Back to Journals » International Journal of Women's Health » Volume 14
Clinical Utility of Romosozumab in the Management of Osteoporosis: Focus on Patient Selection and Perspectives
Authors Lim SY , Bolster MB
Received 31 July 2022
Accepted for publication 5 October 2022
Published 15 December 2022 Volume 2022:14 Pages 1733—1747
DOI https://doi.org/10.2147/IJWH.S315184
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Elie Al-Chaer
Sian Yik Lim,1,2 Marcy B Bolster3
1Hawaii Pacific Health Medical Group, Honolulu, HI, USA; 2Department of Family Medicine, John E Burns School of Medicine, University of Hawaii, Honolulu, HI, USA; 3Division of Rheumatology, Allergy, and Immunology, Massachusetts General Hospital, Associate Professor of Medicine, Harvard Medical School, Boston, MA, USA
Correspondence: Sian Yik Lim, Bone and Joint Center, Straub Clinic, 800 S. King Street, Honolulu, HI, 96813, USA, Tel +1 808-522-4232, Fax +1 808-522-4401, Email [email protected]
Abstract: As one of the most potent osteoanabolic agents with a unique mechanism of action, romosozumab has high efficacy for osteoporosis treatment. It is a monoclonal antibody against sclerostin, a natural inhibitor of the Wnt signaling pathway, and by inhibiting sclerostin, activation of Wnt signaling occurs with a cascade of changes ultimately leading to bone mineral density (BMD) gains. Romosozumab stimulates bone modeling and has a dual effect of activating bone formation while inhibiting bone resorption. With this unique mechanism of action, treatment with romosozumab leads to a rapid and significant gain in BMD; these gains are higher than seen with bisphosphonates, denosumab, or parathyroid hormone (PTH) analogs. The FRAME and ARCH studies represent two pivotal trials demonstrating the efficacy of romosozumab in treating osteoporosis. Treatment with romosozumab should be followed by an antiresorptive agent, as this approach has demonstrated maintenance of or greater increases in BMD and reduced fracture risk even after finishing romosozumab treatment. As an osteoanabolic agent, romosozumab has shown superiority to alendronate in reducing fracture risk, increasing bone density, and potentially more rapid fracture risk reduction. Recent data have suggested that romosozumab prior to antiresorptive therapy may be the ideal treatment sequence, especially in high-risk patients and patients at imminent risk of fracture. Carrying a black box warning, romosozumab should be avoided in patients who have had myocardial infarction or stroke in the past year. Further studies are needed to clarify the increased cardiovascular risk attributed to this drug. Romosozumab has expanded our osteoporosis armamentarium and has enabled novel approaches, including “treat to target.” Future studies are needed to evaluate the optimal use sequence and to assess its safety, especially in patients with cardiovascular risk factors.
Keywords: romosozumab, sclerostin, osteoporosis, BMD
Introduction
Osteoporosis is a skeletal condition characterized by low bone mass, deterioration of bone tissue, and disruption in bone architecture, leading to decreased bone strength and increased risk of fracture.1,2 Osteoporosis-related fractures are associated with high health-care costs, as well as significant morbidity and mortality.3 Treatment for osteoporosis includes antiresorptive agents such as bisphosphonates and denosumab. In recent years, osteoanabolic treatments have been developed, including teriparatide, abaloparatide, and romosozumab. In this article, we discuss the profile of romosozumab, focusing on patient selection and perspectives on the utility of romosozumab in combination with current available therapies in osteoporosis treatment.
Targeting Sclerostin to Activate WNT Signaling Selectively in Bone
Bone homeostasis is controlled by many signaling pathways. The Wnt signaling pathway plays an essential role in osteoblast differentiation, and activating the Wnt-β-catenin pathway leads to increased bone formation and decreased bone resorption.4 Activation of the pathway occurs when the Wnt proteins bind to Frizzled family receptor and low-density lipoprotein receptor-related protein (LRP) 5/6 complexes. A cascade of intracellular changes occurs, ultimately leading to osteoblast maturation and differentiation and osteoclast inhibition through induction of osteoprotegerin (OPG) expression.
Wingless/integrated (Wnt) signaling is complex, and many molecules are involved in controlling the various effects of Wnt signaling on bone cells. Sclerostin, predominately produced by osteocytes and primarily found in bone of the adult skeleton,5 is an extracellular signaling molecule and natural antagonist of the Wnt signaling pathway. Sclerostin binds to the surface of osteoblasts through lipoprotein receptor-related protein (LRP)s, and when bound to LRPs, sclerostin competes with Wnt ligands for interaction with LRPs. This binding exerts an inhibitory effect on Wnt signaling and reduces osteoblastic activity and thus bone formation.
Sclerostin and the Wnt signaling pathway play a critical role in the ability of the skeleton to strengthen bone in response to mechanical stress. In response to mechanical stress, osteocytes produce less sclerostin leading to increased bone formation by osteoblasts.6 Of note, mutation in the canonical Wnt-beta-catenin signaling pathway provides significant insight into the importance of this pathway. Little et al described a family found to have increased bone mass but who were normal phenotypically due to a gain-in-function LRP-5 mutation within the family.7 In osteoporosis-pseudoglioma syndrome, a loss-of-function LRP-5 mutation leads to a unique phenotype of reduced bone mass, skeletal deformities, and fragility fractures in childhood, and it is associated with blindness.8
The SOST gene encodes sclerostin, and genetic abnormalities related to this gene characterize two rare autosomal recessive genetic conditions of sclerosteosis and Van Buchem disease. In sclerosteosis, there is a loss-of-function mutation in the SOST gene. In Van Buchem disease, there is a non-coding deletion that removes a SOST specific regulatory element in bone.9 These genetic abnormalities lead to decreased production of biologically active sclerostin. Patients with these conditions have high bone mass characterized by progressive generalized osteosclerosis in the skull, mandible, ribs, clavicles, and long bones.10,11 Given this sclerostin deficiency, patients with sclerosteosis and Van Buchem’s disease demonstrate a very low fracture risk due to increased bone mass.10–12
Romosozumab: A Monoclonal Antibody That Inhibits Sclerostin
Romosozumab is a humanized IgG2 monoclonal antibody that binds to and inhibits sclerostin (Figure 1). Removal of the inhibition of sclerostin on the Wnt-B-catenin pathway ultimately leads to bone formation and to a lesser degree decreased bone resorption hence its utility in the treatment of osteoporosis. Although it is possible to achieve bone formation by targeting other molecules in the beta-catenin signaling cascade, the ubiquity of the Wnt signaling pathway in different cells raises the concern of untoward systemic side effects.13,14 Sclerostin, secreted extracellularly and primarily localized to the bone tissue, provides an ideal therapeutic target for osteoporosis.
