Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Cutaneous Adverse Events After COVID-19 Vaccination
Authors Weschawalit S, Pongcharoen P, Suthiwartnarueput W, Srivilaithon W , Daorattanachai K, Jongrak P, Chakkavittumrong P
Received 25 March 2023
Accepted for publication 25 May 2023
Published 8 June 2023 Volume 2023:16 Pages 1473—1484
DOI https://doi.org/10.2147/CCID.S410690
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Sinee Weschawalit,1 Padcha Pongcharoen,1 Worapop Suthiwartnarueput,2 Winchana Srivilaithon,3 Kiattichai Daorattanachai,3 Piyanat Jongrak,1 Panlop Chakkavittumrong1
1Division of Dermatology, Department of Internal Medicine, Faculty of Medicine, Thammasat University, Pathumthani, Thailand; 2Department of Pathology, Faculty of Medicine, Thammasat University, Pathumthani, Thailand; 3Department of Emergency Medicine, Faculty of Medicine, Thammasat University, Pathumthani, Thailand
Correspondence: Panlop Chakkavittumrong, Division of Dermatology, Department of Internal Medicine, Faculty of Medicine, Thammasat University, Pathumthani, 12120, Thailand, Tel +6690-9894056, Email [email protected]
Purpose: The morphology and timing of cutaneous reactions after Coronavirus disease (COVID-19) vaccines have been well described; however, data on the rates and risk factors are limited. Therefore, this study aimed to measure the incidence of cutaneous adverse reactions (CARs) after COVID-19 vaccination in Thailand, describe the rash characteristics according to the doses or types of vaccine, and assess the risk factors for developing CARs.
Patients and Methods: This was a prospective observational study of adults who received COVID-19 vaccination and provided informed consent. Cutaneous diagnoses were made by expert dermatologists with supporting skin biopsies, as needed. Data were analyzed using descriptive statistics and logistic regression to examine the independent risk of developing a CAR.
Results: Between July 2021 and January 2022, 7505 participants were vaccinated. Vaccine-related CARs occurred in 92 patients with an overall risk of 1.2%. CARs occurred after the first (n=41), second (n=23), third (n=27), and fourth (n=1) doses. Among the 92, 75 (81%) developed CARs within 7 days and 61 (66%) resolved within 7 days. Urticaria, injection site reaction, and a delayed (≥ 3 days post vaccine) local reaction were the three most common CARs occurring in 59 cases (64%). In total, 51 (55%) patients received only symptomatic and supportive treatment. Underlying urticaria and psoriasis were the independent factors for developing a CAR: adjusted odd rations of 15.63 (6.02– 40.57, p < 0.001) and 5.36 (1.57– 18.36, p = 0.007), respectively. A total of 6/34 (17%) and 4/31 (12%) patients developed urticarial and psoriasis flare post vaccine. Our study found superficial perivascular and intraepidermal eosinophil infiltration, which may be unusual pathological findings in vaccine-induced pemphigus foliaceous.
Conclusion: CARs after COVID-19 vaccination had a low incidence and were mostly mild in severity and transient in nature. Underlying urticaria and psoriasis were risk factors for CAR development.
Keywords: COVID-19 vaccine, adverse event, cutaneous reaction, incidence, psoriasis, urticaria
Introduction
The Coronavirus disease 2019 (COVID-19) pandemic, declared by the WHO, began in March 20201 and the first COVID-19 vaccine, an mRNA vaccine (Pfizer), was approved by the US FDA for Emergency Use Authorization. Currently, nine COVID-19 vaccines are available on the emergency list of World Health Organization (WHO): mRNA (BNT162b2; Pfizer, mRNA-1273; Moderna), viral vectors (ChAdOx1 nCoV-19; AstraZeneca, Ad26.COV2.S; Johnson & Johnson, Ad5-nCoV-S; Convidecia), inactivated vaccines (CoronaVac; Sinovac, BBIBP-CorV; Sinopharm, BBV152; Covaxin) and protein subunit (NVX-COv2373; Novavax).2 As of October 2022, more than five billion people had received the COVID-19 vaccine.3 In March 2021, Sinovac became the initial vaccine to be distributed in Thailand. During the study period, the predominant vaccine option available was from AstraZeneca, with additional options including Pfizer, Sinovac, Moderna, and Sinopharm.
