Back to Journals » Clinical Epidemiology » Volume 14
Cohort Description: Preventing an Opioid Epidemic in Norway – Focusing on Treatment of Chronic Pain (POINT) – A National Registry-Based Study
Authors Hamina A , Odsbu I , Borchgrevink PC, Chen LC , Clausen T, Espnes KA , Gjesdal K , Handal M, Hartikainen S, Hjellvik V, Holter MTS, Høibø T, Kurita GP, Langaas HC , Lid TG , Nøst TH , Sjøgren P, Skurtveit S
Received 25 July 2022
Accepted for publication 25 October 2022
Published 8 December 2022 Volume 2022:14 Pages 1477—1486
DOI https://doi.org/10.2147/CLEP.S382136
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Vera Ehrenstein
Aleksi Hamina,1,* Ingvild Odsbu,2,* Petter Christian Borchgrevink,3,4 Li-Chia Chen,5 Thomas Clausen,1 Ketil Arne Espnes,6,7 Kine Gjesdal,8,9 Marte Handal,1,10 Sirpa Hartikainen,11 Vidar Hjellvik,10 Marianne Therese Smogeli Holter,12 Trond Høibø,13 Geana Paula Kurita,14– 16 Harald Christian Langaas,7 Torgeir Gilje Lid,8,9 Torunn Hatlen Nøst,3,17 Per Sjøgren,15 Svetlana Skurtveit1,2 On behalf of the POINT study group
1Norwegian Centre for Addiction Research (SERAF), Institute of Clinical Medicine, University of Oslo, Oslo, Norway; 2Department of Mental Disorders, Division of Mental and Physical Health, the Norwegian Institute of Public Health, Oslo, Norway; 3Department of Pain and Complex Disorders, St. Olavs Hospital, Trondheim University Hospital, Trondheim, Norway; 4Institute of Circulation and Medical Imaging, Norwegian University of Science and Technology, Trondheim, Norway; 5Division of Pharmacy and Optometry, School of Health Sciences, University of Manchester, Manchester, UK; 6Department of Clinical Pharmacology, St. Olavs Hospital, Trondheim University Hospital, Trondheim, Norway; 7Regional Medicines Information and Pharmacovigilance Centre (RELIS), Department of Clinical Pharmacology, St. Olavs Hospital, Trondheim University Hospital, Trondheim, Norway; 8Faculty of Health Sciences, University of Stavanger, Stavanger, Norway; 9Center for Alcohol and Drug Research (KORFOR), Stavanger University Hospital, Stavanger, Norway; 10Department of Chronic Diseases, Division of Mental and Physical Health, the Norwegian Institute of Public Health, Oslo, Norway; 11School of Pharmacy, University of Eastern Finland, Kuopio, Finland; 12Department of Psychology, Faculty of Social Sciences, University of Oslo, Oslo, Norway; 13Research Unit for General Practice, NORCE Norwegian Research Centre, Bergen, Norway; 14Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; 15Department of Oncology, Rigshospitalet Copenhagen University Hospital, Copenhagen, Denmark; 16Department of Anaesthesiology, Pain and Respiratory Support, Rigshospitalet Copenhagen University Hospital, Copenhagen, Denmark; 17Department of Mental Health, Norwegian University of Science and Technology, Trondheim, Norway
*These authors contributed equally to this work
Correspondence: Ingvild Odsbu, Department of Mental Disorders, Division of Mental and Physical Health, Norwegian Institute of Public Health, PO Box 222 Skøyen, Oslo, 0213, Norway, Tel +47 41454379, Email [email protected]
Aim: The POINT project aims to provide evidence to optimise chronic pain management, prevent adverse consequences of opioids, and improve chronic pain patients’ pain relief, functional capacity, and quality of life. We describe the outline of the project and its work packages. More specifically, we describe a cohort of persons with chronic pain and a cohort of long-term opioid users identified from a national registry linkage.
