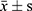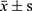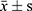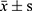Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Differences and Clinical Significance of Serum 25-Hydroxyvitamin D3 and Vasohibin-1 (VASH-1) Levels in Patients with Diabetic Nephropathy and Different Renal Injuries
Authors Liu H, Wang D, Tang J, Yu L, Su S
Received 20 January 2023
Accepted for publication 6 April 2023
Published 19 April 2023 Volume 2023:16 Pages 1085—1091
DOI https://doi.org/10.2147/DMSO.S405554
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Hui Liu,1 Dongyan Wang,1 Jingnan Tang,1 Linlin Yu,2 Shanshan Su1
1Department of Nephrology, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, Shandong, People’s Republic of China; 2Department of Science and Technology, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, Shandong, People’s Republic of China
Correspondence: Shanshan Su, Department of Nephrology, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, Shandong, People’s Republic of China, Email [email protected]
Objective: We investigate the relationship between the changes of serum 25-hydroxyvitamin D3 (25(OH)D3) and vasohibin-1 (VASH-1) and renal function injury in patients with type 2 diabetic nephropathy.
Methods: In this study, 143 patients with diabetic nephropathy (DN) were selected as DN group, and 80 patients with type 2 diabetes mellitus were selected as T2DM group. The serum 25 (OH) D3, VASH-1, blood glucose index, inflammation index and renal function index were compared between the two groups. According to the urinary microalbumin/creatinine ratio (UACR), the DN group was divided into microalbuminuria group (UACR range≥ 30.0mg/g and < 300.0mg/g) and macroalbuminuria group (UACR≥ 300.0mg/g) for stratified comparison. The correlation between 25-hydroxyvitamin D3, VASH-1 and inflammation index and renal function index was analyzed by simple linear correlation analysis.
Results: The level of 25 (OH) D3 in DN group was significantly lower than that in T2DM group (P< 0.05). The levels of VASH-1, CysC, BUN, Scr, 24h urine protein, serum CRP, TGF-β 1, TNF-α and IL-6 in DN group were higher than those in T2DM group (P< 0.05). The level of 25 (OH) D3 in DN patients with massive proteinuria was significantly lower than that in DN patients with microalbuminuria. The level of VASH-1 in DN patients with massive proteinuria was higher than that in DN patients with microalbuminuria (P< 0.05). There was a negative correlation between 25 (OH) D3 and CysC, BUN, Scr, 24h urine protein, CRP, TGF-β 1, TNF-α, IL-6 in patients with DN (P< 0.05). VASH-1 was positively correlated with Scr, 24h urinary protein, CRP, TGF-β 1, TNF-α and IL-6 in patients with DN (P< 0.05).
Conclusion: The level of serum 25 (OH) D3 in DN patients was considerably decreased, and the level of VASH-1 was increased, which was related to the degree of renal function injury and inflammatory response.
Keywords: 25 hydroxyvitamin D3, angiogenesis inhibitor protein 1, type 2 diabetes, diabetic nephropathy, renal function damage, inflammation indicators
Introduction
Diabetes is a metabolic disease characterized by hyperglycemia due to defective insulin secretion or impaired biological function, or both.1,2 The constitution of diabetes in China is mainly type 2 diabetes (T2DM), and long-term elevated blood sugar can lead to eye, kidney, heart, blood vessels, nerve chronic damage and dysfunction.2–4 Diabetic nephropathy is one of the most important complications of diabetic patients, which is featured by progressive reduction of proteinuria and glomerular filtration rate (GFR). There are no obvious symptoms in the early stage of the disease. Once it develops to the end stage, irreversible renal function damage will occur, making the treatment more difficult.5–7 Therefore, early prediction of diabetic nephropathy is particularly important. Serum 25-hydroxyvitamin D3 refers to activated vitamin D3, which must be activated by kidney and liver to achieve calcium absorption. Therefore, serum 25-hydroxyvitamin D3 level can reflect renal function and serve as a serum marker for predicting diabetic nephropathy.8,9 Vasohibin-1 (VASH-1) is considered to be the only protein with negative regulation of angiogenesis derived from endothelial cells, which can be induced by vascular endothelial growth factor 2 and basic fibroblast growth factor 2. VASH-1 inhibits angiogenesis by targeting multiple factors, blocks the synthesis and release of angiogenic factors, and antagonizes their effects. Studies have shown that VASH-1 has a protective effect on renal function in patients with diabetic nephropathy.10 Therefore, this study explored the differences and clinical significance of serum 25-hydroxyvitamin D3 and VASH-1 levels in patients with diabetic nephropathy and different renal injuries.