The reported vaccine-related adverse reactions range from major to minor. The major reactions have included myocarditis, neuritis, or severe cutaneous drug reactions with reported incidence rates of 55/100,000 (myocarditis) and 1/100,000 for neuritis. The rate of anaphylaxis is 11/1,000,000 and only 11 case reports of Stevens-Johnson Syndrome or Toxic Epidermal Necrolysis (SJS/TEN).4–7 Several cutaneous reactions after vaccination have been reported and include injection site reaction, urticaria, maculopapular, eczematous rashes, and urticarial vasculitis.8 Recently, emerging data pertaining to cutaneous adverse reactions (CARs) following administration of booster doses to health-care workers (HCWs) have shown that heterologous booster doses tend to elicit greater reactogenicity than homologous booster doses.9 Although much of the literature has focused on the rash characteristics, some reports have documented low overall rates of CARs eg 0.22%, 0.6% and 1.8% from Farinazzo et al, Rerknimitr et al and Grieco et al, respectively. Moreover, few studies have assessed the incidence rates of vaccine related cutaneous adverse reactions (CARs), the characteristics of systemic symptoms, and the relationship between underlying morbidity and the risk of CARs.
We, therefore, estimated the incidence of CARs following COVID-19 vaccination in a Thai population, described the rash morphologies, and assessed whether the underlying morbidities were risk factors for developing CARs.
Methods
This observational prospective cohort study was conducted at Thammasat University Hospital (TUH), a tertiary care center situated on the outskirts of Bangkok. We recruited adults aged ≥ 18 years who received any COVID-19 vaccine at the TUH between July 2021 and January 2022. Data were collected from standardized case record forms in a Google Form database using LINE accounts; LINE is a popular messaging service similar to WhatsApp. The collected data included demographic data; history of underlying comorbid and cutaneous diseases; allergies; injection fillers; and the date/s, dose, and type of vaccine administered: Pfizer (PZ), Moderna (Mo), AstraZeneca (AZ), Sinovac (SV), and Sinopharm (SP). Prior to enrollment in the case record form, study participants were provided with an information sheet and consent form. Consent was obtained from individuals who indicated their acceptance by checking the designated box.
A cutaneous adverse reaction (CARs) was defined as the new onset of a symptom or sign, or exacerbation of the disease present on the day of vaccination, as determined by the attending dermatologist.
A researcher sent text messages to all participants via a LINE account, 1, 3, and 7 days after vaccination to ask about any reactions. Participants were also asked to send the research team news about any reactions via LINE. A reported reaction resulted in an online consultation, and additional details were noted. If face-to-face consultation was deemed necessary, participants visited the TUH skin clinic. All participants with CARs were asked to send photographs of their rashes. Additional rash data were recorded following a review by a dermatologist, including morphology, clinical diagnosis, onset, any other symptoms, treatment, and duration. Participants were followed up for up to one month after the reaction or as clinically indicated. We also collected data from TUH out-patients who presented to our clinic or were referred with a rash within four weeks after COVID-19 vaccination. If a participant had a CAR after the first vaccine dose, they were followed up after subsequent vaccine doses. Subjects who experienced reactions were educated on the potential risk of CARs and the benefits associated with receiving the subsequent dose. The participants then decided whether to receive the next dose by themselves.
This study was approved by the Thammasat University ethics committee, reference ID MTU-EC-IM-2-163/64. We followed the Strengthening the Reporting of Observational Studies in Epidemiology statement recommendations. We defined “injection site reaction” as pain, swelling and redness at the site of vaccine injection and “delayed local reaction” as injection site reaction which occurs ≥ 3 days after injection.
