEGF and other clinical characteristics to develop a clinical prognostic model for OS prediction; (ii) verify the model internally; (iii) compare the final model with the CNLC stage, AJCC stage, BCLC stage, and the model without “VEGF”; (iv) stratify patients by risk using the established prognostic model.
Materials and Methods
Patients
From October 2018 to March 2021, 182 consecutive patients with newly diagnosed unresectable HCC were enrolled in the First Affiliated Hospital of Air Force Medical University and underwent initial treatment with either TACE monotherapy or TACE plus sorafenib. HCC was diagnosed according to histological examination or imaging criteria, based on practice guidelines established by the AASLD or the EASL.6,7 The inclusion criteria were: (i) an age older than 18 years; (ii) the Eastern Cooperative Oncology Group performance status (ECOG PS) was ≤1; (iii) patients were treated with TACE or TACE plus sorafenib after initial diagnosis of unresectable HCC; (iv) Child-Pugh score ≤7; (v) at least one measurable lesion larger than 1 cm in diameter. The exclusion criteria were: (i) the patients who have received any regional or systemic therapies previously, including ablation, resection, radiation, TACE, or targeted therapy; (ii) spontaneous tumor rupture before treatment; (iii) concomitant with any other malignancies; (iv) contraindications of sorafenib or TACE therapy; (v) liver transplantation or partial hepatectomy after one or more cycles of TACE due to tumor shrinkage or any other reasons. The protocol of the present study was performed according to the Declaration of Helsinki (as revised in 2013) and approved by the Institutional Review Board of Xijing Hospital of Fourth Military Medical University (KY20222265-F-1).
Treatment Protocol
Before the first TACE session, the baseline imaging profile was obtained to evaluate the tumor burden of patients regardless of the treatment modality received (TACE alone or TACE-Sorafenib).
TACE procedure was performed as follows: a 5F-RH arterial catheter was selectively inserted into the hepatic artery to recognize the location, the number of tumors, size, and blood supply of the tumors. Firstly, 1.0g of 5-fluorouracil and 50mg of lobaplatin were injected into the proper hepatic artery through the catheter for infusion chemotherapy. Subsequently, a microcatheter was super-selectively inserted into the segmental or subsegmental tumor-feeding vessels. Finally, DC Bead loaded with 40 mg of epirubicin was injected into the feeder’s vessels for embolization. All procedures were performed by investigators with more than 10 years of experience. 4–6 weeks after the first TACE session, a magnetic resonance imaging (MRI) or contrast agent-enhanced computed tomography (CT) of the abdomen and laboratory examinations were conducted to evaluate the necessity of a consecutive TACE treatment. The second TACE procedure was scheduled if intrahepatic residual viable tumors were shown in follow-up imaging. TACE was discontinued if there was no residual tumor in the imaging data. Patients were re-evaluated by receiving the aforementioned laboratory examinations and the imaging (CT or MRI) at 4-week and 8-week intervals, respectively.
The TACE procedures, types of drugs, and subsequent periodic evaluation for the patients administrated with TACE-Sorafenib therapy were consistent with those described for the patients who received TACE alone. Patients initially underwent sorafenib (Bayer Healthcare, Leverkusen, Germany) at 400 mg twice daily in 3–7 days after the first TACE session. Dose reduction (from 400 mg/day to 400 mg every other day) or treatment interruption owing to sorafenib-related adverse events (AEs) was allowed. Continuing the sorafenib treatment was encouraged unless unacceptable drug toxicity or unmanageable disease progression occurred. To protect liver function, sorafenib was discontinued for 3 days before each on-demand TACE.
Outcomes
The primary endpoint of our study was OS, defined as the date of HCC diagnosis to the date of death due to any cause or to their last follow-up. The secondary endpoint was progression-free survival (PFS), defined as the date of HCC diagnosis to imaging progression based on the modified Response Evaluation Criteria in the Solid Tumors (mRECIST) criteria,30 or to death, or the last follow-up.
Imaging Assessment
Two radiologists (S.W.Z. and L.C.) measured target lesions (n≤ 2) and assessed tumor response through the contrast-enhanced CT or MRI based on the mRECIST criteria. They have no access to the other clinical information of patients, and any inconsistencies between their results were addressed by achieving consensus. Objective response rate (ORR) was defined as the proportion of patients achieving the best response of complete response (CR) or partial response (PR). Disease control rate (DCR) was defined as the proportion of patients achieving a complete response, partial response, or stable disease (SD). AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events 4.0.31 Dose reductions or discontinuation due to AEs and the duration of sorafenib therapy were recorded for each patient who received TACE-Sorafenib.
Serum VEGF-A Assay and Data Collection
Peripheral venous blood samples (Approximately 5mL) were obtained from patients with HCC before the first TACE session and stored at room temperature (37°C) until use, then drawn into a serum separator tube and centrifuged at 3500 rpm for 10 minutes. The supernatant was removed and frozen at −20°C for storage until analysis. Concentrations of serum VEGF-A were detected by using a Vascular Endothelial Growth Factor Assay Kit (chemiluminescence) and JR-1 Chemiluminescent Immunoassay Analyzer (Shandong Weigao Group Medical Polymer Co., Ltd., Weihai, China) based on the manufacturer’s instructions.
To conduct data analyses, baseline characteristics, diagnostic, and treatment information of patients with HCC were collected, including the following: gender, age, ECOG PS, etiologic cause, Child-Pugh score, tumor size, BCLC stage, number of tumors, portal vein tumor thrombus (PVTT), extrahepatic spread (EHS), ascites, alpha-fetoprotein (AFP), total bilirubin, alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), neutrophil-lymphocyte ratio (NLR), and VEGF. There were no missing data for all patients at baseline.