Data Sources: The project utilises data from nationwide healthcare and population registers in Norway. Using the Norwegian Prescription Database, we identified a cohort of persons who have been dispensed drugs reimbursed for chronic pain and a cohort of persons who used opioids long term from 2010 to 2019. Data from the Norwegian Registry for Primary Health Care and the Norwegian Patient Registry (2008– 2019), Cancer Registry (1990– 2018) Cause of Death Registry (2010– 2019) and demographic and socioeconomic registers from Statistics Norway (2010– 2019) were linked to the cohorts.
Study Population: There were 568,869 participants with chronic pain. Sixty-three percent of the cohort was women, and the mean age was 57.1 years. There were 336,712 long-term opioid users (58.6% women; 60.9 years). In chronic pain and long-term opioid user cohorts, the most frequent musculoskeletal diagnosis was back pain diagnosed in primary care (27.6% and 30.7%). Psychiatric diagnoses were also common.
Main Variables: Upcoming studies will utilise psychiatric and somatic diagnoses from the patient registers, drug use from the prescription register, causes of death, demographics, and socioeconomic status (eg, education, income, workability, immigrant status) as exposures or outcomes.
Conclusion and Future Plans: The two cohorts have numerous pain-related diagnoses, especially in the musculoskeletal system, and noticeably frequent somatic and psychiatric morbidity. The POINT project also includes later work packages that explore prescriber and patient perspectives around safe and effective treatment of chronic pain.
Keywords: opioids, chronic pain, healthcare registers
Introduction
Chronic pain, ie, pain lasting more than three months,1 is a common problem in populations worldwide. Based on health survey data, its prevalence among adults in Norway can be as high as 30%.2 It is the most frequent reason for long-term sick leave and disability benefits, highlighting its significant economic impact.3 On an individual level, chronic pain substantially affects health, functioning, and quality of life.4 Opioid use has increased substantially as a treatment for chronic pain, although its benefits in long-term therapy are limited.5,6 In North America, this increase was in part culpable for the ongoing epidemic of opioid use and overdose deaths.7,8
Short-term use of opioids for severe acute pain, palliative care and cancer pain is essential and widely utilised. However, chronic non-cancer pain may be underpinned by different mechanisms, as the pain is not always directly related to tissue damage, changing the nature of the pain. Consequently, the treatment needs to target more than biological mechanisms, and improved quality of life and maintaining functioning may be more appropriate goals than complete pain elimination.5 Moreover, although pain is always associated with biological, psychological, and social factors, the potential relative contribution of psychosocial factors increases as the pain persists. Perhaps unsurprisingly, then, no robust evidence supports opioids’ effectiveness in improving functioning or quality of life during long-term use, and the treatment of chronic pain often requires a more multidisciplinary approach.5,9–11 Long-term opioid treatment is also problematic due to the potential for serious adverse effects and events, such as cognitive impairment, falls and fractures, tolerance development, addiction, and the risk of overdoses, all of which have considerable effects on patients’ functional capacity and quality of life.10,12
“The Preventing an Opioid Epidemic In Norway, Focusing on Treatment of Chronic pain (POINT)” project was developed to assist healthcare providers in optimising chronic pain management, preventing adverse consequences of opioids, and increasing patients’ quality of life. POINT is an interdisciplinary project that incorporates epidemiological methods on healthcare register-based data, surveys, qualitative, interview-based methods on patient and prescriber perspectives, and development of non-pharmacological treatment programs in primary care.13
Here, we describe the data sources and the two main cohorts utilised in the register-based part of the project: a cohort of persons with chronic pain and a cohort of long-term opioid users identified from nationwide healthcare and population registers.
Materials and Methods
A Brief Overview of the POINT Project
The POINT project consists of four work packages, each investigating different aspects of the treatment of chronic pain and especially opioid use in chronic pain management (Figure 1). This cohort description article covers the study populations of work packages 1 and 2. We also briefly introduce work packages 3 and 4 to understand the first two work packages in their context.