Study Size
The incidence of reported CARs was 0.4–0.7%.10 One-sample comparison of proportions was compared with 90% power and 5% alpha error (two-sided test). The estimated sample size was 7292 patients.
Statistical Methods
Descriptive data are summarized as mean and standard deviation (SD), median and interquartile range (IQR), or percentage, as appropriate. All variables were compared between patients who experienced rashes and those who did not. The unpaired t-test was used for continuous variables and the chi-square test or exact probability test was used for categorical variables. Multivariable logistic regression analysis was used to demonstrate the relationship between comorbidities and CAR development. A p value less than 0.05 was statistically significant. STATA program version 14 (StataCorp LLC, College Station, TX, USA) was used for the data analysis.
Results
Figure 1 shows the flowchart of patient recruitment. Of the 44,242 individuals who received the COVID-19 vaccination at TUH during the study period, 7621 were included in the study. After excluding duplicated and missing data, 7505 participants were analyzed.
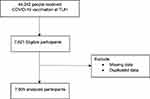 |
Figure 1 Flowchart of patient recruitment. |
Of the 7505 participants, 6841 (91.15%) received AZ, 449 SV, 193 PZ, 11 Mo, and 11 SP (Table 1). Females accounted for 67%. The overall median (IQR) age was 35 (26–47) and the majority of recipients were in the 20–60 years old group. A total of 4428, 2655, 419 and two participants received the first, second, third, and fourth doses of the vaccine, respectively.
 |
Table 1 Clinical Characteristics of Participants with Rash and No-Rash, and Results from the Univariable Analysis |
Overall, 92/7505 participants (1.2%) had CARs after COVID-19 vaccination: 41/92 (44.57%) had a reaction after the first dose of the vaccine; 23/92 (25%), 27/92 (29.34%), and 1/92 (1.1%) had CARs after the second dose, third, and fourth doses, respectively. The median time to rash onset was 2.5 days (IQR1-4) and 81.5% (75/92) of cases had CARs within 7 days. The median CAR duration was 4 days (IQR 2.5–15) and in 61/92 (66.3%) cases, the CAR lasted ≤7 days. A total of 14/92 (15%) recipients had CARs that lasted > 30 days (Table 2 and Table 3).
 |
Table 2 Characteristics of Participants with Rash According to Types of Vaccine |
 |
Table 3 Characteristic of Onset and Duration According to the Sequence of Vaccine |
Of the 92 participants, 69 (75%) decided to receive the next vaccine dose and of these, 62 (89.85%) did not have a recurrent rash. Of the six (6/69, 8.6%) patients with a recurrent rash, four had the same reaction, and two had different reactions compared to the antecedent dose; five of the six participants received a different vaccine compared to the antecedent vaccine. Therapeutic management was supportive for most of the six participants. Methotrexate and narrowband UVB (NB-UVB) were required in one case of psoriasis to control the disease (Table 4).
 |
Table 4 Characteristics of Rash in Participants Who Have Rash Following Subsequent Vaccination and Management |
The three most common reactions were urticaria, injection site reactions, and delayed local reactions, accounting for 32/92 (34.7%), 16/92 (17.4%), and 11/92 (11.9%) cases, respectively (Table 1 and Table 2). Of the 31 participants with known psoriasis, four (12.9%) had an exacerbation of their disease, and two developed urticaria. There were two patients with new onset psoriasis. Of the 34 vaccinees known to have chronic urticaria, six (17.6%) developed a post vaccine urticarial rash and one developed erythema multiforme. Only two (1.6%) of 170 atopic participants had CARs in our study (eczema and urticaria). We also observed a small number (n=3, 3.2%) of individuals who developed herpes zoster or herpes simplex reactivation.