Data and Methods
General Information
In this study, 143 patients with diabetic nephropathy (DN) diagnosed in our hospital were selected as DN group, and 80 patients with simple type 2 diabetes diagnosed in the same period were selected as T2DM group. This study complied with the Declaration of Helsinki.
Inclusion criteria: (1) The age range of the subjects selected in this study was 50–79 years old. (2) Diagnostic criteria for type 2 diabetes7 reference to the people’s health press, the ninth edition of the ‘internal medicine’11 standard, diabetic nephropathy in patients with urinary albumin creatinine ratio (UACR)≥30.0mg/g. (3) All included patients were willing to accept the relevant examination of this study. (4) Patients signed informed consent before the implementation of this study. This study was approved by the Medical Ethics Committee of Affiliated Hospital of Shandong University of Traditional Chinese Medicine. Exclusion criteria: (1) Patients with malignant tumor. (2) With primary kidney disease (pyelonephritis, chronic glomerulonephritis, immune system diseases, etc.). (3) Patients with kidney stones. (4) Patients with severe infection. (5) Drug-induced renal injury. (6) Recent use of drugs affecting renal function.
Observation Indicators and Detection Methods
According to urine PCR, the patients were divided into microalbuminuria group and macroalbuminuria group. Serum 25 (OH) D3, VASH-1, fasting plasma glucose (FPG), fasting insulin (Fins), glycosylated hemoglobin (HbA1c), C-reactive protein (CRP), transforming growth factor-β1 (TGF-β1), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), cystatin C (CysC), urea nitrogen (BUN), serum creatinine (Scr) and 24-h urine protein were compared between the two groups.
Fasting blood samples were collected from patients in the morning, centrifuged at 1000r/min with a radius of 10cm for 20min to separate serum. Serum 25 (OH) D3 level was detected by serum 25 (OH) D3 kit (provided by Suzhou Boyuan Medical Technology Co., Ltd.). VASH-1 level was detected by VASH-1 kit (provided by Suzhou Boyuan Medical Technology Co., Ltd.). Enzyme-linked immunosorbent assay kit (provided by Suzhou Boyuan Medical Technology Co., Ltd.) was used to detect FPG, Fins, HbA1 c, CRP, TGF-β1, TNF-α, IL-6, CysC, BUN, Scr and 24 h urine protein levels.
Statistical Method
The data were processed by SPSS21.0. The normal distribution of 25 (OH) D3, VASH-1, CRP, TGF-β1, TNF-α, IL-6 and other counting data collected in this study were described by (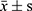 ). The independent sample t test was used to compare the hypothesis test between the two groups. Count data (gender, personal history, combined disease) were described by the number of cases (percentage). Non-hierarchical count data between groups were analyzed by χ2 test and Pearson method for correlation analysis.
). The independent sample t test was used to compare the hypothesis test between the two groups. Count data (gender, personal history, combined disease) were described by the number of cases (percentage). Non-hierarchical count data between groups were analyzed by χ2 test and Pearson method for correlation analysis.