Statistical Analysis
Statistical analyses were conducted using R version 4.1.1 (The R Foundation for Statistical Computing) and SPSS version 26.0 (IBM Corp). Continuous variables were expressed as mean ± standard deviations (SDs) or median (interquartile range), and categorical variables were expressed as frequencies with percentages. Differences between the two treatments were compared with the χ2 or Fisher exact test for categorical variables and t-tests or Mann–Whitney U-test for continuous variables. Univariate and multivariate Cox regression was conducted to determine whether VEGF was an independent prognostic factor for OS or PFS. Kaplan–Meier curves were drawn, and Log rank tests were used to compare the survival of patients with high and low VEGF. Maximally selected rank statistics were performed to obtain the optimal cutoff for baseline VEGF,32 which showed the most significant association with OS. The median was recognized as the cutoff value for other continuous laboratory variables: 400ng/mL for AFP, 17.5μmol/L for total bilirubin, 122.5 U/L for ALP, and 2.94 for NLR.
Development of the Prognostic Model
Before the variable screening, the relationship between candidate continuous variables and each relative hazard of death was evaluated with 3-knot restricted cubic splines. The non-linearity was tested, and the continuous variable was transformed into a categorical variable when the p value was <0.05. Subsequentially, univariate Cox regression was performed to assess the prognostic value of candidate variables. Those with a p value <0.05 were included in the least absolute shrinkage and selection operator (LASSO) regression model. The optimal penalization coefficient lambda and variables entered into the final prediction model were identified with 10-fold cross-validation to improve prediction performance. Finally, the Cox regression model was used to refit the model to obtain the coefficients for each variable. The total number of primary endpoint events (OS) reached 92, and we took into account a ratio of 10 events per variable.
Validation of the Prognostic Model
We internally validated the final model using an enhanced bootstrap method described by Harrell,33 which calculates the optimism corrected value by correcting for the overestimation of model performance, such that unbiased estimates could be obtained. The predictive performance of the model is based on the following evaluation metrics: (1) discrimination: assessed by the overall C-index and area under the curve (AUC) of time-dependent receiver operating characteristic (ROC) curves. (2) calibration: the calibration curves with 100 bootstrap resamples based on different time points were drawn to evaluate the degree to which the predicted probability is consistent with the actual observed outcomes. (3) model comparison: the new model without the variable “VEGF” was compared with the original final model by Harrell’s C-index, The AUC of the time-dependent ROC, area under the decision curve analysis (AUDC), integrated discrimination improvement (IDI), and continuous net reclassification index (NRI). These tests were exploited to identify changes in the model’s predictive ability and the contribution of baseline serum VEGF levels to the model.
Risk Stratification
Risk stratification was performed according to the risk scores of each patient by the X-Tile program,34 which divided the patients into low-, medium-, and high-risk subgroups, and their survival differences were subsequently assessed.
Results
Patient Characteristics
After screening for inclusion and exclusion criteria, a total of 182 patients who underwent TACE alone (n= 83) or TACE-Sorafenib (n= 99) were included in the study (Figure 1). There were no statistical differences in baseline demographics and clinical characteristics between patients receiving the two treatment modalities (Table 1). Chronic hepatitis B virus (HBV) infection was the major etiology of HCC (75.3%).
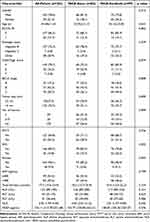 |
Table 1 Baseline Demographics and Clinical Characteristics in All-Patient |
 |
Figure 1 The flowchart of the study. |
The median follow-up time was 20.0 (1.3–35.5) months for all patients, 18.5 (1.3–35.4) months for the patients treated with TACE alone, and 20.0 (3.0–35.5) months for the patients treated with TACE-Sorafenib. The median OS for all patients was 17.0 (95% CI = 11.8–22.2) months. The median duration of sorafenib therapy was 6.7 (8.2–13.8) months. The objective response rate (ORR) was 69.7%, and the disease control rate (DCR) was 93.9%.
Sorafenib-Related Adverse Events
The adverse events (AEs) due to sorafenib in patients who received TACE-Sorafenib were listed in Table S1. 76 (76.8%) of the 99 patients experienced a total of 104 AEs. The majority of AEs were hand-foot skin reactions (54.5%), diarrhea (24.2%), and rashes (5.1%). 49 (49.5%) patients had dose reductions or temporary drug interruptions. No treatment-related deaths occurred during TACE-Sorafenib therapy.
Prognostic Value of VEGF for Unresectable HCC
We analyzed the prognostic value of baseline VEGF and other variables in patients with unresectable HCC using univariate and multivariate Cox regression.
Univariate analysis demonstrated that high serum VEGF was associated with poorer survival (Table 2). The median OS of patients with low and high VEGF was not reached (NR) and 10.1 months in all-patient (Figure 2A), 21.8 and 6.1 months in the TACE group (Figure 2B), and NR and 16.7 months in the TACE-Sorafenib group (Figure 2C). Multivariate analysis revealed that VEGF was an independent prognostic factor for OS in all-patient, the TACE group, and the TACE-Sorafenib group (Table 3).
 |
Table 2 Identification of Prognostic Factors for OS in All-Patient, the TACE Group, and the TACE-Sorafenib Group Based on Univariate Cox Regression |
 |
Table 3 Identification of Independent Prognostic Factors for OS in All-Patient, the TACE Group, and the TACE-Sorafenib Group Based on Multivariate Cox Regression |
Based on univariate analysis, we found that high serum VEGF was correlated significantly with worse PFS in all-patient and the TACE-Sorafenib group (Table S2). The median PFS of patients with low and high VEGF were 13.3 and 5.6 months in all all-patient (Figure 2D) and 13.1 and 5.3 months in the TACE-Sorafenib group (Figure 2E). Multivariate analysis showed that VEGF was an independent prognostic factor for PFS in all-patient and the TACE-Sorafenib group (Table S3). While in the TACE group, there was no significant difference in PFS between HCC patients with high and low VEGF (Figure 2F).