At the end of the study period, 6/92 (6.5%) patients had continuing rashes: guttate psoriasis, erythrodermic psoriasis, urticaria, urticarial vasculitis, alopecia areata, and pityriasis rubra pilaris, which persisted for between 84–220 days (12–31 weeks)
The CARs were generally mild. Half of the patients (51/92) received supportive or topical treatments. Other treatments for psoriasis, erythromelagia, and urticarial vasculitis included oral prednisolone, aspirin, ibuprofen, cyclosporine, methotrexate, colchicine, acitretin, and ixekizumab (Table 1). One case of Stevens-Johnson Syndrome (SJS) was observed. The patient was referred from another hospital with a diagnosis of SJS that developed 4 days after Sinopharm and was treated with intravenous dexamethasone and oral prednisolone. The CARs resolved within 18 days. The physician recommended administration of a vaccine of a different type but the patient decided not to proceed with her subsequent vaccination dose.
Aside from a rash, 77/92 (83.6%) participants also complained of localized and/or systemic symptoms (Supplementary Table 1). Any known comorbid diseases and known dermatologic diseases were found in a large minority of cases, 34/92 (36.9%) and 18/92 (19.6%) respectively. Six participants had a history of filler injections; however, none experienced a filler reaction after vaccination.
By multivariable analysis, having underlying urticaria and psoriasis were significantly associated with an increased risk of developing a CAR: adjusted odds ratio (aOR) 15.63 (95% CI 6.02–40.57, p < 0.001) and aOR 5.36 (95% CI 1.57–18.36, p = 0.007), respectively. Diabetes mellitus, hypertension, dyslipidemia, and malignancy were not significant explanatory variables for a CAR (Table 5).
 |
Table 5 Results from Multivariable Logistic Regression Analysis Demonstrate the Relationship Between Comorbid Disease and the Development of Rash |
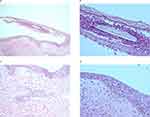
A skin biopsy was performed to confirm diagnoses in ten cases: urticarial vasculitis, erythema multiforme (Supplementary Figure 1), alopecia areata, pustular psoriasis (Supplementary Figure 2), erythrodermic psoriasis, pityriasis rubra pilaris (Supplementary Figure 3) and pemphigus foliaceous. We found superficial perivascular infiltration with eosinophils and a few intraepidermal eosinophils in the participant with pemphigus foliaceous but direct immunofluorescence in this case confirmed the presence of an intercellular space pattern with IgG and C3 (Figure 2A–D).
Discussion
In our large prospective study, we found that the incidence of a CAR was low (1.2%) and that only a minority of participants went on to have a recurrent rash. To date, several studies have reported vaccine-related CARs after COVID-19 vaccination. CARs incidence rates reported by Grieco et al and Rerknimitr et al were 1.8%11 and 0.6%,12 respectively, consistent with our 1.2%. Rerknimitr et al reported the incidences from SV were 0.94% and 0.7% after the first and second doses of vaccine, whereas those of AZ were 1% and 0.52%, respectively. Recurrent rashes after subsequent doses occurred in 6/69 (9.6%) of our redosed participants. This proportion is consistent with the 8.5% reported by Gireco et al and the 9% following the AZ vaccine but lower than the 32.26% following the SV.12
In most cases in our study, the onset and duration of CARs occurred within and lasted for less than 7 days, which is consistent with previously published studies.11–14
The most common rash in our study was urticaria, which is in the top 5 most common CARs reported post COVID-19 vaccine in systematic reviews and observational studies; these CARs are injection site reaction (34.05%), urticaria/angioedema (10.89%), eczematous rashes (6.95%) herpes zoster reactivation(2.69%) and maculopapular rash (1.78%).11–16 Less common CARs we encountered included alopecia areata, psoriasis, and autoimmune bullous diseases, which have also been reported following different COVID-19 vaccinations in many studies. The small number of urticarial flares (17.6%) in our study is consistent with previous study by Tuchinda et al (15%)17 which additionally reported concurrent thyroid disease as an independent risk factor.