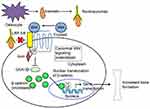 |
Figure 1 Mechanism of action of romosozumab. Notes: Romosozumab is a human monoclonal antibody that binds sclerostin (an inhibitor of the Wnt pathway signaling). When romosozumab binds to sclerostin, sclerostin cannot bind to the LRP-5 and LRP-6 receptors and is unable to exert its inhibitory effect. Wnt binds to LRP-5 or LRP-6 coreceptors and specific Frizzled family receptor leading to activation of the Wnt signalling pathway and bone formation. Adapted with permission from Dove Medical Press. Shah AD, Shoback D, Lewiecki EM. Sclerostin inhibition: a novel therapeutic approach in the treatment of osteoporosis. Int J Womens Health. 2015:7:565–58054 and adapted with permission from Dove Medical Press. Lim SY. Bolster M. Profile of romosozumab and its potential in the management of osteoporosis. Drug Des Devel Ther. 2017; 11:1221–123114. |
Romosozumab is given as two subcutaneous injections (210mg) once monthly for 12 months. Each injection is in a single-use, prefilled syringe containing 105mg of the medication. It is administered subcutaneously by a health-care provider into the abdomen, thigh, or upper arm.15 Romosozumab, like other monoclonal antibodies, is absorbed through the lymphatic system when administered subcutaneously. Systemic absorption occurs through convective transport in the lymphatic vessels and diffusion across the blood vessels.16,17 When administered subcutaneously, 50–70% absorption occurs, and romosozumab has a half-life of 6–7 days.18 Monoclonal antibodies are too large in molecular size to be filtered in the kidneys, and biliary excretion is minimal. Monoclonal antibodies are eliminated through intracellular catabolism, pinocytosis a non-specific fluid phase endocytosis and a specific receptor-mediated endocytosis process.16
Romosozumab: A Potent Osteoanabolic Agent That Stimulates Bone Formation and Inhibits Bone Resorption
Romosozumab is one of the most potent osteoanabolic agents developed for the treatment of osteoporosis. In a Phase 2 randomized, placebo-controlled, parallel-group, eight-group study (Study ID NCT00896532), the investigators enrolled 419 postmenopausal women ages 55–85 years with a lumbar spine, total hip, or femoral neck T-score between −2.0 and −3.5. Patients were randomized to receive monthly subcutaneous romosozumab (70, 140, 210mg), every three-months romosozumab (140, 210mg), placebo, oral alendronate (70mg weekly), or subcutaneous teriparatide (20 μg daily). The study’s primary endpoint was percentage change from baseline of lumbar spine bone mineral density (BMD) at 12 months.19 Patients who received romosozumab had significantly increased BMD at all sites (lumbar spine, total hip, and femoral neck). Patients who received romosozumab, 210mg subcutaneously once per month demonstrated the highest increases in BMD (11.3% in the lumbar spine, 4.1% in the total hip, 3.7% in the femoral neck). Romosozumab 210mg subcutaneously monthly led to considerably more bone density gains than the active comparators of teriparatide or alendronate.19
In the subgroups receiving romosozumab, serum P1NP (procollagen 1 intact N-terminal propeptide; bone formation marker) levels were increased and peaked after one month of treatment. Depending on the dose of romosozumab, serum P1NP decreased back to baseline by months 2–9. Serum CTX (C-terminal telopeptide of type 1 collagen; bone resorption marker) decreased and remained below baseline for the full 12 months of treatment. The pattern of change in bone turnover markers reflects the unique mechanism of action with romosozumab treatment. Initially, there is an increase in bone formation followed by a prolonged decrease in bone resorption. In contrast, with PTH analogs such as teriparatide and abaloparatide, bone resorption is increased simultaneously with bone formation, while with alendronate, an antiresorptive agent, both bone resorption and bone formation are decreased. The pattern of change in bone turnover markers also suggests that the osteoanabolic effects of romosozumab decrease with time, while the antiresorptive effects persist. This attenuation of the osteoanabolic effect explains why romosozumab is only used for one year in clinical trials, with subsequent transition to other osteoporosis treatments.20
Romosozumab primarily stimulates modeling-based bone formation in cancellous and endocortical surfaces.21,22 Modeling occurs when osteoblasts initiate bone formation on quiescent bone surfaces, while with remodelling osteoblast activity is dependent on bone resorption related to prior osteoclast activity.20,21 Romosozumab strengthens bone microarchitecture by improving trabecular architecture and increasing bone mass in humans.23 Due to the ability to improve bone architecture and increase bone mass, romosozumab provides potential advantages as a treatment for osteoporosis as compared to antiresorptive agents such as bisphosphonates and denosumab that primarily work by preventing further deterioration of bone architecture.