Results
Comparison of Baseline Data Between DN Group and T2DM Group
The baseline data of the two groups are compared in Table 1, and there was good comparability and balance between the two groups.
 |
Table 1 Comparison of Baseline Data Between Two Groups of Patients |
Comparison of 25 (OH) D3 and VASH-1 Levels Between DN Group and T2DM Group
After a comparative analysis, the level of 25 (OH) D3 in the DN group was significantly lower than that in the T2 DM group, and the level of VASH-1 in the DN group was higher than that in the T2 DM group (P<0.05). See Table 2.
 |
Table 2 Comparison of 25 (OH) D3 and VASH-1 Levels Between DN Group and T2DM Group ( |
Comparison of Blood Glucose and Renal Function Indexes Between DN Group and T2 DM Group
After comparative analysis, CysC, BUN, Scr, 24h urine protein of DN group were higher than T2DM group (P<0.05). The levels of FPG, Fins and HbA1c were compared between DN group and T2DM group (P>0.05). See Table 3.
 |
Table 3 Comparison of Blood Glucose and Renal Function Indexes Between DN Group and T2 DM Group ( |
Comparison of Inflammatory Markers Between DN Group and T2DM Group
After a comparative analysis, the levels of serum CRP, TGF-β1, TNF-α and IL-6 in DN group were higher than those in T2DM group (P<0.05). See Table 4.
 |
Table 4 Comparison of Inflammatory Markers Between DN Group and T2DM Group ( |
Comparison of 25 (OH) D3 and VASH-1 Levels in DN Patients with Different Conditions
After a comparative analysis, the level of 25 (OH) D3 in DN patients with massive proteinuria was significantly lower than that in DN patients with microalbuminuria, and the level of VASH-1 in DN patients with massive proteinuria was higher than that in DN patients with microalbuminuria (P<0.05). See Table 5.
 |
Table 5 Comparison of 25 (OH) D3 and VASH-1 Levels in DN Patients with Different Conditions ( |
Correlation of 25 (OH) D3 and VASH-1 Levels with Inflammatory Indexes and Renal Function Indexes in DN Group
There was a negative correlation between 25 (OH) D3 and CysC, BUN, Scr, 24h urine protein, CRP, TGF-β1, TNF-α, IL-6 in patients with DN (P<0.05). VASH-1 was positively correlated with Scr, 24h urinary protein, CRP, TGF-β1, TNF-α and IL-6 in patients with DN (P<0.05). See Table 6 and Table 7.
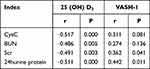 |
Table 6 Correlation Between 25 (OH) D3, VASH-1 and Renal Function Indexes in Patients with DN |
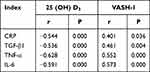 |
Table 7 Correlation Between 25 (OH) D3, VASH-1 Levels and Inflammatory Markers in Patients with DN |
Discussion
Diabetes is a high blood sugar caused by insulin resistance and a relative lack of insulin. The typical symptoms are ‘three more and one less’, that is, more drinking, more eating, more urine but weight loss.12,13 The disease is easy to cause complications such as kidney disease, retinopathy, and diabetic foot. Among them, diabetic nephropathy is the most common one, which is generally related to genetic and metabolic abnormalities caused by hyperglycemia. Diabetic nephropathy must be controlled in the early stage. If it develops to the end stage, it will cause renal failure, thus increasing the difficulty of treatment and mortality.14–17 Therefore, early use of serum indicators to predict diabetic nephropathy is particularly important. Studies have shown that 25 (OH) D3 can be used as a predictor of diabetic nephropathy.18 However, there are few studies on the value of VASH-1 in diabetic nephropathy.