Alopecia areata, an organ-specific autoimmune disease that can be exacerbated by oxidative stress18 is one of the most common (2.2%) new-onset skin diseases during or after COVID-19 infection19 and has been reported after COVID-19 vaccination. It may be found in localized areas, affect the entire scalp (alopecia totalis) and in some cases, all body hair (alopecia universalis). The treatment for vaccine-induced alopecia areata was not different from that for alopecia areata in the general population. In the localized form, intralesional triamcinolone is often used and gives good results; for more extensive disease, immunotherapy or oral tofacitinib may be given.20 Our three cases of alopecia areata have been treated with intralesional triamcinolone and desoximetasone lotion and showed good results. Full hair regrowth was observed within 3–5 months.
Vaccine-induced psoriasis has been reported from influenza and BCG vaccines. The forms of psoriasis have included plaque, guttae, pustular or erythrodermic, both as exacerbations or new-onset. Time for onset is between 1–90 days, but often occurs within 7 days.21 Multi-modality of treatments such as methotrexate, acitretin, narrow-band UVB, and the biologicals are usually effective within 4 months.21,22 Pemphigus foliaceous is a rare reaction that has also been reported following COVID-19, influenza, hepatitis B and rabies vaccines.23 It usually responds well to oral corticosteroids.24 In the case of our patient, azathioprine was initiated at a dose of 50 mg/day following a three-month course of prednisolone at a dose of 15 mg/day. Symptomatic improvement was observed; subsequently, the prednisolone dosage was gradually tapered until completely discontinued over a period of eight months. However, symptom recurrence was observed after four months, leading to the reintroduction of prednisolone at a dose of 30 mg/day. At present, the patient’s symptoms are being managed with a daily regimen of prednisolone 15 mg in conjunction with azathioprine 50 mg/day.
We observed a herpes zoster reactivation rate of 3.2% which appears lower than 13.8% of both herpes zoster and simplex reported by Catala et al in their smaller study of 405 individuals whose mean age was 50 years old.14 Known risk factors for herpes zoster reaction include advanced age, immunocompromise (disease or drugs) and psychological stress. However, immune dysregulation or transient lymphopenia 6–8 days after COVID-19 vaccination may have triggered the zoster reactivation seen in our 23 old, healthy individuals.25,26
Urticarial vasculitis (UV) has been reported after H1N1 vaccination27 and also COVID-19 vaccine.28–30 Nazzaro documented a case of UV that occurred subsequent to the first dose of the Moderna vaccine, which required treatment with oral methylprednisolone at a dose of 32 mg/day for a period of two months in order to manage the symptom.31 In our study, two participants diagnosed with UV were treated with differing therapeutic regimens. One patient required oral administration of antihistamines and colchicine, whereas the other necessitated a five-week course of oral prednisolone at a dose of 15 mg/day in conjunction with colchicine to effectively control the disease.
To date, severe cutaneous adverse reactions (SCAR) after Covid-19 vaccinations have rarely been reported.32,33 There have been only 11 case reports of SJS or TEN, which have occurred after several COVID-19 vaccines, for example, PZ, Mo, AZ, SP, and Janssen, started 1 to 14 days post vaccine and lasted 7 to 35 days.7,34 There were no deaths, and all patients had a good outcome after treatment with prednisolone, dexamethasone, etanercept, and IVIG. In our study, one participant experienced SJS 4 days after the SP vaccine. She had no history of any medications in the previous 8 weeks and was well. We did not exclude a very mild form of Mycoplasma pneumoniae (no chest radiograph, no PCR, or serology)35 so the relationship with SP is possible.
In addition to clinical data, skin biopsies were performed in selected cases. Pathological results in pemphigus foliaceous, which have not been mentioned in previous vaccine-induced cases, are unusual for this condition. This may be explained by vaccine-related conditions, but further studies are needed to shed light on the skin pathophysiology.