Romosozumab: Efficacy in the Treatment of Osteoporosis in Postmenopausal Women
The efficacy of romosozumab for osteoporosis treatment is supported by robust data from the pivotal studies of the FRAME (FRActure study in postmenopausal woMen with ostEoporosis) study and the ARCH (Active-contRolled fraCture Study in postmenopausal women with osteoporosis at High risk of fracture) study. Other studies have provided invaluable information in treatment of men: BRIDGE (placeBo-contRolled study evaluatIng the efficacy anD safety of romosozumab in treatinG mEn with osteoporosis),24 and the optimal sequence of romosozumab use in osteoporosis treatment: STRUCTURE (An Open-label, Randomized, Teriparatide-controlled Study to Evaluate the Effect of Treatment with Romosozumab in Postmenopausal Women With Osteoporosis Previously Treated with Bisphosphonate Therapy). Table 1 summarizes the trial design, study participants, comparator groups, primary endpoints, and results of these pivotal trials.
 |
Table 1 Key Randomized Control Trials of Romosozumab |
FRAME was a multicenter, international, randomized, double-blind, placebo-controlled, parallel-group study that compared two groups of patients over two years of treatment. One group received monthly subcutaneous injections of romosozumab 210mg in year one, followed by every 6-month subcutaneous injections of denosumab 60mg (romosozumab-denosumab group). The second group received one-year treatment with placebo followed by 6-month subcutaneous injections of denosumab 60mg (placebo-denosumab group) (Figure 2). The study included 7180 postmenopausal women, aged 55–90 years. The study participants had a total hip or femoral neck BMD T-score of −2.5 to −3.5. Women with a hip fracture history and one severe or more than two moderate vertebral fractures were excluded. The primary endpoints for this study were cumulative incidence of new vertebral fracture at 12 months and 24 months.25
 |
Figure 2 The study design of the FRAME and ARCH trials. Data from these studies.25,26 |
The average age of patients in the study was approximately 71 years, with well-matched baseline characteristics between treatment arms. The mean T-score for patients was ≤ −2.5 in the lumbar spine, total hip, and femoral neck. Approximately 18% of patients had a prevalent vertebral fracture, mostly mild-moderate in severity. Approximately 22% of patients had a previous nonvertebral fracture. Ninety percent of patients completed year 1, and nearly 85% completed year 2 of the study. The primary endpoint of vertebral fracture risk reduction in the FRAME study was achieved. At 12 months, romosozumab led to 73% reduction in new vertebral fractures, and at 24 months, after the transition from romosozumab to denosumab, new vertebral fractures were reduced by 75%.25
The ARCH study included a population at higher risk for fracture compared to the FRAME trial by enrolling patients with osteoporosis and one or more prior fragility fracture. Inclusion criteria included patients with a T-score of −2.5 or less at the total hip or femoral neck and either one or more moderate or severe vertebral fractures, or two or more mild vertebral fractures. Patients were also included with a T-score of −2.0 or less at the total hip or femoral neck and either two or more moderate or severe vertebral fractures or a fracture of the proximal femur sustained 3–24 months before randomization. The ARCH study randomized patients into two groups: the first group received monthly subcutaneous injections of romosozumab 210mg followed by open-label oral alendronate (romosozumab-alendronate group) until the time of primary analysis. The second group received weekly oral alendronate 70mg weekly in year 1, followed by open-label alendronate (alendronate-alendronate group) until the time of primary analysis (Figure 2).
Investigators performed the primary analysis (at 33 months) when incident clinical fractures (nonvertebral and symptomatic vertebral fracture) had been confirmed in at least 330 patients, and all patients completed the month-24 visit. The study’s primary outcome was cumulative incidence of new vertebral fractures at 24 months and cumulative incidence of clinical fractures (symptomatic vertebral fractures + nonvertebral fractures) at the time of primary analysis. The ARCH study met the primary endpoint of reduced vertebral fractures; new vertebral fractures were reduced by 48% in the romosozumab-alendronate group.
The average age of patients in the study was 74 years, with baseline characteristics between treatment arms being well matched. Patients’ mean T-score was within the osteoporotic range in the lumbar spine, total hip, and femoral neck. Most patients had a prior fracture; approximately 96% of patients had a prevalent vertebral fracture (65% classified as severe and 27% classified as moderate), and nearly 38% had a previous nonvertebral fracture. Study completion rates were high with 89.3% of patients completing year 1, and approximately 77% finishing the primary analysis period. The ARCH study met the primary endpoints. At 24 months, romosozumab led to 48% reduction in new vertebral fractures. At the time of primary analysis clinical fractures were reduced by 27% in the romosozumab-alendronate group compared to the alendronate-alendronate group.
Efficacy of 12-Month Treatment of Romosozumab
In the FRAME study, twelve months of romosozumab treatment led to a 73% relative risk reduction (RRR) of vertebral fractures (p < 0.001) compared to placebo. Fracture reduction was noted rapidly after six months of treatment with romosozumab. Between 6 months and 12 months, only two additional patients had vertebral compression fractures, compared to 33 patients in the placebo group. At 12 months, romosozumab treatment led to a 36% RRR of clinical fractures (p = 0.008) (composite of nonvertebral fracture and symptomatic vertebral fracture) compared to the placebo group. Compared to placebo, 12 months of romosozumab treatment increased BMD by 13.3% in the lumbar spine and 6.9% in the total hip.25
In the ARCH study, the study with a population at higher fracture risk, the romosozumab group showed a 37% risk reduction of vertebral compression fractures compared to alendronate at 12 months of treatment. Patients who received romosozumab had more significant BMD gains from baseline than those who received alendronate alone at all time points. At 12 months, patients who received romosozumab had BMD gains of 13.7% (lumbar spine) and 6.2% (total hip), compared to 5.0% (lumbar spine) and 2.8% (total hip) in patients who received alendronate.26
Transitioning to Antiresorptive Agent After 12 Months of Romosozumab Treatment: Maintaining Fracture Reduction Efficacy
The benefits and utility of transitioning to an antiresorptive agent after the completion of anabolic therapy are well established. Studies clearly show that treatment with romosozumab for two years results in markedly increased BMD at the lumbar spine and total hip.27 Patients who transitioned from romosozumab to denosumab continued to gain BMD; however, in patients who received placebo after romosozumab, BMD returned to pretreatment levels.27
The FRAME study demonstrated the utility of transitioning from 1 year of romosozumab treatment to denosumab. BMD continued to increase at 24 months with the transition to denosumab after 12 months of romosozumab (Table 1).25 The romosozumab-denosumab group demonstrated a 75% relative rate reduction of vertebral fractures (p < 0.0001). The reduction in clinical fracture incidence and nonvertebral fracture incidence at 24 months were not statistically significant, but there were numerically fewer fractures in the group receiving romosozumab-denosumab compared to the placebo-denosumab group. Transitioning to denosumab maintained the fracture protection achieved after one year of romosozumab treatment. In the extension study, patients who received a second year of denosumab demonstrated stable fracture reduction (Table 1).28
The ARCH study demonstrated the utility of transitioning from romosozumab to alendronate. From bone density gains achieved in the first year of romosozumab, bone density continued to increase subsequently when patients transitioned to alendronate (Table 1). At 24 months, the fracture reduction benefit persisted or was increased in the 2nd year after patients transitioned to alendronate in the romosozumab-alendronate group (RRR vertebral fractures 48%, p < 0.001). At the time of primary analysis (33 months), the romosozumab-alendronate group had 27% fewer clinical fractures (p < 0.001), 19% fewer nonvertebral fractures (p < 0.04), and 18% fewer hip fractures (P < 0.02) than the alendronate-alendronate group.