In this study, the levels of 25 (OH) D3 and VASH-1 were compared between the two groups. The results showed that the level of 25 (OH) D3 in DN group was significantly lower than that in T2DM group, and the level of VASH-1 was higher than that in T2DM group. Vitamin D3 and its derivatives can effectively protect renal function and reduce proteinuria. The level of 25 (OH) D3 is closely related to obesity, diabetes, urinary protein and urinary inflammatory cytokines. Vitamin D is synthesized by human skin after ultraviolet irradiation and intake from food or supplements. Vitamin D enters the human body binds to vitamin D receptors and is transported to the kidney, which is hydroxylated into 1,25-dihydroxyvitamin D3 under the action of 1-αhydroxylase in the kidney. Therefore, in the event of renal injury, patients will show a significant reduction in serum 25 (OH) D3 levels, and the results of this study were consistent with those of previous studies.19–21 At the same time, VASH-1 is an endothelial-derived secretory protein regulated by angiogenic factors, which can promote the proliferation and metastasis of endothelial cells and affect new angiogenesis.22 The progression of diabetic nephropathy is often accompanied by new angiogenesis. Therefore, VASH-1 is increased in this process to protect the kidney from stress response and promote the proliferation of vascular endothelial inhibitors.23,24
In this study, the renal function indexes of the two groups were compared. The results showed that CysC, BUN, Scr and 24 h urine protein in the DN group were significantly higher than those in the T2 DM group, indicating that the renal function of patients with diabetic nephropathy was worse than that of patients with simple diabetes. CysC, BUN, Scr and 24 h urine protein are stable indicators for evaluating renal function.25,26
In this study, the inflammatory indexes of the two groups were compared. The results showed that the levels of serum CRP, TGF-β1, TNF-α and IL-6 in the DN group were significantly higher than those in the T2DM group, indicating that the inflammation level of diabetic nephropathy patients was higher than that of simple diabetic patients.27,28 CRP is an acute reactive protein and a marker of inflammation in the body.29,30 TNF-α is a cytokine that can cause hemorrhagic necrosis in a variety of tumors. TGF-β is a cytokine that regulates cell growth and differentiation. IL-6 is a small cytokine or signal protein secreted by cells. Among them, there is often a high level of TGF-β in the active tissue of cell differentiation, such as osteoblasts, kidneys, bone marrow and hematopoietic cells of fetal liver.31,32 When inflammation occurs in human organs, TGF-β can promote the production of IL-6 in human fibroblasts, promote the expression of extracellular matrix through the regulation of IL-6 gene transcription, and induce cell proliferation and differentiation.33,34 Therefore, when renal function is damaged, cytokines have a protective response to stress and promote factor proliferation and differentiation, which is manifested by a significant increase in serum CRP, TGF-β1, TNF-α, and IL-6 levels.
This study showed that the level of 25 (OH) D3 in DN patients with massive proteinuria was significantly lower than that in DN patients with microalbuminuria, and the level of VASH-1 was higher than that in DN patients with microalbuminuria. Studies have shown that in patients with vitamin D deficiency, there will be trace or large proteinuria risk, and over time, proteinuria levels will gradually increase.35–41 Because vitamin D needs to bind to the receptor, it is hydroxylated to 25 (OH) D3 under the action of 1-αhydroxylase, so vitamin D is insufficient, and the 25 (OH) D3 level of DN patients with massive proteinuria will be significantly reduced.42,43 VASH-1 binds to receptors on renal mesangial cells and reduces urinary protein production by activating the NK-κB pathway, whereas VASH-1 consumes large amounts of secreted proteins to reduce proteinuria.44–46 Therefore, the level of VASH-1 in DN patients with massive proteinuria will be significantly increased.
This study showed that 25 (OH) D3 in DN patients was significantly negatively correlated with CysC, BUN, Scr, 24 h urine protein, CRP, TGF-β1, TNF-α and IL-6 (P<0.05). VASH-1 was positively correlated with Scr, 24 h urine protein, CRP, TGF-β1, TNF-α and IL-6, indicating that 25 (OH) D3 and VASH-1 levels were related to renal function and inflammatory indicators and were related to the degree of renal function injury. The results further confirmed the above inference, indicating that serum 25 (OH) D3 and VASH-1 can be used as indicators to evaluate the degree of renal function damage.
In summary, the level of serum 25 (OH) D3 in patients with DN was significantly decreased, and the level of VASH-1 was increased, which was related to the degree of renal function injury and inflammatory response.
Data Sharing Statement
All data included in this manuscript are available from the corresponding author upon reasonable request.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interest.