The mechanisms underlying CARs remain incompletely explained and focus on immune- or non-immune reactions to vaccine components.36 Increases in interleukin 6 and Th17 cells following vaccination have been hypothesized as the pathogenesis of new onset psoriasis and psoriatic flares. In chronic urticaria, mast cell degranulation is the main mechanism following several triggers, including vaccines or drugs.37 The potential molecular mimicry between the viral antigen in COVID-19 vaccines and human components (as yet unknown) and the subsequent induction of pathological autoantibodies may explain autoimmune-mediated alopecia areata following COVID-19 vaccine.38
Interestingly, our study found an association between pre-existing urticaria and psoriasis and an increased risk of CARs. The incidence of psoriasis and urticaria in the general population is estimated to be approximately 1%. In our study, the number of participants with psoriasis and urticaria was 31/7505 (0.41%) and 34/7505 (0.45%), respectively. As a result, the association between comorbidities and the development of CAR, as observed in our multivariable logistic regression analysis, may be extrapolated to the general population. Underlying metabolic syndrome and malignancy were not independent explanatory factors in our large analysis; physicians could inform patients with urticaria and psoriasis about this potential risk before receiving the vaccination. However, further investigation with the primary objective of exploring this association should be conducted to validate these results, considering that the advantages of preventing severe SARS-CoV-2 infections outweigh the risks of CARs.
Although our study had many strengths, such as large sample size, prospective design with close follow-up, and expert dermatologist diagnoses, it had several limitations. AZ was the main type of vaccine (accounting for ~90%) in this study so the data cannot be generalized to other vaccines with confidence. Most of our patients were in the 20–60 years age bracket, and the total number of skin rashes was only 92.
Conclusion
In summary, this study has shown a low incidence of CARs (1.2%), and most were of mild severity. A small proportion, some 10% had a recurrent rash following a subsequent vaccine dose. Our findings suggest that urticaria and psoriasis may be potential risk factors for CARs. Further research is required to validate this association.
Ethics Approval and Informed Consent
This study was approved by the Thammasat University ethics committee, reference ID MTU-EC-IM-2-163/64. We confirmed that our study complied with the Declaration of Helsinki.
Acknowledgments
This study was supported by Thammasat University Research Fund, Contract No. TUFT 89/2564. We greatly appreciate all the members of the Thammasat University Hospital for their efforts and devotion during the COVID-19 crisis.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Funding
This study was supported by the Thammasat University Research Fund, Contract No. TUFT 89/2564.
Disclosure
The authors declare that they have no competing interests.
References
1. World Health Organization. coronavirus disease (COVID-19) pandemic; 2023. Available from: https://www.who.int/europe/emergencies/situations/covid-19#:~:text=This%20led%20WHO%20to%20declare,pandemic%20on%2011%20March%202020.
2. World Health Organization. COVID-19 vaccines with WHO emergency use listing; 2023. Available from: https://extranet.who.int/pqweb/vaccines/vaccinescovid-19-vaccine-eul-issued.