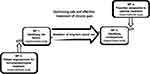 |
Figure 1 The four work packages of the POINT project. |
The first two work packages of the POINT project study the risk factors for long-term opioid use, utilising a data linkage of several nationwide healthcare and population registers. Work package 1 explores the factors that can help prescribers identify patients at risk of becoming long-term opioid users. As long-term opioid use may not be inherently problematic, work package 2 explores the consequences of long-term opioid use, investigating which predictors are associated with the adverse consequences in long-term opioid users.
Work package 3 investigates the patients’ perspectives on opioid use and their experiences with the treatment they receive from the healthcare services. Additionally, the work package explores the potential for non-pharmacological treatment alternatives. Subprojects of the work package include studies on patients with iatrogenic opioid use disorder, investigation on an automated e-health intervention supporting pain management and opioid reduction, and a non-pharmacological, multidisciplinary treatment alternative program in primary healthcare on the aspects of living with chronic pain.
Work package 4 investigates healthcare personnel’s perspectives on opioid prescribing recommendations and regulations and how they understand and manage associated risks. A questionnaire survey will investigate patient assessment when general practitioners (GPs) are prescribing opioids to patients with chronic non-cancer pain and GPs’ views on non-pharmacological treatment alternatives for chronic pain. Subprojects of the work package include a quantitative and qualitative evaluation of an academic detailing intervention14 focusing on opioid prescribing for chronic, non-cancer pain and an exploration of healthcare personnel’s experiences with treating patients with iatrogenic opioid use disorder with a particular focus on multidisciplinary teams.
Study Setting
In 2019, the Norwegian population was around 5.348 million people. Residents of Norway have universal health and social insurance coverage, and enrolment is automatic. Private medical insurance is limited. Prescription drugs are paid out-of-pocket or by co-payment (39% of the total price and not more than 520 NOK per prescription) up to an annual ceiling (2369 NOK in 2019) for reimbursed drugs. Drugs can be reimbursed for predefined chronic conditions requiring long-term drug treatment, ie, at least three months a year. Reimbursed drugs are approved in advance with closely defined areas of use and terms and conditions, or individual reimbursement can be granted if the areas of use or terms and conditions are not met. Reimbursed drugs are usually dispensed for three months, and the prescription is valid for one year.
Data Sources
We retrieved the data for these cohorts from national healthcare and population registers in Norway. The registers are mandatory, ie, they are not based on informed consent, and data are collected prospectively.15 All physicians and pharmacies are obliged by law to report data on inpatient and outpatient care visits and dispensed prescriptions, respectively. The registers have been widely utilised in epidemiological research. We provide an overview of the data sources included in the POINT project in Table 1.
 |
Table 1 Overview of the Mandatory Health- and Population Registers Included in the POINT Project |
A unique personal identification number (PIN) is assigned to everyone born in Norway and anyone who settles in Norway for a period exceeding six months.15 Data from the national health- and population registers can be linked via the unique PIN, which makes it possible to follow an individual over time and to combine information on medical diagnoses, surgical procedures, medication use, sociodemographic characteristics, labour market participation, social benefits, and death, for example.
The Norwegian Prescription Database (NorPD) contains complete information (>99%) on all dispensed prescription drugs from all pharmacies in Norway.16,17 Data on drugs sold over-the-counter (OTC) and drugs administered in hospitals and nursing homes are unavailable. Drugs are classified according to the Anatomic Therapeutic Chemical (ATC) classification system.18
The Norwegian Patient Registry (NPR) contains information on all patient contacts with public specialist health care services, including private institutions and medical specialists contracted to the regional health authorities.19 Diagnoses are recorded according to the International Classification of Diseases 10th revision (ICD-10), and surgical and medical procedures are recorded according to the NOMESCO Classification of Surgical Procedures (NCSP) and Norwegian Classification of Medical Procedures (NCMP), respectively. The completeness of the register is considered to be high, but does vary depending on the diagnosis or condition being studied.19 As for primary care, the Norwegian Registry for Primary Health Care (NRPHC) captures all patient contacts with primary health care services, including Accident and Emergency services (A&E) and GP visits.19,20 Diagnoses from primary care are registered according to the International Classification of Primary Care, 2nd edition (ICPC-2).