References
1. Faselis C, Katsimardou A, Imprialos K, Deligkaris P, Kallistratos M, Dimitriadis K. Microvascular complications of type 2 diabetes mellitus. Curr Vasc Pharmacol. 2020;18(2):117–124. doi:10.2174/1570161117666190502103733
2. Ye YW, Yan ZY, He LP, Li CP. More studies are necessary to establish the effectiveness of Jinhuang powder in the treatment of diabetic foot. World J Diabetes. 2022;13(7):581–583. doi:10.4239/wjd.v13.i7.581
3. He LP, Song YX, Zhu T, Gu W, Liu CW. Progress in the Relationship between Vitamin D Deficiency and the Incidence of Type 1 Diabetes Mellitus in Children. J Diabetes Res. 2022;2022:5953562. doi:10.1155/2022/5953562
4. Shen W, He LP, Zhou LL. Obesity is associated with colitis in women but not necessarily causal relationship. World J Clin Cases. 2022;10(26):9542–9544. doi:10.12998/wjcc.v10.i26.9542
5. Bonner R, Albajrami O, Hudspeth J, Upadhyay A. Diabetic Kidney Disease. Prim Care. 2020;47(4):645–659. doi:10.1016/j.pop.2020.08.004
6. Yamanouchi M, Furuichi K, Hoshino J, Ubara Y, Wada T. Nonproteinuric diabetic kidney disease. Clin Exp Nephrol. 2020;24(7):573–581. doi:10.1007/s10157-020-01881-0
7. Zhou B, Jin YQ, He LP. Loss of skeletal muscle mass is not specific to type 2 diabetes. World J Diabetes. 2022;13(8):665–667. doi:10.4239/wjd.v13.i8.665
8. Koch EAT, Nakhoul R, Nakhoul F, Nakhoul N. Autophagy in diabetic nephropathy: a review. Int Urol Nephrol. 2020;52(9):1705–1712. doi:10.1007/s11255-020-02545-4
9. Ren XH, Ye YW, He LP. Baseline differences may impact on relationship between dietary tryptophan and risk of obesity and type 2 diabetes. World J Clin Cases. 2022;10(21):7617–7619. doi:10.12998/wjcc.v10.i21.7617
10. Perez-Lopez L, Boronat M, Melian C, Brito-Casillas Y, Wagner AM. Animal Models and Renal Biomarkers of Diabetic Nephropathy. Adv Exp Med Biol. 2021;1307:521–551. doi:10.1007/5584_2020_527
11. Yasuda I, Hasegawa K, Sakamaki Y, et al. Pre-emptive Short-term Nicotinamide Mononucleotide Treatment in a Mouse Model of Diabetic Nephropathy. J Am Soc Nephrol. 2021;32(6):1355–1370. doi:10.1681/ASN.2020081188
12. Saini DC, Kochar A, Poonia R. Clinical correlation of diabetic retinopathy with nephropathy and neuropathy. Indian J Ophthalmol. 2021;69(11):3364–3368. doi:10.4103/ijo.IJO_1237_21
13. Farber E, Hanut A, Tadmor H, Ruth A, Nakhoul F, Nakhoul N. Autophagy and Diabetic Nephropathy. Harefuah. 2021;160(11):740–745.