3. Database CoDC. Vaccines for COVID-19[Internet]; 2022. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html.
4. Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144(6):471–484. doi:10.1161/CIRCULATIONAHA.121.056135
5. Woo EJ, Mba-Jonas A, Dimova RB, Alimchandani M, Zinderman CE, Nair N. Association of receipt of the Ad26.COV2.S COVID-19 vaccine with presumptive guillain-barré syndrome, February-July 2021. JAMA. 2021;326(16):1606–1613. doi:10.1001/jama.2021.16496
6. Shimabukuro T, Nair N. Allergic reactions including anaphylaxis after receipt of the first dose of pfizer-BioNTech COVID-19 vaccine. JAMA. 2021;325(8):780–781. doi:10.1001/jama.2021.0600
7. Siripipattanamongkol N, Rattanasak S, Taiyaitieng C, et al. Toxic epidermal necrolysis after first dose of Pfizer-BioNTech (BNT162b2) vaccination with pharmacogenomic testing. Pediatr Dermatol. 2022;39(4):601–605. doi:10.1111/pde.15074
8. Maronese CA, Zelin E, Avallone G, et al. Cutaneous vasculitis and vasculopathy in the era of COVID-19 pandemic. Front Med. 2022;9:996288. doi:10.3389/fmed.2022.996288
9. Avallone G, Cavallo F, Astrua C, et al. Cutaneous adverse reactions following SARS-CoV-2 vaccine booster dose: a real-life multicentre experience. J Eur Acad Dermatol Venereol. 2022;36(11):e876–e879. doi:10.1111/jdv.18386
10. Menni C, Klaser K, May A, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID symptom study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21(7):939–949. doi:10.1016/S1473-3099(21)00224-3
11. Grieco T, Maddalena P, Sernicola A, et al. Cutaneous adverse reactions after COVID-19 vaccines in a cohort of 2740 Italian subjects: an observational study. Dermatol Ther. 2021;34(6):e15153.
12. Rerknimitr P, Puaratanaarunkon T, Wongtada C, et al. Cutaneous adverse reactions from 35,229 doses of sinovac and AstraZeneca COVID-19 vaccination: a prospective cohort study in healthcare workers. J Eur Acad Dermatol Venereol. 2022;36(3):e158–e161. doi:10.1111/jdv.17761
13. McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after moderna and pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85(1):46–55. doi:10.1016/j.jaad.2021.03.092
14. Català A, Muñoz-Santos C, Galván-Casas C, et al. Cutaneous reactions after SARS-CoV-2 vaccination: a cross-sectional Spanish nationwide study of 405 cases. Br J Dermatol. 2022;186(1):142–152. doi:10.1111/bjd.20639
15. Avallone G, Quaglino P, Cavallo F, et al. SARS-CoV −2 vaccine-related cutaneous manifestations: a systematic review. Int J Dermatol. 2022;61(10):1187–1204. doi:10.1111/ijd.16063
16. Qaderi K, Golezar MH, Mardani A, et al. Cutaneous adverse reactions of COVID-19 vaccines: a systematic review. Dermatol Ther. 2022;35(5):e15391. doi:10.1111/dth.15391
17. Tuchinda P, Kulthanan K, Chularojanamontri L, Pongkittilar B, Pochanapan O, Rujitharanawong C. Disease activity of patients with chronic urticaria receiving COVID-19 vaccines. Asian Pac J Allergy Immunol. 2022. doi:10.12932/AP-160322-1345
18. Acharya P, Mathur MC. Oxidative stress in alopecia areata: a systematic review and meta-analysis. Int J Dermatol. 2020;59(4):434–440. doi:10.1111/ijd.14753
19. Chularojanamontri L, Tuchinda P, Rujitharanawong C, et al. New-onset and exacerbated skin diseases after COVID-19 infection: a systematic review. J Dermatol. 2022;49(11):e419–e421. doi:10.1111/1346-8138.16501
20. Scollan ME, Breneman A, Kinariwalla N, et al. Alopecia areata after SARS-CoV-2 vaccination. JAAD Case Rep. 2022;20:1–5. doi:10.1016/j.jdcr.2021.11.023