The Cancer Registry of Norway contains information on all incident malignancies and certain benign tumours.21 The register is considered close to complete as it includes 99% of all neoplasms diagnosed in the country.22 The Cause of Death Registry contains information on all deaths occurring in Norway, including deaths of Norwegian citizens while abroad.23 Information on underlying and contributing causes of death is available and coded according to ICD-10. The register captures around 98% of the deaths every year.
The complete, linked data have been available for our research group since November 2021. Most data cover the period 2010–2019, except for the NPR and NRPHC data (2008–2019) and the data from the Cancer Registry (1990–2018).
Study Participants with Chronic Pain
All individuals aged 15 years or older with a valid personal identification number (PIN) from 2010 to 2019 were eligible for inclusion in the cohort. Identified from the NorPD, the inclusion criterion was at least one dispensation of drugs (opioids or non-opioids) reimbursed for chronic pain (dispensations with reimbursement code −71). Under the reimbursement scheme for chronic pain, a patient can be prescribed reimbursed non-opioids (eg, paracetamol, NSAIDs, gabapentin, pregabalin, amitriptyline, carbamazepine) or opioids for the treatment of moderate-to-severe chronic pain regardless of the underlying diagnosis when in need of drug treatment for more than three months a year. To be eligible for reimbursed opioids, the patient’s pain and risk of addiction need to be carefully assessed, and a concrete treatment plan must be established. For conditions other than active cancer, it must also be documented that the patient has tried at least two other non-opioid medications without satisfactory effect.
Reimbursement requires an application by the physician documenting these criteria on behalf of the patient to the health authorities. Initially, only anaesthesiologists and physicians at pain clinics could prescribe reimbursed opioids. Starting in 2016, GPs could apply for approval of reimbursed opioids for specific patients on the criteria mentioned above. There is a limit on the amount of opioids that can be prescribed. Anaesthesiologists and physicians at pain clinics can apply for the approval of reimbursed prescriptions of up to 300 mg oral morphine equivalents (OMEQ) per day. In contrast, primary care physicians may apply for approval of reimbursed prescriptions of up to 100 mg OMEQ per day. If a higher daily dosage is required, the extra amount must be prescribed without reimbursement. Opioids can also be reimbursed for palliative care (reimbursement code −90). Patients must pay out-of-pocket for opioids if neither palliative care nor chronic pain criteria are met.
Study Participants with Long-Term Opioid Use
We analysed dispensation data to identify the cohort of long-term opioid users. All 2,265,900 people aged 15 or over who purchased opioids in Norway during 2010–2019 were eligible. To define long-term opioid use, we utilised a previously described method, according to which use persisting over three months is regarded as long-term use. We excluded those who had less than 91 days of follow-up, ie, people who died within three months of their first opioid dispensation and those who began opioid use within the last three months of 2019 (Supplemental Figure 1).
Long-term users were defined as those who 1) had one opioid dispensation and at least one more within the 91 to 180 days following the first dispensation and 2) were dispensed at least 90 administration units (eg, tablets) within 90 days following the first dispensation. We included both prevalent and incident opioid users in this cohort description, but the number of long-term users among incident opioid users was also analysed separately (Supplemental Figure 1). Incident opioid use was defined by a washout period of 365 days, during which no opioid dispensations were allowed. See Hamina et al24 for a detailed explanation of the cohort formation methods.