Development and Validation of the VEGF-Associated Model
The total number of primary events that occurred in all patients was 92. By the results of the restricted cubic splines, VEGF (p=0.02) and ALP (p=0.001) presented a non-linear profile, while NLR presented a linear profile (p=0.20) (Figure 3A-C). Thus, we converted ALP and VEGF into dichotomous variables according to the respective cut-off values that were previously determined (122.5 U/L for ALP and 172.48pg/mL for VEGF).
After identifying prognostic factors for OS based on univariate Cox regression analysis (Table 2), we used the LASSO regression (Figure 3D and E) to conduct variable screening, and a Cox regression was performed to refit the model immediately followed. The final model contained eight elements, treatment modalities (TACE or TACE-Sorafenib), number of tumors (1, 2–3, >3), PVTT (Yes, No), EHS (Yes, No), AFP (>400, ≤400 ng/mL), ALP (>122.5, ≤122.5U/L), NLR (Continuous data), and VEGF (>172.48, ≤172.48pg/mL). The HRs and coefficients are shown in Table 4. Accordingly, we created a nomogram for predicting the survival probabilities of 6-month, 1-year, and 2-year in patients with HCC (Figure 4). We provide a specific example of the point system, patient A, who has HCC and PVTT, will earn 35 points. Furthermore, he has no symptoms of EHS (0 points) and has a net count of four tumors (50 points). Patient A received treatment of sorafenib (0 points) after the first TACE treatment, and his laboratory test results showed a baseline AFP of 193 ng/mL (0 points), an ALP of 253 U/L (24 points), an NLR of 4 (25 points) and a VEGF of 182.4pg/mL (37 points); according to the nomogram, patient A totaled 171 points, which suggest that the patient’s 6-month, 1-year, and 2-year survival probabilities were 0.81, 0.38, and 0.11, respectively.
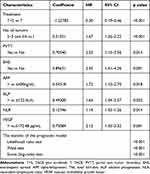 |
Table 4 Variables Included in the Final Model After LASSO Regression Analysis |
Upon calibration of the final model, after 100 bootstrap resamples, the brier score of the final model before and after correction at 6-month, 1-year, and 2-year intervals were 0.1071 (Figure 5A) and 0.1153, 0.1367 (Figure 5B) and 0.1514, 0.1516 (Figure 5C) and 0.1711, respectively. As for discrimination, we found that the overall C-index of the model before and after correction for optimistic estimates was 0.8083 and 0.7892, respectively. The AUC of the final model before and after correction for optimistic estimates at 6-month, 1-year, and 2-year were 0.861 and 0.843, 0.882 and 0.860, 0.862 and 0.833 (Figure 5D), respectively.
Comparisons of the predictive power of different Models
We compared the power to predict OS of the VEGF-associated model with that of CNLC stage, TNM stage (AJCC), BCLC stage, and the model without the “baseline VEGF” variable in 6-month, 1-year, and 2-year. The results are shown in Table 5 and Figure 6. Both the Harrell’s C-index and the time-dependent AUC of the ROC of the final model at different cutoff times were higher than that of CNLC stage (Figure 6A), AJCC stage (Figure 6B), BCLC stage (Figure 6C) and the model without the “baseline VEGF” (Figure 6D). Similarly, the VEGF-associated model also achieved a higher area under the decision curve analysis (AUDC) than other models in 6-month (Figure 6E), 1-year (Figure 6F), and 2-year (Figure 6G).
 |
Table 5 Comparison of the Predictive Power Between the Different Model |
In addition, we evaluated the improvement in the performance and predictive ability of the final model compared to other models by using integrated discrimination improvement (IDI) and continuous net reclassification index (NRI) (Table 5). According to the IDI, the final model showed significant improvement in predicting 1-year and 2-year OS compared to AJCC stage (Figure S1A and B) and BCLC stage (Figure S1C and D). According to the continuous NRI, the final model performed better for predicting OS of all time points compared to the model without the “baseline VEGF” (Figure S1E-G) and better for predicting 1-year and 2-year OS compared to CNLC stage (Figure S1H and I), AJCC stage (Figure S1A and B), and BCLC stage (Figure S1C and D).
Model Stratification and Subgroup Analysis
We scored each patient in the dataset based on the nomogram mentioned above. We stratified the patients into three stratification groups: low-, medium-, and high-risk, based on the optimal cutoff value set by the X-Tile program. The low-risk group was defined as patients with a total point ≤148, the medium-risk group with a net point ranging from 148 to 215, and the high-risk group with a total point >215. The number of patients in the three groups was 109 (59.9%), 49 (26.9%), and 24 (13.2%), respectively. The median OS of the low-, medium-, and high-risk groups were not reached (NR), 8.9 months and 5.3 months, respectively (Figure 7A). The median PFS of the low-, medium-, and high-risk groups were 15.2 months, 5.6 months, and 3.4 months, respectively (Figure 7B).
Subsequently, we analyzed the performance of the final model stratification in a subgroup of patients aged ≤55 or >55 years. The result indicated that the model stratification was consistently robust and capable in the subgroup analysis (Figure 7C-F).