21. Wei N, Kresch M, Elbogen E, Lebwohl M. New onset and exacerbation of psoriasis after COVID-19 vaccination. JAAD Case Rep. 2022;19:74–77. doi:10.1016/j.jdcr.2021.11.016
22. Wu PC, Huang IH, Wang CW, Tsai CC, Chung WH, Chen CB. New onset and exacerbations of psoriasis following COVID-19 vaccines: a systematic review. Am J Clin Dermatol. 2022;23(6):775–799. doi:10.1007/s40257-022-00721-z
23. Tavakolpour S. Pemphigus trigger factors: special focus on pemphigus vulgaris and pemphigus foliaceus. Arch Dermatol Res. 2018;310(2):95–106. doi:10.1007/s00403-017-1790-8
24. Calabria E, Canfora F, Mascolo M, Varricchio S, Mignogna MD, Adamo D. Autoimmune mucocutaneous blistering diseases after SARS-Cov-2 vaccination: a case report of pemphigus vulgaris and a literature review. Pathol Res Pract. 2022;232:153834. doi:10.1016/j.prp.2022.153834
25. Van Dam CS, Lede I, Schaar J, Al-Dulaimy M, Rösken R, Smits M. Herpes zoster after COVID vaccination. Int J Infect Dis. 2021;111:169–171. doi:10.1016/j.ijid.2021.08.048
26. Özdemir AK, Kayhan S, Çakmak SK. Herpes zoster after inactivated SARS-CoV-2 vaccine in two healthy young adults. J Eur Acad Dermatol Venereol. 2021;35(12):e846–e847. doi:10.1111/jdv.17577
27. Hughes R, Lacour JP, Baldin B, Reverte M, Ortonne JP, Passeron T. Urticarial vasculitis secondary to H1N1 vaccination. Acta Derm Venereol. 2010;90(6):651–652. doi:10.2340/00015555-0950
28. Dash S, Behera B, Sethy M, Mishra J, Garg S. COVID-19 vaccine-induced urticarial vasculitis. Dermatol Ther. 2021;34(5):e15093. doi:10.1111/dth.15093
29. Ono H, Yamaguchi R, Shimizu A. Urticarial vasculitis after COVID-19 vaccination: a case report and literature review. Dermatol Ther. 2022;35(8):e15613. doi:10.1111/dth.15613
30. Baraldi C, Boling LB, Patrizi A, et al. Unique case of urticarial skin eruptions after COVID-19 vaccination. Am J Dermatopathol. 2022;44(3):198–200. doi:10.1097/DAD.0000000000002036
31. Nazzaro G, Maronese CA. Urticarial vasculitis following mRNA anti-COVID-19 vaccine. Dermatol Ther. 2022;35(3):e15282. doi:10.1111/dth.15282
32. Agaronov A, Makdesi C, Hall CS. Acute generalized exanthematous pustulosis induced by moderna COVID-19 messenger RNA vaccine. JAAD Case Rep. 2021;16:96–97. doi:10.1016/j.jdcr.2021.08.013
33. O’Connor T, O’Callaghan-Maher M, Ryan P, Gibson G. Drug reaction with eosinophilia and systemic symptoms syndrome following vaccination with the AstraZeneca COVID-19 vaccine. JAAD Case Rep. 2022;20:14–16. doi:10.1016/j.jdcr.2021.11.028
34. Marcelino J, Vieira J, Ferreira F, et al. Stevens-Johnson syndrome related with comirnaty® coronavirus disease 2019 vaccine. Asia Pac Allergy. 2022;12(3):e30. doi:10.5415/apallergy.2022.12.e30
35. Heymann WR. More than mycoplasma-induced rash and mucositis: the potential role of mycoplasma pneumoniae in Stevens-Johnson syndrome/toxic epidermal necrolysis. J Am Acad Dermatol. 2022;86(4):746–747. doi:10.1016/j.jaad.2021.12.037
36. Gambichler T, Boms S, Susok L, et al. Cutaneous findings following COVID-19 vaccination: review of world literature and own experience. J Eur Acad Dermatol Venereol. 2022;36(2):172–180. doi:10.1111/jdv.17744
37. Hennino A, Bérard F, Guillot I, Saad N, Rozières A, Nicolas JF. Pathophysiology of urticaria. Clin Rev Allergy Immunol. 2006;30(1):3–11. doi:10.1385/CRIAI:30:1:003
38. Bardazzi F, Guglielmo A, Abbenante D, Sacchelli L, Sechi A, Starace MVR. New insights into alopecia areata during COVID-19 pandemic: when infection or vaccination could play a role. J Cosmet Dermatol. 2022;21(5):1796–1798. doi:10.1111/jocd.14864
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.