The two cohorts were not mutually exclusive, ie, both may include persons from the other cohort. For the cohort of people with chronic pain, we measured time-varying demographic characteristics at first dispensation with reimbursement for chronic pain during the study follow-up and considered this as the index date. For long-term opioid users, the index date was the first dispensation during the long-term use period. Here, we report relevant principal and secondary diagnoses registered in the NRPHC or NPR in primary (ICPC-2) or secondary care and the A&E services (ICD-10). Moreover, cancer diagnoses were extracted from the Cancer Registry of Norway. Pain-related diagnoses were extracted at the index date or a maximum of 365 days before it, and diagnoses of other chronic conditions at the index date or any time before it (during 2008–2019). The reported diagnoses were selected according to frequency and relevance. Some participants had missing socioeconomic data from Statistics Norway; these numbers vary according to variables and are shown in Table 2.
 |
Table 2 Demographic Characteristics of the Cohorts at Index Date. Note That One Person Can Be Included in Both Cohorts |
Characteristics of Study Participants
The Cohort of People with Chronic Pain
During the study follow-up, 568,869 people were dispensed drugs reimbursed for chronic pain, forming the cohort of people with chronic pain. Of the people in the cohort, 469,856 (82.6%) were dispensed opioids at some point, but only 33,821 (5.9%) of those were reimbursed for chronic pain. Similarly, 554,523 (97.5%) were dispensed either NSAIDs or paracetamol. Of these, 425,396 (74.8%) were reimbursed for chronic pain. A majority (63.2%) of the complete cohort were women, and the mean age at cohort entry was 57.1 years (SD 18.2 years) (Table 2). Approximately 14.0% were born outside Norway, and 19.5% had received higher education at the index date.
In the cohort of people with chronic pain, musculoskeletal system diagnoses were widespread in both primary and secondary care (Table 3). The most frequent individual musculoskeletal diagnosis during the whole follow-up period was back pain (primary care: 27.6%; secondary care: 5.9%). Thirteen per cent were diagnosed with cancer. Additionally, mental and behavioural disorders were common in the cohort (55.1% in primary care and 22.7% in secondary care).
 |
Table 3 Primary and Secondary Care Diagnoses in the Cohorts on the Index Date or Any Time Before It in 2008–2019 |
The Cohort of Long-Term Opioid Users
From 2010 to 2019, 336,712 people used opioids for more than three months, forming the cohort of long-term opioid users (Table 2). Fifty-eight per cent were women, and the average age at first long-term opioid use was 60.9 years (SD 17.9). Approximately 9.0% were born outside of Norway, and 15.7% had received higher education. Similar to the cohort of people with chronic pain, musculoskeletal diagnoses were widespread during the follow-up period among long-term opioid users (Table 3). Back pain had been diagnosed in 30.7% of the cohort in primary care and 4.5% in secondary care. Cancer was diagnosed among 5.5%. The prevalence of diagnoses for mental and behavioural disorders was 53.8% in primary care and 21.7% in secondary care.
Summary
In brief, the two cohorts include various pain-related diagnoses, especially in the musculoskeletal system. Although both groups are very heterogeneous in nature, mental disorders and somatic comorbidity are noticeably frequent.
Strengths and Limitations of the Cohorts
The cohorts established for work packages 1 and 2 are based on nationwide registers and do not include selection bias stemming from regional differences.16 The registers have been used in research for decades, and their characteristics are well known.16 Due to legal requirements, the databases are comprehensive and include the entire Norwegian population.17 A specific limitation of the cohort of chronic pain patients is that it comprises persons treated with analgesics or other prescription drugs, thus excluding persons utilising only non-pharmacological treatment or who are not in medical treatment. Similarly, our data sources are collected for administrative purposes and include little data on clinical metrics, such as the severity of pain, pain mechanisms, or laboratory findings. We also do not have data on the exact diagnosis for which analgesics were prescribed, and non-reimbursed prescriptions do not include any information on treatment indications. Additionally, some participants have missing data from Statistics Norway. The specific consequences of these limitations need to be assessed depending on the research question at hand.