Discussion
HCC accounts for more than 80% of primary liver cancer cases.3 It acts as a tremendous global threat to human health.35 Although technological advances have changed the landscape of HCC treatment, the long-term prognosis of patients remains unsatisfactory, especially for those with unresectable HCC. For those patients who are ineligible for resection, TACE is a standard locoregional treatment for intermediate-stage HCC. It can increase their survival significantly, even though it is a palliative option.36 Sorafenib is the first multikinase inhibitor approved by FDA to treat advanced HCC, and it could inhibit neoangiogenesis by blocking VEGF receptors. According to the EASL Clinical Practice Guidelines,6 TACE was the only recommended standard treatment for intermediate-stage HCC (BCLC stage B), and sorafenib was the frontline treatment option for advanced HCC (BCLC stage C). However, based on the CNLC staging system,37 TACE-Sorafenib combination therapy was recommended in CNLC stage IIb patients (belongs to stage B in the BCLC system) because of the reported prolonged PFS in the TACTICS Trial.23 In addition, some studies have revealed a significant survival benefit in HCC patients with PVTT (equivalent to BCLC stage C) who received TACE,38–40 the TACE in combination with system therapy was also allowed in patients with advanced HCC, which is recognized as a contraindication for TACE in Western countries.37,41
Regarding the OS of HCC patients, there were two main reasons for the difference between our results and the TACTICS trial.23 Firstly, the inclusion criteria were different between the two studies. The tumors of patients included in the TACTICS trial were confined to the liver, while in our study, we also included patients with PVTT and patients with BCLC stage B. A retrospective study reported that TACE-Sorafenib could significantly prolong the OS in HCC patients with PVTT compared to those with TACE administration.21 Secondly, the time point of initial treatment with sorafenib was also different between the two studies. In the TACTICS trial, patients received pretreatment with sorafenib for 2–3 weeks before the first TACE session and continued to use it after TACE. However, treatment with sorafenib was scheduled after the initial TACE in our study; the protocol was the same as the retrospective study mentioned above.21 The difference in the treatment order may explain the inconsistency between the results of the two studies. Of course, this speculation needs further confirmation. For PFS, our results are consistent with a phase-III trial,42 and the calculated DCR and ORR trends are also similar to the findings of the SPACE trial.24 Notably, the high DCR and ORR in all patients were probably due to the super-selective nature of the TACE treatment that was performed.
VEGF and its receptors are the most well-known and fully discovered regulators of angiogenesis in HCC. In mammals, the VEGF family comprises five members: VEGF-A, B, C, D, and placental growth factor.43 Circulating VEGA levels were identified as an independent OS predictor of HCC,44 and were associated with HCC growth and metastasis.45 A previous study showed that the decrease in serum VEGF after treatment with sorafenib was significantly correlated with OS and PFS.46 Nevertheless, by secreting VEGF, TACE induces an angiogenic response, resulting in new blood vessel growth and, eventually, tumor procession and recurrence.47 Thus, there was believed to be a close association between an elevated serum VEGF level and distant metastasis and unfavorable outcomes in HCC patients treated with TACE.48 We also showed that baseline VEGF was an independent prognostic factor for OS in unresectable HCC patients treated with TACE or TACE-Sorafenib. Therefore, to illustrate the clinical value of VEGF, we developed a VEGF-included clinical prognostic model via LASSO regression to predict HCC-related death and validated the model internally. As a result of our study, we were able to confirm the robustness of the scoring algorithm and its prognostic value.
Our novel multiparameter model incorporates eight factors. It has consistently been supported that the number of tumors is an important prognostic factor for HCC.49 Similarly, PVTT and EHS were also associated with a dismal prognosis.50 Laboratory parameters such as VEGF and AFP were the highest-ranked factors. The tumor burden of HCC may be quite heterogeneous at the unresectable stage, and AFP concentration can influence prognosis.51 Serum AFP levels after the treatment of oxaliplatin chemotherapy or sorafenib often predict an improved therapeutic response and prolonged OS,52,53 but the causal relationship is not known clearly. It was shown that AFP was inhibited at 0.1–1 µmol sorafenib in two AFP-positive human HCC cell lines.54 However, another study concluded that AFP expression in HCC cells did not change significantly after treatment with sorafenib, indicating that the decline in serum AFP level was most likely attributable to the tumor regression after sorafenib intervention.55 The risk scoring nomogram also incorporated NLR. High NLR was predictive of poor tumor response and resulted in shorter PFS after treatment with TACE. In addition, the tumors of patients with HCC with high NLR were more aspherical, as shown by imaging results. This finding indicates that they may be more invasive and metastatic.56 In a previous study, the magnitude of benefit produced by sorafenib was greater in patients with lower NLR.52 This may be because, in the context of chronic inflammation caused by HCC, NLR is a systemic indicator of immune status. It is possible that sorafenib could provide better survival benefits against tumors with higher adaptive immune infiltrations or lower innate immune infiltrations. The specific mechanisms of NLR’s action in HCC remain largely unknown, but its mechanisms may be related to tumor immune microenvironment and inflammatory factors. However, the coefficient of NLR (≈0.13) and HR value (≈1.14) in our final model are the smallest among all factors, so it is reasonable that the predictive effect of NLR in patients with HCC is limited. An elevated serum ALP value usually indicates liver damage in patients with cholestasis or parenchymal disease.57 Several possible mechanisms have been proposed for how ALP enhances cancer cell proliferation, invasion, and metastasis.58,59 A meta-analysis included 27 studies and found that higher serum ALP levels in HCC patients may be associated with a worse prognosis.60 Abnormally heightened ALP level was found to have predicted a worse survival rate.61 Here, we again confirmed the potential roles of ALP in predicting the prognosis of patients with HCC.