14. Russo G, Piscitelli P, Giandalia A, et al. Atherogenic dyslipidemia and diabetic nephropathy. J Nephrol. 2020;33(5):1001–1008. doi:10.1007/s40620-020-00739-8
15. Ji J, Tao P, Wang Q, Li L, Xu Y. SIRT1: mechanism and Protective Effect in Diabetic Nephropathy. Endocr Metab Immune Disord Drug Targets. 2021;21(5):835–842. doi:10.2174/1871530320666201029143606
16. Tsai IT, Wu CC, Hung WC, et al. FABP1 and FABP2 as markers of diabetic nephropathy. Int J Med Sci. 2020;17(15):2338–2345. doi:10.7150/ijms.49078
17. Lin ZJ, Zhang QW, Yu XL, Zhou B, Liu CW, He LP. Different nutrient compositions in diet and taking hypoglycemic drugs can modulate gut microbial flora. World J Diabetes. 2022;13(9):799–801. doi:10.4239/wjd.v13.i9.799
18. Popykhova EB, Ivanov AN, Stepanova TV, Lagutina DD, Savkina AA. Diabetic Nephropathy. - possibilities of early laboratory diagnostics and course prediction (review of literature). Klin Lab Diagn. 2021;66(10):593–602. doi:10.51620/0869-2084-2021-66-10-593-602
19. Salem M, Sallam AM, Abdel-Aleem E, El-Mesallamy HO. Effect of Lisinopril and Verapamil on Angiopoietin 2 and Endostatin in Hypertensive Diabetic Patients with Nephropathy: a Randomized Trial. Horm Metab Res. 2021;53(7):470–477. doi:10.1055/a-1517-6643
20. Jing Z, Hu L, Su Y, Ying G, Ma C, Wei J. Potential signaling pathway through which Notch regulates oxidative damage and apoptosis in renal tubular epithelial cells induced by high glucose. J Recept Signal Transduct Res. 2021;41(4):357–362. doi:10.1080/10799893.2020.1810706
21. Lee YH, Kim KP, Park SH, et al. Urinary chemokine C-X-C motif ligand 16 and endostatin as predictors of tubulointerstitial fibrosis in patients with advanced diabetic kidney disease. Nephrol Dial Transplant. 2021;36(2):295–305. doi:10.1093/ndt/gfz168
22. Kobayashi H, Looker HC, Satake E, et al. Results of untargeted analysis using the SOMAscan proteomics platform indicates novel associations of circulating proteins with risk of progression to kidney failure in diabetes. Kidney Int. 2022;102(2):370–381. doi:10.1016/j.kint.2022.04.022
23. Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol. 2020;16(7):377–390. doi:10.1038/s41581-020-0278-5
24. Chertow GM, Vart P, Jongs N, et al. Effects of Dapagliflozin in Stage 4 Chronic Kidney Disease. J Am Soc Nephrol. 2021;32(9):2352–2361. doi:10.1681/ASN.2021020167
25. Yamazaki T, Mimura I, Tanaka T, Nangaku M. Treatment of Diabetic Kidney Disease: current and Future. Diabetes Metab J. 2021;45(1):11–26. doi:10.4093/dmj.2020.0217
26. Frampton JE. Finerenone: first Approval. Drugs. 2021;81(15):1787–1794. doi:10.1007/s40265-021-01599-7
27. Piperidou A, Loutradis C, Sarafidis P. SGLT-2 inhibitors and nephroprotection: current evidence and future perspectives. J Hum Hypertens. 2021;35(1):12–25. doi:10.1038/s41371-020-00393-4
28. Barrera-Chimal J, Lima-Posada I, Bakris GL, Jaisser F. Mineralocorticoid receptor antagonists in diabetic kidney disease - mechanistic and therapeutic effects. Nat Rev Nephrol. 2022;18(1):56–70. doi:10.1038/s41581-021-00490-8
29. Barutta F, Bellini S, Canepa S, Durazzo M, Gruden G. Novel biomarkers of diabetic kidney disease: current status and potential clinical application. Acta Diabetol. 2021;58(7):819–830. doi:10.1007/s00592-020-01656-9
30. Ueki K, Sasako T, Okazaki Y, et al. Multifactorial intervention has a significant effect on diabetic kidney disease in patients with type 2 diabetes. Kidney Int. 2021;99(1):256–266. doi:10.1016/j.kint.2020.08.012