Findings to Date and Future Plans
The POINT project started in June 2021. All data are available for the research group for work packages 1 and 2, and we are currently characterising the two cohorts in terms of demographics, comorbidities, co-medication, and socioeconomic status. Our prior research on the chronic pain cohort showed that the number of people receiving reimbursed opioids for the treatment of chronic pain increased considerably within a ten-year period.25 Although higher than recommended doses of >300 mg OMEQ were rare, almost half (48%) of the cohort were dispensed opioids every year for nine years, indicating highly persistent use.
Odsbu et al studied the use of benzodiazepines and benzodiazepine-related drugs (BZRD) among those in the chronic pain cohort who had received reimbursed opioids.26 Among prevalent opioid users, 54% were dispensed BZRD in addition to opioids in 2019, which is strongly discouraged by pain treatment guidelines due to the high risk of adverse effects. Utilising the methodology for defining long-term opioid use described in this article, Hamina et al found that one in seven persons who were dispensed opioids used them longer than three months at some time point during 2004–2019, with little change in the prevalence and incidence of long-term opioid use during the follow-up.24 The work to identify risk factors for becoming a long-term opioid user and the consequences of long-term opioid use will continue throughout the coming years.
As for work packages 3 and 4, we are currently specifying the study design for exploring the perspectives of patients and professionals. We are also developing the study design of the non-pharmacological, multidisciplinary treatment alternative program in primary healthcare. The e-health intervention will be partly based on an e-health intervention previously developed for behaviour change,27 and the task of adapting it to the current purpose is planned to begin in late 2022. Study protocols for work packages 3 and 4 will be developed separately.
Collaboration
We have ongoing international collaborations and are open to new proposals. Due to data protection regulations, the linked register data are only available to selected POINT project members and may not be shared with other collaborators. However, we encourage collaborations to harmonise study protocols and analysis plans.
Patient and Public Involvement
The project includes patient participation in the form of two patient representatives, and the project is performed in cooperation with the Norwegian Chronic Pain Patients’ Organization and the Norwegian Forum of Disabled Peoples’ Organizations.13
Disclaimer
Data from the Norwegian Patient Registry and the Cancer Registry of Norway have been used in this publication. The interpretation and reporting of these data are the sole responsibility of the authors, and no endorsement by the Norwegian Patient Registry or the Cancer Registry of Norway is intended nor should be inferred.
Data Sharing Statement
The register-based cohorts are based on individual-level data from the national health and population registers. The authors are not allowed, by law, to publicly share this data. Therefore, the authors cannot make these data fully available to the public. The authors may share statistical code.
Ethics
The Norwegian Institute of Public Health has completed and approved a Data Protection Impact Assessment for the register-based studies in work packages 1 and 2, in accordance with the General Data Protection Regulation (GDPR). The Regional Committee for Medical and Health Research Ethics (REC South-East) has also approved the studies in work packages 1 and 2 (2019/656/REC South-East C). The data for work packages 1 and 2 have been pseudonymised and stored at Services for Sensitive data (TSD) at the University of Oslo, dedicated explicitly to storing sensitive research data. Only selected members of the research group will have access to the data. All disseminations from the studies will be aggregated; thus, no individual participants will be identifiable. In the qualitative studies, participants will be pseudonymised, and effort will be taken to protect their anonymity in publications. We expect to apply for ethical approval for the studies in work packages 3 and 4 in 2022–2023.