To validate our VEGF-included model, the enhanced bootstrap resampling was applied. Our predictive model was discovered to be consistently robust regardless of the length of prognostic time, with an overall C-index of 0.7892 and Brier scores lower than 0.2. Additionally, the AUC of ROC at 6-month, 1-year, and 2-year exceeded 0.8. Based on available objective clinical parameters and statistical methods, our survival prediction model incorporates relevant, statistically significant factors to provide accurate predictions for advanced patients with HCC treated with TACE alone or TACE-Sorafenib. Several prediction models have been introduced to predict the survival of patients undergoing TACE monotherapy,62–64 and those undergoing TACE plus sorafenib treatment.65,66 Encouragingly, our model showed demonstrated a better ability to discriminate than models. The largest AUC in the models mentioned above is 0.851, with the highest C-index of only 0.68. Our model evaluation index is higher than these values. In addition, our model was mainly constructed based on laboratory and imaging indicators, so our model was relatively simple and had more clinical applicability than many models or signatures constructed by various gene expressions or modifications.67,68
To further evaluated the prediction performance of the VEGF-associated model, we compared it with CNLC stage, AJCC stage, BCLC stage, and the model without “VEGF”. The results showed that the performance of the final model was superior to the other models at each time point, and we confirmed that VEGF is indispensable to this model. Moreover, our current model includes two treatment options, and clinicians can pre-judge the prognosis by assuming clinical parameters to aid in planning treatment strategies for patients with inoperable HCC. Notably, an ideal prognosis predictive model requires not only appropriate development methods but also conciseness for clinical application.68 Thus, we stratified the cases into three prognostic strata according to scores drawn by the nomogram to meet this demand. Significant differences in survival were demonstrated among patients divided into three categories, with the median OS of NR, 8.9 months, and 5.3 months, respectively. Using the same prognostic index, PFS was also found to be significant. In subgroup analyses categorized by age, the survival rate of the three strata by scores was consistently discriminative. The accuracy of risk stratification in patients treated with TACE or TACE-Sorafenib may lead to more appropriate clinical management, such as enabling more effective communication with the patient’s family, selectively filtering patients who still have a poor prognosis after TACE plus sorafenib treatment, decreasing the unnecessary expenditure of medical resources, and reduce the patients’ medical financial burden. Furthermore, we can first adjust variables within the model to attempt to improve the prognosis of patients who are otherwise predicted to have a poor prognosis to a relatively satisfactory prognosis.
The current study is characterized by the following strengths and novelties: (i) based on the hypervascular nature of the vast majority of HCCs and the importance of VEGF for prognosis, we included VEGF and constructed a completely novel prognostic model for HCC, which was validated to perform favorably as compared to previous models, and VEGF was proved to be essential for the model; (ii) we established the first model specifically for patients receiving the above two treatment modalities. It is available to a broader range of objects and consists of continuous and other categorized factors to provide individualized and stratified survival estimates for clinical trials and clinical practice. (iii) This study is the first to validate the prognostic significance of VEGF in patients with advanced hepatocellular carcinoma after receiving TACE in combination with Sorafenib. Immune checkpoint inhibitors in combination with VEGF inhibitors can overcome primary drug resistance that may occur after immune checkpoint inhibitors treatment,69 while it is unknown whether VEGF has an indicative role in the treatment of immune checkpoint inhibitors. We believe that in the years to come, with deepening research of the heterogeneity of the tumor itself and the differences in patient sensitivity to drugs, ICIs-based therapy combined with other modalities, such as TACE, sorafenib, and regorafenib, will be the mainstream treatment for patients with intermediate to advanced HCC. Our study may be instructive in this area.
The study that we have conducted has some limitations that must be noted. Firstly, this is a retrospective single-center study. Under this premise, the retrospective confounding bias is inevitable. Only basal variables are accounted for in the current model, and other clinical or laboratory parameters that may affect HCC prognosis are not included. For instance, alterations in the microenvironment of the tumor were acquired after receiving TACE or sorafenib treatment,70,71 and it contributes to predicting the prognosis and therapeutic refractoriness. However, our model did not incorporate factors related to the tumor microenvironment, such as CD4+/CD8+ T cells72 or interleukin-6 and 873 into the model. Including these factors could help optimize the predictive power of this model, despite their potential risk towards parameterizing the model. Secondly, the majority of HCC patients in our study were HBV-related. However, in other countries, HCV-related or NAFLD-related HCC is more common.74 Differences in etiology may result in differences in clinical symptoms, tumor burden, liver function, and some of the important factors in our model. In addition, all patients included in this study were Chinese; different ethnicity, social environment, and living habits may make our model not applicable to HCC patients from other countries or cultures. These limitations may impede our model’s scope of application, and how to increase its applicability will be one of the priorities of our subsequent studies. Thirdly, we only performed an internal validation because of the paucity of external data. It is, however, necessary to further validate and refine this model with external validation in another setting.
Conclusion
In conclusion, from 182 patients with HCC who underwent TACE alone or TACE plus sorafenib therapy, we constructed an easy-to-use prognostic model that incorporated laboratory parameters, image characters, and treatments. Because of the accessibility and availability of the factors found within this model, utilizing this scoring model to predict the clinical outcomes of patients and stratify patients is highly desirable. Further requirements for validating and optimizing this model with external data, especially for different etiologies, remain unmet.
Abbreviations
VEGF(R), vascular endothelial growth factor (receptor); HCC, hepatocellular carcinoma; TACE, transcatheter arterial chemoembolization; CNLC, China Liver Cancer; AJCC, American Joint Committee on Cancer; BCLC, Barcelona Clinic Liver Cancer; EASL, European Association for the Study of Liver; AASLD, American Association for the Study of Liver Disease; OS, overall survival; PDGFR, platelet-derived growth factor receptor; TTP, improved time to progression; PFS, progression-free survival; ICIs, immune checkpoint inhibitors; ECOG PS, Eastern Cooperative Oncology Group performance status; MRI, magnetic resonance imaging; CT, computed tomography; AEs, adverse events; mRECIST, modified Response Evaluation Criteria in the Solid Tumors; ORR, objective response rate; CR, complete response; PR, partial response; DCR, disease control rate; SD, stable disease; PVTT, portal vein tumor thrombus; EHS, extrahepatic spread; AFP, alpha-fetoprotein; ALP, alkaline phosphatase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; NLR, neutrophil-lymphocyte ratio; LASSO, least absolute shrinkage and selection operator; AUC, area under the curve; ROC, receiver operating characteristic; AUDC, area under the decision curve analysis; IDI, integrated discrimination improvement; NRI, net reclassification index.