31. Cao Z, Huang D, Tang C, et al. Pyroptosis in diabetes and diabetic nephropathy. Clin Chim Acta. 2022;531:188–196. doi:10.1016/j.cca.2022.04.011
32. Wu L, Liu C, Chang DY, et al. Annexin A1 alleviates kidney injury by promoting the resolution of inflammation in diabetic nephropathy. Kidney Int. 2021;100(1):107–121. doi:10.1016/j.kint.2021.02.025
33. Packer M. Role of Impaired Nutrient and Oxygen Deprivation Signaling and Deficient Autophagic Flux in Diabetic CKD Development: implications for Understanding the Effects of Sodium-Glucose Cotransporter 2-Inhibitors. J Am Soc Nephrol. 2020;31(5):907–919. doi:10.1681/ASN.2020010010
34. Yuan Y, Yuan L, Li L, et al. Mitochondrial transfer from mesenchymal stem cells to macrophages restricts inflammation and alleviates kidney injury in diabetic nephropathy mice via PGC-1alpha activation. Stem Cells. 2021;39(7):913–928. doi:10.1002/stem.3375
35. Jung SW, Moon JY. The role of inflammation in diabetic kidney disease. Korean J Intern Med. 2021;36(4):753–766. doi:10.3904/kjim.2021.174
36. Jiang WJ, Xu CT, Du CL, et al. Tubular epithelial cell-to-macrophage communication forms a negative feedback loop via extracellular vesicle transfer to promote renal inflammation and apoptosis in diabetic nephropathy. Theranostics. 2022;12(1):324–339. doi:10.7150/thno.63735
37. Pan C, He L. Blood Lipids Are Not Specific for Stroke Risk. Biol Trace Elem Res. 2023;201(2):527–528. doi:10.1007/s12011-022-03184-9
38. Ren XH, Pan RJ, Li ZP, He LP. The Difference in Body Type May Modify the Relationship Between Dietary Mineral Intake and Hypertension Among Korean Adults. Biol Trace Elem Res. 2023;201(4):1670–1671. doi:10.1007/s12011-022-03310-7
39. Shi J, He L. Long-Term Use of Anti-Coronary Heart Disease Medications May Impact the Serum Zinc Concentration. Biol Trace Elem Res. 2023;201(1):1. doi:10.1007/s12011-022-03115-8
40. Song YX, He LP, Li CP. The Relationship between Serum Calcium Level and Risk Factor of Pregnancy-Induced Hypertension: a Meta-Analysis. Clin Exp Obstet Gyn. 2023;50(3):66.
41. Wang Y, Pan R, He L. Taking Medication for Diabetes May Modify the Link Between Serum Zinc Concentrations and Prediabetes and Diabetes in the General Population. Biol Trace Elem Res. 2023;201:1118–1119. doi:10.1007/s12011-022-03251-1
42. Guo X, Wu Y, Zhang C, Wu L, Qin L, Liu T. Network Pharmacology Analysis of ZiShenWan for Diabetic Nephropathy and Experimental Verification of Its Anti-Inflammatory Mechanism. Drug Des Devel Ther. 2021;15:1577–1594. doi:10.2147/DDDT.S297683
43. Ma L, Wu F, Shao Q, Chen G, Xu L, Lu F. Baicalin Alleviates Oxidative Stress and Inflammation in Diabetic Nephropathy via Nrf2 and MAPK Signaling Pathway. Drug Des Devel Ther. 2021;15:3207–3221. doi:10.2147/DDDT.S319260
44. He LP, Zhou ZX, Li CP. Narrative review of ferroptosis in obesity. J Cell Mol Med. 2023;27(7):920–926. doi:10.1111/jcmm.17701
45. Su WY, Li Y, Chen X, et al. Ginsenoside Rh1 Improves Type 2 Diabetic Nephropathy through AMPK/PI3K/Akt-Mediated Inflammation and Apoptosis Signaling Pathway. Am J Chin Med. 2021;49(5):1215–1233. doi:10.1142/S0192415X21500580
46. Satari M, Bahmani F, Reiner Z, et al. Metabolic and Anti-inflammatory Response to Melatonin Administration in Patients with Diabetic Nephropathy. Iran J Kidney Dis. 2021;1(1):22–30.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.