There is also data to support the utility of intravenous zoledronic acid after romosozumab treatment. A phase 2 study (Study ID NCT00896532, Extension, Week 48–72) evaluated the safety and efficacy of a single dose of intravenous zoledronic acid versus placebo in follow up to one year of romosozumab treatment. In patients receiving no additional treatment, BMD decreased to slightly above baseline in the lumbar spine and near baseline in the total hip. Intravenous zoledronic acid maintained BMD when it followed one year of romosozumab treatment (Table 1).29
These data support the importance of using an antiresorptive agent after a one-year course of romosozumab (Figure 3).25–27,29 After transitioning from romosozumab to an antiresorptive agent (denosumab or bisphosphonates), increases or maintenance of BMD were achieved. Furthermore, fracture protection is maintained two years after the transition from romosozumab to denosumab or bisphosphonates.15
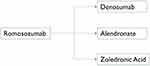 |
Figure 3 Romosozumab treatment should be followed by potent anti-resorptive treatment. |
Efficacy of Romosozumab in the Treatment of Osteoporosis in Men and Other Specific Populations
Men
The BRIDGE study evaluated the efficacy of romosozumab in men (Table 1). In the BRIDGE study, 245 men aged 55–90 years, with BMD T-score at the lumbar spine, total hip, or femoral neck of ≤-2.5 or ≤-1.5, with a history of fragility nonvertebral or vertebral fracture were randomized 2:1 to receive subcutaneous romosozumab (210mg) monthly or placebo for 12 months.24 The study’s primary endpoint was percentage increase from baseline lumbar spine BMD at 12 months. At one year, the study found greater gains in the lumbar spine and total hip BMD in the romosozumab group as compared to placebo; the increases at the lumbar spine were comparable to those observed in women in the FRAME and ARCH studies (Table 1).24–26
Chronic Kidney Disease
The ARCH and FRAME trials included patients with chronic kidney disease. Post hoc analyses divided patients based on estimated glomerular filtration rate (eGFR) into normal renal function (eGFR equal to or more than 90 cc/min), mild renal insufficiency (eGFR 60–89 cc/min), and moderate renal insufficiency (eGFR 30–59 cc/min).30 In patients with lowerD eGFR, there was a trend toward lesser increases in BMD, although BMD differences between romosozumab and comparator groups remained statistically significant. Importantly, the investigators found fracture prevention efficacy across the three groups of patients. In the FRAME and ARCH, at 12 months, the reduction of incident new vertebral compression fractures was similar across patient populations with normal kidney function, mild renal insufficiency, and moderate renal insufficiency respectively.30
End-Stage Renal Disease
Data regarding romosozumab in end-stage renal disease are very limited. In a Japanese cohort of 96 patients on hemodialysis who are at high risk for fracture, patients treated with romosozumab showed significant increases in lumbar spine (15.3%± 12.9%) and femoral neck BMD (7.2% ± 8.3%). At the same time, there was no change in BMD in patients not treated with romosozumab.31 Fracture efficacy data was not available; thus, future studies on the fracture risk reduction with romosozumab in patients with end-stage renal disease are needed.
Safety
Adverse events and serious adverse events in the ARCH and FRAME were well balanced between active romosozumab and comparator groups.25,26 Adverse events occurring in more than 10% of subjects included nasopharyngitis and back pain. Injection site reactions were seen more commonly in patients who received romosozumab compared to placebo (romosozumab-denosumab: 5.3%; placebo-denosumab: 2.9%) or compared to alendronate (romosozumab-alendronate 4.4% versus alendronate-alendronate 2.6%).
Osteonecrosis of the Jaw and Atypical Femoral Fractures
Osteonecrosis of the jaw and atypical femoral fractures have been reported in patients receiving romosozumab. In the FRAME study, where the comparator was placebo, there were 2 cases of osteonecrosis of the jaw in the romosozumab group. One case occurred after 12 months of romosozumab treatment. The second case occurred after 12 months of romosozumab treatment following one dose of denosumab. One atypical femoral fracture occurred after treatment with romosozumab for three months.25 In the ARCH study, one case of osteonecrosis of the jaw was noted in a patient who received romosozumab followed by alendronate. There were two cases of atypical femoral fractures in patients who received romosozumab followed by alendronate. Among patients who received alendronate for two years in the ARCH study, there was one case of osteonecrosis of the jaw and four cases of atypical femoral fractures,26 and this likely reflects that while romosozumab is an anabolic agent, it has antiresorptive properties. Therefore, caution is required when monitoring patient for adverse events.