Data Sharing Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Approval and Informed Consent
The protocol of the present study was performed according to the Declaration of Helsinki (as revised in 2013) and approved by the Institutional Review Board of Xijing Hospital of Fourth Military Medical University (KY20222265-F-1). The requirement of informed consent was exempted mainly based on the following reasons. This was a retrospective, non-intervention study, which did not interfere with the diagnosis and treatment and carried no additional risk to the patients. In order to protect the privacy of patients, clinical data were collected anonymously, and our results did not contain any information that may lead to patient identification.
Consent for Publication
All the authors confirm that the details of any images, videos, recordings, etc. can be published.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL., et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Llovet JM, Castet F, Heikenwalder M, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19(3):151–172. doi:10.1038/s41571-021-00573-2
3. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604. doi:10.1038/s41575-019-0186-y
4. Kloeckner R, Galle PR, Bruix J. Local and regional therapies for hepatocellular carcinoma. Hepatology. 2021;73(Suppl S1):137–149. doi:10.1002/hep.31424
5. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi:10.1016/s0140-6736(18)30010-2
6. Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150(4):835–853. doi:10.1053/j.gastro.2015.12.041
7. Yang JD, Roberts LR. Hepatocellular carcinoma: a global view. Nat Rev Gastroenterol Hepatol. 2010;7(8):209–249. doi:10.3322/caac.21660
8. Sangro B, Salem R. Transarterial chemoembolization and radioembolization. Semin Liver Dis. 2014;34(4):435–443. doi:10.1055/s-0034-1394142
9. Rou WS, Lee BS, Moon HS, Lee ES, Kim SH, Lee HY. Risk factors and therapeutic results of early local recurrence after transcatheter arterial chemoembolization. World j Gastroenterol. 2014;20(22):6995–7004. doi:10.3748/wjg.v20.i22.6995
10. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi:10.1056/NEJMoa0708857
11. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a Phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi:10.1016/s1470-2045(08)
12. Zhu YJ, Zheng B, Wang HY, Chen L. New knowledge of the mechanisms of sorafenib resistance in liver cancer. Acta Pharmacol Sin. 2017;38(5):614–622. doi:10.1038/aps.2017.5
13. Rizzo A, Nannini M, Novelli M, Dalia Ricci A, Scioscio VD, Pantaleo MA. Dose reduction and discontinuation of standard-dose regorafenib associated with adverse drug events in cancer patients: a systematic review and meta-analysis. Ther Adv Med Oncol. 2020;12:1758835920936932. doi:10.1177/1758835920936932
14. Chao Y, Wu C-Y, Kuo C-Y, et al. Cytokines are associated with postembolization fever and survival in hepatocellular carcinoma patients receiving transcatheter arterial chemoembolization. Hepatol Int. 2013;7(3):883–892. doi:10.1007/s12072-012-9409-9
15. Chan SL, Yeo W, Mo F, et al. A Phase 2 study of the efficacy and biomarker on the combination of transarterial chemoembolization and axitinib in the treatment of inoperable hepatocellular carcinoma. Cancer. 2017;123(20):3977–3985. doi:10.1002/cncr.30825
16. Kaseb AO, Hassan MM, Lin E, et al. V-CLIP: integrating plasma vascular endothelial growth factor into a new scoring system to stratify patients with advanced hepatocellular carcinoma for clinical trials. Cancer. 2011;117(11):2478–2488. doi:10.1002/cncr.25791
17. Cai R, Song R, Pang P, et al. Transcatheter arterial chemoembolization plus sorafenib versus transcatheter arterial chemoembolization alone to treat advanced hepatocellular carcinoma: a meta-analysis. BMC Cancer. 2017;17(1):714. doi:10.1186/s12885-017-3707-5
18. Zhang X, Wang K, Wang M, et al. Transarterial chemoembolization (TACE) combined with sorafenib versus TACE for hepatocellular carcinoma with portal vein tumor thrombus: a systematic review and meta-analysis. Oncotarget. 2017;8(17):29416–29427. doi:10.18632/oncotarget.15075
19. Geschwind J-F, Kudo M, Marrero JA, et al. TACE Treatment in Patients with Sorafenib-treated Unresectable Hepatocellular Carcinoma in Clinical Practice: final Analysis of GIDEON. Radiology. 2016;279(2):630–640. doi:10.1148/radiol.2015150667
20. Li J, Zhang F, Yang J, et al. Combination of individualized local control and target-specific agent to improve unresectable liver cancer managements: a matched case-control study. Target Oncol. 2015;10(2):287–295. doi:10.1007/s11523-014-0338-5
21. Zhu K, Chen J, Lai L, et al. Hepatocellular Carcinoma with Portal Vein Tumor Thrombus: treatment with Transarterial Chemoembolization Combined with Sorafenib—A Retrospective Controlled Study. Radiology. 2014;272(1):284–293. doi:10.1148/radiol.14131946
22. Li L, Zhao W, Wang M, et al. Transarterial chemoembolization plus sorafenib for the management of unresectable hepatocellular carcinoma: a systematic review and meta-analysis. BMC Gastroenterol. 2018;18(1):138. doi:10.1186/s12876-018-0849-0
23. Kudo M, Ueshima K, Ikeda M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69(8):1492–1501. doi:10.1136/gutjnl-2019-318934
24. Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: the SPACE trial. J Hepatol. 2016;64(5):1090–1098. doi:10.1016/j.jhep.2016.01.012
25. Rizzo A, Ricci AD, Gadaleta-Caldarola G, Brandi G. First-line immune checkpoint inhibitor-based combinations in unresectable hepatocellular carcinoma: current management and future challenges. Expert Rev Gastroenterol Hepatol. 2021;15(11):1245–1251. doi:10.1080/17474124.2021.1973431
26. Rizzo A, Ricci AD, Di Federico A, et al. Predictive Biomarkers for Checkpoint Inhibitor-Based Immunotherapy in Hepatocellular Carcinoma: where Do We Stand? Front Oncol. 2021;11:803133. doi:10.3389/fonc.2021.803133
27. Bolondi L, Burroughs A, Dufour J-F, et al. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32(4):348–359. doi:10.1055/s-0032-1329906
28. Kadalayil L, Benini R, Pallan L, et al. A simple prognostic scoring system for patients receiving transarterial embolisation for hepatocellular cancer. Ann Oncol. 2013;24(10):2565–2570. doi:10.1093/annonc/mdt247
29. Yamakado K, Miyayama S, Hirota S, et al. Subgrouping of intermediate-stage (BCLC stage B) hepatocellular carcinoma based on tumor number and size and Child–Pugh grade correlated with prognosis after transarterial chemoembolization. Jpn J Radiol. 2014;32(5):260–265. doi:10.1007/s11604-014-0298-9
30. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi:10.1055/s-0030-1247132
31. Rodriguez-Canales J, Parra-Cuentas E, Wistuba II. Diagnosis and Molecular Classification of Lung Cancer. Cancer Treat Res. 2016;170:25–46. doi:10.1007/978-3-319-40389-2_2
32. Hothorn T, Zeileis A. Generalized maximally selected statistics. Biometrics. 2008;64(4):1263–1269. doi:10.1111/j.1541-0420.2008.00995.x
33. de Sousa VML, Carvalho L. Heterogeneity in Lung Cancer. Pathobiology. 2018;85(1–2):96–107. doi:10.1159/000487440
34. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–7259. doi:10.1158/1078-0432.Ccr-04-0713
35. Komoll R-M, Hu Q, Olarewaju O, et al. MicroRNA-342-3p is a potent tumour suppressor in hepatocellular carcinoma. J Hepatol. 2021;74(1):122–134. doi:10.1016/j.jhep.2020.07.039
36. Fu Z, Li X, Zhong J, et al. Lenvatinib in combination with transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma (uHCC): a retrospective controlled study. Hepatol Int. 2021;15(3):663–675. doi:10.1007/s12072-021-10184-9
37. Xie D-Y, Ren Z-G, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020;9(4):452–463. doi:10.21037/hbsn-20-480
38. Pinter M, Hucke F, Graziadei I, et al. Advanced-stage hepatocellular carcinoma: transarterial chemoembolization versus sorafenib. Radiology. 2012;263(2):590–599. doi:10.1148/radiol.12111550
39. Chung GE, Lee J-H, Kim HY, et al. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology. 2011;258(2):627–634. doi:10.1148/radiol.10101058
40. Luo J, Guo R-P, Lai ECH, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol. 2011;18(2):413–420. doi:10.1245/s10434-010-1321-8
41. Kudo M, Matsui O, Izumi N, et al. JSH Consensus-Based Clinical Practice Guidelines for the Management of Hepatocellular Carcinoma: 2014 Update by the Liver Cancer Study Group of Japan. Liver Cancer. 2014;3(3–4):458–468. doi:10.1159/000343875
42. Meyer T, Fox R, Ma YT, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, Phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2(8):565–575. doi:10.1016/S2468-1253(17)30156-5
43. Morse MA, Sun W, Kim R, et al. The Role of Angiogenesis in Hepatocellular Carcinoma. Clin Cancer Res. 2019;25(3):912–920. doi:10.1158/1078-0432.CCR-18-1254
44. Lacin S, Yalcin S. The Prognostic Value of Circulating VEGF-A Level in Patients With Hepatocellular Cancer. Technol Cancer Res Treat. 2020;19:1533033820971677. doi:10.1177/1533033820971677
45. Jin-no K, Tanimizu M, Hyodo I, et al. Circulating vascular endothelial growth factor (VEGF) is a possible tumor marker for metastasis in human hepatocellular carcinoma. J Gastroenterol. 1998;33(3):376–382. doi:10.1007/s005350050099
46. Godin C, Bodeau S, Saidak Z, et al. Early decrease in serum amphiregulin or vascular endothelial growth factor levels predicts sorafenib efficacy in hepatocellular carcinoma. Oncol Rep. 2019;41(3):2041–2050. doi:10.3892/or.2018.6922
47. Lin Z-H, Jiang J-R, Ma X-K, et al. Prognostic value of serum HIF-1α change following transarterial chemoembolization in hepatocellular carcinoma. Clin Exp Med. 2021;21(1):109–120. doi:10.1007/s10238-020-00667-8
48. Shim JH, Park J-W, Kim JH, et al. Association between increment of serum VEGF level and prognosis after transcatheter arterial chemoembolization in hepatocellular carcinoma patients. Cancer Science. 2008;99(10):2037–2044. doi:10.1111/j.1349-7006.2008.00909.x
49. Horwitz E, Stein I, Andreozzi M, et al. Human and mouse VEGFA -amplified hepatocellular carcinomas are highly sensitive to sorafenib treatment. Cancer Discov. 2014;4(6):730–743. doi:10.1158/2159-8290.CD-13-0782
50. Liu X, Lu J, Zhang G, et al. A Machine Learning approach yields a multiparameter prognostic marker in liver cancer. Cancer Immunol Res. 2021;9(3):337–347. doi:10.1158/2326-6066.CIR-20-0616
51. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
52. Bruix J, Cheng A-L, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: analysis of two phase III studies. J Hepatol. 2017;67(5):999–1008. doi:10.1016/j.jhep.2017.06.026