Acknowledgments
The POINT project is a collaboration between partners from the research sector (University of Oslo (SERAF), The Norwegian Institute of Public Health, and the Center for Alcohol and Drug Research (KORFOR)), partners from outside the research sector (Stavanger Municipality, Stavanger University Hospital, St. Olavs Hospital, and Trondheim University Hospital), and two user organisations (Norwegian Chronic Pain Patients’ Organization (FKS) and Norwegian Forum of Disabled Peoples’ Organizations (SAFO)). The authors acknowledge all of the other members of the POINT study group: Ann Oldervoll (Norwegian Centre for Addiction Research (SERAF), University of Oslo), Christian Tjagvad (Norwegian Centre for Addiction Research (SERAF), University of Oslo), Håvar Brendryen (Department of Psychology, University of Oslo), Ashley Muller (Norwegian Institute of Public Health), Dagfinn Glad (Norwegian Forum of Disabled Peoples’ Organizations (SAFO)), Eva Skovlund (Norwegian University of Science and Technology), Håkon Nestvold (Norwegian Centre for Addiction Research (SERAF), University of Oslo), Jørgen Møllerop (Stavanger Municipality), Jorunn Arnesen (Norwegian Chronic Pain Patients’ Organization (FKS)), Line Cecilie Christiansen (Stavanger Municipality), Line Cecilie Gjerde (Norwegian Institute of Public Health), Linn Gjersing (Norwegian Institute of Public Health), Olav Fredheim (Norwegian University of Science and Technology, St. Olavs Hospital/Trondheim University Hospital, Akershus University Hospital), Olof A Steingrimsdottir (Norwegian Institute of Public Health), Ottar Ness (Norwegian University of Science and Technology), Stine Sveingård (Norwegian University of Science and Technology), and Svein Skeie (Helse Stavanger HF). The authors further thank Ann Oldervoll for editing the language of the manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The POINT project is supported by the Norwegian Research Council (grant no. 320360).
Disclosure
GK has received grants from Novo Nordisk Foundation, The Danish Cancer Society, and European Commission, Horizon Europe Framework Programme (HORIZON). SH has received lecture fees from Eisai Ltd. Dr Vidar Hjellvik reports grants from Norwegian Research Council, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Treede R, Rief W, Barke A, Aziz Q, Pain MB. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the international classification of diseases (icd-11). Pain. 2018;160(1):19–27. doi:10.1097/j.pain.0000000000001384
2. Landmark T, Romundstad P, Dale O, Borchgrevink PC, Kaasa S. Estimating the prevalence of chronic pain: validation of recall against longitudinal reporting (the HUNT pain study). Pain. 2012;153(7):1368–1373. doi:10.1016/j.pain.2012.02.004
3. Nielsen CS. Chronic pain is strongly associated with work disability. Scand J Pain. 2013;4(4):180–181. doi:10.1016/J.SJPAIN.2013.08.002
4. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287. doi:10.1016/j.ejpain.2005.06.009
5. Hylands-White N, Duarte RV, Raphael JH. An overview of treatment approaches for chronic pain management. Rheumatol Int. 2017;37(1):29–42. doi:10.1007/s00296-016-3481-8
6. Birke H, Ekholm O, Sjøgren P, Kurita GP, Højsted J. Long-term opioid therapy in Denmark: a disappointing journey. Eur J Pain. 2017;21(9):1516–1527. doi:10.1002/ejp.1053
7. Manchikanti L, Helm S, Fellows B, et al. Opioid epidemic in the United States. Pain Physician. 2012;15(3 Suppl):ES9–38. doi:10.36076/ppj.2012/15/ES9
8. Clausen T. What lessons from Norway’s experience could be applied in the United States in response to the addiction and overdose crisis? Addiction. 2022;117(5):1192–1194. doi:10.1111/add.15845
9. Busse JW, Wang L, Kamaleldin M, et al. Opioids for chronic noncancer pain: a systematic review and meta-analysis. JAMA. 2018;320(23):2448–2460. doi:10.1001/jama.2018.18472
10. Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a national institutes of health pathways to prevention workshop. Ann Intern Med. 2015;162(4):276–286. doi:10.7326/M14-2559
11. National Institute for Healthcare and Clinical Excellence. Chronic pain (primary and secondary) in over 16s: assessment of all chronic pain and management of chronic primary pain. NICE Guidelines; 2021.