Cardiovascular Risk
Romosozumab carries a black box warning related to avoiding its use in patients who have had a stroke or myocardial infarction in the past year. Consideration should be given to its potential cardiovascular risks. There were no differences in adverse cardiovascular events in the FRAME study, which was one of the largest trials (7180 patients) comparing romosozumab to placebo, followed by one year of denosumab.25 However, in the smaller studies: ARCH (4093 patients)26 and BRIDGE (245 patients), numerically more cardiovascular events were noted in patients receiving romosozumab. In the first year of the ARCH study, a higher frequency of serious cardiovascular adverse events was noted. Further analysis showed a higher number of patients with cardiac ischemic events and cerebrovascular events in patients who received romosozumab. Heart failure, noncoronary revascularization, and peripheral vascular ischemic events not requiring revascularization were numerically lower in the romosozumab-alendronate group. The BRIDGE trial noted serious cardiovascular adverse events in 8 patients who received romosozumab, and 2 patients who received placebo. However, interpretation of this data is difficult due to the low number of serious cardiovascular events.24
While increased numbers of cardiovascular events in the ARCH and BRIDGE studies were concerning, these findings need to be interpreted cautiously, and also in the context of the fracture prevention benefit romosozumab. The number of cardiovascular events was very small because the trials were powered to assess romosozumab fracture reduction efficacy, and not cardiovascular outcomes. Furthermore, data of cardiovascular risk factors (for example, lipid levels), and cardiovascular outcomes were limited.32 During the drug approval process, in addition to thoroughly evaluating clinical trial data, the Federal Drug Administration (FDA) evaluated preclinical studies, and genetic studies.33 It was noted that the role of sclerostin in atherosclerosis was unclear. Investigations of the relationships between sclerostin and cardiovascular dysfunction surrogate markers, and sclerostin and cardiovascular outcomes yielded conflicting data. Additionally, patients with sclerosteosis and Van buchem disease did not exhibit high risk of cardiovascular events.34
Nevertheless, adjudication of cardiovascular events in the ARCH by Duke Clinical research Institute, did show an increased risk of major adverse cardiac events (MACE), a vital end point used in clinical trials. The MACE (comprises cardiovascular death, nonfatal myocardial infarction or nonfatal stroke) hazard ratio in the ARCH was 1.87 (95% CI 1.11, 1.34), demonstrating increased risk of serious myocardial infarction and stroke.33 However, a meta-analysis of the ARCH, FRAME, and BRIDGE trials did not show a statistically significant increase in MACE events.33 Given the black box warning and the need for further information, romosozumab should not be used in patients who have had a myocardial infarction or stroke within the previous year. Consideration needs to be given, and discussion with the patient is important regarding the risks and benefits of treatment, particularly if the patient has other cardiovascular risk factors. Further surveillance and data are needed to monitor and better delineate the cardiovascular risks associated with romosozumab.
Romosozumab: Guidelines
After completion of 1 year of romosozumab, the guidelines recommend transitioning to an antiresorptive therapy to maintain BMD gains and reduce fracture risk.12,35 The 2020 American Association of Clinical Endocrinology guidelines and 2020 Endocrine Society Guidelines recommend romosozumab for patients at very high risk for fractures (Table 2). The International Osteoporosis Foundation (IOF) and the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) recommend osteoanabolic treatments in patients at very high risk for fractures as well. The IOF-ESCEO guidelines defined very high risk as a fracture probability that lies above the upper assessment threshold of the FRAX assessment, with or without the inclusion of BMD.36
 |
Table 2 Definition of Very High Risk of Fracture American Association of Clinical Endocrinology Guidelines 2020, Endocrine Society Guidelines 2020 |
There is increasing evidence supporting the use of osteoanabolic agents as an initial agent, such as romosozumab, in treating very high-risk patients, given the superior gains in BMD and superior anti-fracture efficacy.26 As previously noted, one year of romosozumab treatment followed by alendronate is superior in preventing vertebral and clinical fractures compared to alendronate alone.26 One year of romosozumab treatment, followed by 2 years of denosumab or 1 year of intravenous zoledronic acid, have been shown to be effective for osteoporosis treatment. In patients at very high risk for fracture, for example, those who sustained a recent osteoporotic fracture, the risk for a subsequent fracture is increased several-fold within the first two years after this index fracture.37 Compared to antiresorptive medications, osteoanabolic agents such as teriparatide, abaloparatide, or romosozumab can act more rapidly by increasing BMD and reducing vertebral and nonvertebral fractures. Therefore, they may serve as a “rescue drug” to rapidly reduce fracture risk for patients at very high risk for fracture.12
Romosozumab: Sequencing of Treatment Considerations and Treat to Target
In the treat-to-target approach, the goal is to identify a treatment target representing an acceptable proxy for fracture risk and initiate treatment with a medication likely to reach the target. Treatment is continued if the patient is on a path to achieving the target, and treatment is changed if the target is not attainable with initial therapy. In a treat-to-target approach, careful therapy selection is critical, and the sequence of various osteoporosis medications may be clinically relevant as they may have different effects on T-score and BMD.38,39 Data from the ARCH trial showed that total hip T-score achieved with alendronate or romosozumab treatment (6–24 months) correlated with subsequent fracture risk.40 These findings support using the T-score, or BMD, as a proxy for fracture risk in a treat-to-target approach for osteoporosis. Future studies may identify yet other proxies for fracture risk and thus provide additional treat-to-target goals.