53. Chou W-C, Lee C-L, Yang T-S, et al. Changes in serum α-fetoprotein level predicts treatment response and survival in hepatocellular carcinoma patients and literature review. J Formosan Med Assoc. 2018;117(2):153–163. doi:10.1016/j.jfma.2017.03.010
54. Carr BI, D’Alessandro R, Refolo MG, et al. Effects of low concentrations of regorafenib and sorafenib on human HCC cell AFP, migration, invasion, and growth in vitro. J Cell Physiol. 2013;228(6):1344–1350. doi:10.1002/jcp.24291
55. Chen T, Dai X, Dai J, et al. AFP promotes HCC progression by suppressing the HuR-mediated Fas/FADD apoptotic pathway. Cell Death Dis. 2020;11(10):822. doi:10.1038/s41419-020-03030-7
56. Schobert IT, Savic LJ, Chapiro J, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictors of tumor response in hepatocellular carcinoma after DEB-TACE. Eur Radiol. 2020;30(10):5663–5673. doi:10.1007/s00330-020-06931-5
57. Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med. 2000;342(17):1266–1271. doi:10.1056/NEJM200004273421707
58. Yu M-C, Chan K-M, Lee C-F, et al. Alkaline phosphatase: does it have a role in predicting hepatocellular carcinoma recurrence? J Gastrointest Surg. 2011;15(8):1440–1449. doi:10.1007/s11605-011-1537-3
59. Zhu Y, Xu D, Zhang Z, et al. A new laboratory-based algorithm to predict microvascular invasion and survival in patients with hepatocellular carcinoma. Int J Surg. 2018;57:45–53. doi:10.1016/j.ijsu.2018.07.011
60. Sun P, Chen S, Li Y. The association between pretreatment serum alkaline phosphatase and prognosis in hepatocellular carcinoma: a meta-analysis. Medicine. 2020;99(11):e19438. doi:10.1097/MD.0000000000019438
61. Chen C, Qiu H, Yao Y, et al. Comprehensive predictive factors for CalliSpheres® microspheres (CSM) drug-eluting bead-transarterial chemoembolization and conventional transarterial chemoembolization on treatment response and survival in hepatocellular carcinoma patients. Clin Res Hepatol Gastroenterol. 2021;45(2):101460. doi:10.1016/j.clinre.2020.05.008
62. Jia F, Wu B, Yan R, Li L, Wang K, Han D. Prediction Model for Intermediate-Stage Hepatocellular Carcinoma Response to Transarterial Chemoembolization. J Magn Reson Imaging. 2020;52(6):1657–1667. doi:10.1002/jmri.27189
63. Chen R-X, Gan Y-H, Ge N-L, et al. A new prediction model for prognosis of patients with intermediate-stage HCC after conventional transarterial chemoembolization: an internally validated study. J Cancer. 2019;10(26):6535–6542. doi:10.7150/jca.34064
64. Wang Q, Xia D, Bai W, et al. Development of a prognostic score for recommended TACE candidates with hepatocellular carcinoma: a multicentre observational study. J Hepatol. 2019;70(5):893–903. doi:10.1016/j.jhep.2019.01.013
65. Chu HH, Chun S-Y, Kim JH, et al. A prediction model for overall survival after transarterial chemoembolization for hepatocellular carcinoma invading the hepatic vein or inferior vena cava. Eur Radiol. 2021;31(6):4232–4242. doi:10.1007/s00330-020-07536-8
66. Li J-R, Wu M-J, Wang T, et al. A prognostic score model for predicting the survival benefits of patients undergoing sorafenib plus transarterial chemoembolization for hepatocellular carcinoma with portal vein invasion. Abdom Radiol. 2021;46(5):1967–1976. doi:10.1007/s00261-020-02897-6
67. Fako V, Martin SP, Pomyen Y, et al. Gene signature predictive of hepatocellular carcinoma patient response to transarterial chemoembolization. Int J Biol Sci. 2019;15(12):2654–2663. doi:10.7150/ijbs.39534
68. Tang Y, Wu Y, Xue M, Zhu B, Fan W, Li J. A 10-Gene Signature Identified by Machine Learning for Predicting the Response to Transarterial Chemoembolization in Patients with Hepatocellular Carcinoma. J Oncol. 2022;2022:3822773. doi:10.1155/2022/3822773
69. Di Federico A, Rizzo A, Carloni R, et al. Atezolizumab-bevacizumab plus Y-90 TARE for the treatment of hepatocellular carcinoma: preclinical rationale and ongoing clinical trials. Expert Opin Investig Drugs. 2022;31(4):361–369. doi:10.1080/13543784.2022.2009455
70. Xia S, Pan Y, Liang Y, Xu J, Cai X. The microenvironmental and metabolic aspects of sorafenib resistance in hepatocellular carcinoma. EBioMedicine. 2020;51:102610. doi:10.1016/j.ebiom.2019.102610
71. He Q, Yang J, Jin Y. Development and Validation of TACE Refractoriness-Related Diagnostic and Prognostic Scores and Characterization of Tumor Microenvironment Infiltration in Hepatocellular Carcinoma. Front Immunol. 2022;13:869993. doi:10.3389/fimmu.2022.869993
72. Ren Z, Yue Y, Zhang Y, et al. Changes in the Peripheral Blood Treg Cell Proportion in Hepatocellular Carcinoma Patients After Transarterial Chemoembolization With Microparticles. Front Immunol. 2021;12:624789. doi:10.3389/fimmu.2021.624789
73. Loosen SH, Schulze-Hagen M, Leyh C, et al. IL-6 and IL-8 Serum Levels Predict Tumor Response and Overall Survival after TACE for Primary and Secondary Hepatic Malignancies. Int J Mol Sci. 2018;19(6):Jun. doi:10.3390/ijms19061766
74. Fujiwara N, Friedman SL, Goossens N, Hoshida Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol. 2018;68(3):526–549. doi:10.1016/j.jhep.2017.09.016
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