12. Virnes RE, Tiihonen M, Karttunen N, van Poelgeest EP, van der Velde N, Hartikainen S. Opioids and falls risk in older adults: a narrative review. Drugs Aging. 2022;39(3):199–207. doi:10.1007/s40266-022-00929-y
13. POINT project information web page; 2022. Available from: https://www.med.uio.no/klinmed/english/research/projects/point-preventing-opioid-epidemic-in-norway/.
14. Dyrkorn R, Langaas HC, Giverhaug T, Espnes KA, Rowett D, Spigset O. Academic detailing as a method of continuing medical education. Adv Med Educ Pract. 2019;10:717–725. doi:10.2147/AMEP.S206073
15. Laugesen K, Ludvigsson JF, Schmidt M, et al. Nordic health registry-based research: a review of health care systems and key registries. Clin Epidemiol. 2021;13:533–554. doi:10.2147/CLEP.S314959
16. Furu K, Wettermark BB, Andersen M, et al. The Nordic countries as a cohort for pharmacoepidemiological research. Basic Clin Pharmacol Toxicol. 2010;106(2):86–94. doi:10.1111/j.1742-7843.2009.00494.x
17. Norwegian Institute of Public Health. Driftsrapport 2019 helseregistre ved folkehelseinstituttet [operational report 2019 health registers at the national institute of public health]; 2020.
18. World Health Organization Collaborating Center for Drug Statistics Methodology. Norwegian Institute of Public Health. The anatomical therapeutic chemical classification system; 2022. Available from: http://www.whocc.no/atc_ddd_index/.
19. Bakken IJ, Ariansen AMS, Knudsen GP, Johansen KI, Vollset SE. The Norwegian patient registry and the Norwegian registry for primary health care: research potential of two nationwide healthcare registries. Scand J Public Health. 2020;48(1):49–55. doi:10.1177/1403494819859737
20. Norwegian Directorate of Health. KUHR-databasen; 2022. Available from: https://www.helsedirektoratet.no/tema/statistikk-registre-og-rapporter/helsedata-og-helseregistre/kuhr.
21. Pukkala E, Engholm G, Højsgaard Schmidt LK, et al. Nordic cancer registries – an overview of their procedures and data comparability. Acta Oncol. 2018;57(4):440–455. doi:10.1080/0284186X.2017.1407039
22. Larsen IK, Småstuen M, Johannesen TB, et al. Data quality at the cancer registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer. 2009;45(7):1218–1231. doi:10.1016/j.ejca.2008.10.037
23. Norwegian Institute of Public Health. Tall fra Dødsårsaksregisteret for 2020; 2020. Available from: https://www.fhi.no/hn/helseregistre-og-registre/dodsarsaksregisteret/tall-fra-dodsarsaksregisteret-for-2020/.
24. Hamina A, Hjellvik V, Handal M, Odsbu I, Clausen T, Skurtveit S. Describing long‐term opioid use utilizing Nordic prescription registers—A Norwegian example. Basic Clin Pharmacol Toxicol. 2022;130:481–491. doi:10.1111/bcpt.13706
25. Skurtveit S, Hjellvik V, Sakshaug S, et al. Forskrivning av opioider på blå resept mot langvarige smerter. Tidsskrift for Den norske legeforening. 2020;140(15):1–8. doi:10.4045/tidsskr.20.0153
26. Odsbu I, Handal M, Hjellvik V, et al. Bruk av andre vanedannende legemidler blant opioidbrukere med langvarige smerter. Norsk Epidemiologi. 2021;29(1–2):45–53. doi:10.5324/nje.v29i1-2.4045
27. Holter MTS, Johansen A, Brendryen H. How a fully automated eHealth program simulates three therapeutic processes: a case study. J Med Internet Res. 2016;18(6):e176. doi:10.2196/JMIR.5415
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