As discussed in prior sections, romosozumab followed by antiresorptive treatments maintains bone density gains or even yields further increases.25,26 The fracture risk reduction persisted or improved after the transition from an osteoanabolic agent to an antiresorptive agent.25,26 The ARCH and FRAME enrolled patients with a significant wash-out period if they had been taking bisphosphonates. However, anabolic responsiveness may differ when osteoanabolics are used after an antiresorptive, such as a bisphosphonate or denosumab. Data suggests that the anabolic effect of osteoanabolics, when used after antiresorptive medication, may be blunted and variable based on the antiresorptive used before the course of osteoanabolic agents.41,42 For example, when teriparatide was used after denosumab or alendronate, there was transient (12–24 months) BMD loss in the hip, while the gain in BMD in the spine was attenuated.43,44
The STRUCTURE study provided valuable information regarding the effects of romosozumab after antiresorptive treatment (Table 1). In the STRUCTURE study, 436 postmenopausal women aged 55–90 years, who had received oral bisphosphonates for more than three years and alendronate during the year before screening, were randomized to receive either romosozumab or teriparatide. In both romosozumab and teriparatide groups, there was improved spine BMD after 12 months of treatment, although gain in BMD in the spine was more significant in the romosozumab group. However, for hip BMD, at 12 months, BMD gain and hip strength (based on finite element analysis) decreased in the group randomized to teriparatide compared to a 2.9% increase in total hip BMD, with associated increased hip bone strength in the romosozumab group.45 Because data has suggested that more significant increases in BMD are associated with an enhanced reduction in fracture risk,46 these data suggest that romosozumab may be a better treatment option than teriparatide for patients who are at high risk for fracture (low hip T-score) when transitioning from bisphosphonate to osteoanabolic agent.47
To evaluate the effect of treatment sequence on the anabolic effect of romosozumab, Cosman et al48 compared reviewed data from studies where romosozumab was administered before an antiresorptive agent (FRAME and ARCH)25,26 and compared them to studies where romosozumab was administered after antiresorptive therapy (STRUCTURE, phase 2 extension).45,49 When romosozumab was administered before antiresorptive treatment, one year of romosozumab treatment led to an increase in total hip BMD of 6.0–6.2% and a gain in lumbar spine BMD of 13.1–13.7%. When one year of romosozumab treatment was preceded by alendronate, total hip BMD increased by 2.9%, while lumbar spine BMD increased by 9.8%. When romosozumab treatment was preceded by denosumab, total hip BMD increased by 0.9%, while lumbar spine BMD increased by only 5.3%. Overall, these studies demonstrate that over two years, one year of romosozumab, followed by one year of denosumab or alendronate, led to more significant improvement in BMD improvement as compared to 1 year of denosumab followed by one year of romosozumab. There were more considerable BMD gains with romosozumab use before antiresorptive rather than romosozumab use following antiresorptive therapy. Since more significant increases in BMD are associated with a more efficacious reduction in fracture risk, romosozumab followed by antiresorptive may thus result in more substantial fracture risk reduction efficacy.48 Nevertheless, if indicated, osteoanabolics, especially romosozumab, can be used after antiresorptive medications as they do provide bone density gain and fracture risk protection.45
Comparison of Romosozumab with Other Medications for Osteoporosis Treatment: Head-to-Head Studies
Fracture Reduction Superiority of Romosozumab as Compared to Alendronate
Most osteoporosis treatment trials use a placebo as a comparator. The ARCH study is one of the few head-to-head trials showing the fracture benefit of one osteoporosis drug over another. The ARCH study showed that at 12 months, the romosozumab group showed a 37% risk reduction of vertebral compression fractures and a 28% risk reduction of clinical fractures compared to the alendronate group. The superior fracture protection benefit was maintained after the transition from romosozumab in year 1 to alendronate in year 2, where the romosozumab-alendronate group vertebral fracture relative risk reduction was 37%. The clinical fracture relative risk reduction was similarly beneficial at 28% compared to 2 years of alendronate treatment.
BMD Improvement Comparisons as Compared to Alendronate and Teriparatide
In comparing romosozumab with alendronate (ARCH trial), more significant gains in BMD at the hip and spine after one year of romosozumab were seen compared to 1 year of alendronate. Similar findings were noted in two phase 2 studies of romosozumab. In these phase 2 studies, romosozumab was compared to teriparatide and alendronate. There were more significant increases in BMD in patients who received romosozumab than alendronate.50,51 In the aforementioned phase 2 studies, one year of romosozumab was associated with greater BMD increases at the hip and spine compared to teriparatide.50 There are emerging data that more significant bone density gains are associated with greater fracture risk reduction.46 These data might suggest that romosozumab may provide a unique benefit to high-risk patients in the treatment of osteoporosis.
Cost Considerations
Health economic considerations also play an essential role in determining the agent of choice for the treatment of osteoporosis. At this point, in the United States, economic analyses do not strongly support the use of romosozumab compared to antiresorptive medications in all patients with osteoporosis.15 Osteoanabolic agents, such as romosozumab, are branded medications and cost significantly more than generic antiresorptive medications, such as alendronate. A study from the National Institute for Health Research in the United Kingdom showed that incremental cost-effectiveness ratios (ICER) of osteoanabolics, such as romosozumab, generally were more than the commonly used willingness to pay thresholds of £20,000–30,000 per quality-adjusted life-year (QALY).15 In patients with a very high-risk fracture in Sweden, sequential treatment with romosozumab followed by alendronate was found to have an ICER of €33,732, compared to alendronate alone. At a Swedish reference willingness-to-pay per QALY of €60,000, romosozumab to alendronate had a 97.9% probability of being cost-effective against alendronate alone. The authors concluded that romosozumab followed by alendronate might be a cost-effective option in the treatment of severe osteoporosis with high risk of fracture in postmenopausal women.52 In Canada, cost analysis in postmenopausal women with very high risk of fracture has found that romosozumab transitioned to 4 years of alendronate is likely to be cost effective at any decision-maker threshold, including the commonly quoted 50,000 per QALY gained in Canada as compared to 5 years of alendronate alone, or 5 years of risedronate alone.53 Romosozumab transitioned to bisphosphonates may be a cost-effective treatment for postmenopausal women with history of osteoporotic fracture who are at very high risk for fracture.52,53
Summary
Romosozumab is one of the most potent bone anabolic agents available to date, and it holds significant potential to increase our ability to treat osteoporosis. Romosozumab should be avoided in patients who had stroke or myocardial infarction in the preceding year, although further studies are required to clarify increased cardiovascular risk attributable to romosozumab. Romosozumab has demonstrated potent osteoanabolic effect and improves bone architecture. Romosozumab when used in initially followed by currently available antiresorptive agents demonstrates significant fracture reduction potential. Romosozumab may be most beneficial in patients at very high risk or who are at imminent risk for fracture (due to a recent fragility fracture). Recent studies have provided invaluable information into how to use romosozumab in combination with other osteoporosis medications. Future studies are needed to provide information regarding safe and optimal use romosozumab to most benefit patients with osteoporosis.
Disclosure
Dr Marcy B Bolster reports grants from Corbus, Cumberland, Inc, Mitsubishi, Genentech, and Rheumatology Research Foundation; serves as the advisory board for Novartis, honorarium from American Board of Internal Medicine and The Merck Manual; associate editor and reports honorarium from PracticeUpdate, and investment from Johnson & Johnson, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Sozen T, Ozisik L, Basaran NC. An overview and management of osteoporosis. Eur J Rheumatol. 2017;4(1):46–56. doi:10.5152/eurjrheum.2016.048
2. Nih Consensus Development Panel on Osteoporosis Prevention D, Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285(6):785–795. doi:10.1001/jama.285.6.785
3. Lim SY, Bolster MB. Current approaches to osteoporosis treatment. Curr Opin Rheumatol. 2015;27(3):216–224. doi:10.1097/BOR.0000000000000169
4. Shahi M, Peymani A, Sahmani M. Regulation of bone metabolism. Rep Biochem Mol Biol. 2017;5(2):73–82.
5. Delgado-Calle J, Sato AY, Bellido T. Role and mechanism of action of sclerostin in bone. Bone. 2017;96:29–37. doi:10.1016/j.bone.2016.10.007
6. Galea GL, Lanyon LE, Price JS. Sclerostin’s role in bone’s adaptive response to mechanical loading. Bone. 2017;96:38–44. doi:10.1016/j.bone.2016.10.008
7. Little RD, Carulli JP, Del Mastro RG, et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70(1):11–19. doi:10.1086/338450
8. Gong Y, Slee RB, Fukai N, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107(4):513–523. doi:10.1016/S0092-8674(01)00571-2
9. Sebastian A, Loots GG. Genetics of Sost/SOST in sclerosteosis and van Buchem disease animal models. Metabolism. 2018;80:38–47. doi:10.1016/j.metabol.2017.10.005
10. Balemans W, Ebeling M, Patel N, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet. 2001;10(5):537–543. doi:10.1093/hmg/10.5.537
11. Moester MJ, Papapoulos SE, Lowik CW, van Bezooijen RL. Sclerostin: current knowledge and future perspectives. Calcif Tissue Int. 2010;87(2):99–107. doi:10.1007/s00223-010-9372-1
12. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr Pract. 2020;26(Suppl1):1–46. doi:10.4158/GL-2020-0524SUPPL
13. Baron R, Gori F, Leder BZ. Sclerostin inhibition in the treatment of osteoporosis. In: Contemporary Endocrinology. Humana, Cham; 2020:375–389.
14. Lim SY, Bolster MB. Profile of romosozumab and its potential in the management of osteoporosis. Drug Des Devel Ther. 2017;11:1221–1231. doi:10.2147/DDDT.S127568
15. McClung MR. Role of bone-forming agents in the management of osteoporosis. Aging Clin Exp Res. 2021;33(4):775–791. doi:10.1007/s40520-020-01708-8
16. Ryman JT, Meibohm B. Pharmacokinetics of monoclonal antibodies. CPT Pharmacometrics Syst Pharmacol. 2017;6(9):576–588. doi:10.1002/psp4.12224
17. Zhao L, Ji P, Li Z, Roy P, Sahajwalla CG. The antibody drug absorption following subcutaneous or intramuscular administration and its mathematical description by coupling physiologically based absorption process with the conventional compartment pharmacokinetic model. J Clin Pharmacol. 2013;53(3):314–325. doi:10.1002/jcph.4
18. Solling ASK, Harslof T, Langdahl B. The clinical potential of romosozumab for the prevention of fractures in postmenopausal women with osteoporosis. Ther Adv Musculoskelet Dis. 2018;10(5–6):105–115. doi:10.1177/1759720X18775936
19. McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370(5):412–420. doi:10.1056/NEJMoa1305224
20. Ferrari SL. Osteoporosis: romosozumab to rebuild the foundations of bone strength. Nat Rev Rheumatol. 2018;14(3):128. doi:10.1038/nrrheum.2018.5
21. Langdahl B, Ferrari S, Dempster DW. Bone modeling and remodeling: potential as therapeutic targets for the treatment of osteoporosis. Ther Adv Musculoskelet Dis. 2016;8(6):225–235. doi:10.1177/1759720X16670154
22. Ominsky MS, Libanati C, Niu QT, et al. Sustained modeling-based bone formation during adulthood in cynomolgus monkeys may contribute to continuous BMD gains with denosumab. J Bone Miner Res. 2015;30(7):1280–1289. doi:10.1002/jbmr.2480
23. Chavassieux P, Chapurlat R, Portero-Muzy N, et al. Bone-forming and antiresorptive effects of romosozumab in postmenopausal women with osteoporosis: bone histomorphometry and microcomputed tomography analysis after 2 and 12 months of treatment. J Bone Miner Res. 2019;34(9):1597–1608. doi:10.1002/jbmr.3735
24. Lewiecki EM, Blicharski T, Goemaere S, et al. A Phase III randomized placebo-controlled trial to evaluate efficacy and safety of romosozumab in men with osteoporosis. J Clin Endocrinol Metab. 2018;103(9):3183–3193. doi:10.1210/jc.2017-02163
25. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375(16):1532–1543. doi:10.1056/NEJMoa1607948
26. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377(15):1417–1427. doi:10.1056/NEJMoa1708322
27. McClung M, Chines A, Brown J, et al. Effects of 2 years of treatment with romosozumab followed by 1 year of denosumab or placebo in postmenopausal women with low bone mineral density. Ann Rheumatic Dis. 2015;(74):166–167. doi:10.1136/annrheumdis-2015-eular.2291
28. Lewiecki EM, Dinavahi RV, Lazaretti-Castro M, et al. One Year of Romosozumab Followed by Two Years of Denosumab Maintains Fracture Risk Reductions: results of the FRAME Extension Study. J Bone Miner Res. 2019;34(3):419–428. doi:10.1002/jbmr.3622
29. McClung MR, Bolognese MA, Brown JP, et al. A single dose of zoledronate preserves bone mineral density for up to 2 years after a second course of romosozumab. Osteoporos Int. 2020;31(11):2231–2241. doi:10.1007/s00198-020-05502-0
30. Miller PD, Adachi JD, Albergaria BH, et al. Efficacy and safety of romosozumab among postmenopausal women with osteoporosis and mild-to-moderate chronic kidney disease. J Bone Miner Res. 2022;37(8):1437–1445. doi:10.1002/jbmr.4563
31. Sato M, Inaba M, Yamada S, Emoto M, Ohno Y, Tsujimoto Y. Efficacy of romosozumab in patients with osteoporosis on maintenance hemodialysis in Japan; an observational study. J Bone Miner Metab. 2021;39(6):1082–1090. doi:10.1007/s00774-021-01253-y
32. Cummings SR, McCulloch C. Explanations for the difference in rates of cardiovascular events in a trial of alendronate and romosozumab. Osteoporos Int. 2020;31(6):1019–1021. doi:10.1007/s00198-020-05379-z
33. Food and Drug Administration. Division of bone, reproductive, and urologic products office of drug evaluation iii multidisciplinary review and evaluation document, 2018.Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/761062Orig1s000MultidisciplineR.pdf.
34. Food and Drug Administration. FDA briefing document: meeting of the bone, reproductive and urologic drugs advisory committee, BLA 761062 Romosozumab, Amgen, Inc; 2019. Available from: https://www.fda.gov/media/121257/download.
35. Shoback D, Rosen CJ, Black DM, Cheung AM, Murad MH, Eastell R. Pharmacological management of osteoporosis in postmenopausal women: an endocrine society guideline update. J Clin Endocrinol Metab. 2020;105(3):587–594. doi:10.1210/clinem/dgaa048
36. Kanis JA, Harvey NC, McCloskey E, et al. Algorithm for the management of patients at low, high and very high risk of osteoporotic fractures. Osteoporos Int. 2020;31(1):1–12. doi:10.1007/s00198-019-05176-3
37. Balasubramanian A, Zhang J, Chen L, et al. Risk of subsequent fracture after prior fracture among older women. Osteoporos Int. 2019;30(1):79–92. doi:10.1007/s00198-018-4732-1
38. Lewiecki EM, Cummings SR, Cosman F. Treat-to-target for osteoporosis: is now the time? J Clin Endocrinol Metab. 2013;98(3):946–953. doi:10.1210/jc.2012-3680
39. Lewiecki EM, Kendler DL, Davison KS, et al. Western osteoporosis alliance clinical practice series: treat-to-target for osteoporosis. Am J Med. 2019;132(11):e771–e777. doi:10.1016/j.amjmed.2019.04.044
40. Cosman F, Lewiecki EM, Ebeling PR, et al. T-score as an indicator of fracture risk during treatment with romosozumab or alendronate in the ARCH trial. J Bone Miner Res. 2020;35(7):1333–1342. doi:10.1002/jbmr.3996
41. Obermayer-Pietsch BM, Marin F, McCloskey EV, et al. Effects of two years of daily teriparatide treatment on BMD in postmenopausal women with severe osteoporosis with and without prior antiresorptive treatment. J Bone Miner Res. 2008;23(10):1591–1600. doi:10.1359/jbmr.080506
42. Miller PD, Delmas PD, Lindsay R, et al. Early responsiveness of women with osteoporosis to teriparatide after therapy with alendronate or risedronate. J Clin Endocrinol Metab. 2008;93(10):3785–3793. doi:10.1210/jc.2008-0353
43. Cosman F. Anabolic and antiresorptive therapy for osteoporosis: combination and sequential approaches. Curr Osteoporos Rep. 2014;12(4):385–395. doi:10.1007/s11914-014-0237-9
44. Leder BZ, Tsai JN, Uihlein AV, et al. Two years of Denosumab and teriparatide administration in postmenopausal women with osteoporosis (The DATA Extension Study): a randomized controlled trial. J Clin Endocrinol Metab. 2014;99(5):1694–1700. doi:10.1210/jc.2013-4440
45. Langdahl B, Libanati C, Crittenden DB, et al. Superior gains in bone mineral density and estimated strength at the hip for romosozumab compared with teriparatide in women with postmenopausal osteoporosis transitioning from bisphosphonate therapy: results of the Phase 3 open-label structure study. Endocr Rev. 2016;5. doi:10.1530/boneabs.5.HT5
46. Bouxsein ML, Eastell R, Lui LY, et al. Change in bone density and reduction in fracture risk: a meta-regression of published trials. J Bone Miner Res. 2019;34(4):632–642. doi:10.1002/jbmr.3641
47. Lewiecki EM. Romosozumab, clinical trials, and real-world care of patients with osteoporosis. Ann Transl Med. 2020;8(15):974. doi:10.21037/atm.2020.03.196
48. Cosman F, Kendler DL, Langdahl BL, et al. Romosozumab and antiresorptive treatment: the importance of treatment sequence. Osteoporos Int. 2022;33(6):1243–1256. doi:10.1007/s00198-021-06174-0
49. McClung MR, Bolognese MA, Brown JP, et al. Skeletal responses to romosozumab after 12 months of denosumab. JBMR Plus. 2021;5(7):e10512. doi:10.1002/jbm4.10512
50. McClung MR, Grauer A, Boonen S. Romosozumab in postmenopausal women with osteopenia. N Engl J Med. 2014;370(17):1664–1665. doi:10.1056/NEJMc1402396
51. Ishibashi H, Crittenden DB, Miyauchi A, et al. Romosozumab increases bone mineral density in postmenopausal Japanese women with osteoporosis: a phase 2 study. Bone. 2017;103:209–215. doi:10.1016/j.bone.2017.07.005
52. Soreskog E, Lindberg I, Kanis JA, et al. Cost-effectiveness of romosozumab for the treatment of postmenopausal women with severe osteoporosis at high risk of fracture in Sweden. Osteoporos Int. 2021;32(3):585–594. doi:10.1007/s00198-020-05780-8
53. Goeree R, Burke N, Jobin M, et al. Cost-effectiveness of romosozumab for the treatment of postmenopausal women at very high risk of fracture in Canada. Arch Osteoporos. 2022;17(1):71. doi:10.1007/s11657-022-01106-9
54. Shah AD, Shoback D, Lewiecki EM. Sclerostin inhibition: a novel therapeutic approach in the treatment of osteoporosis. Int J Womens Health. 2015;7:565–580.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
