Back to Journals » Clinical Interventions in Aging » Volume 18
The Relationship Between Serum 25-Hydroxyvitamin D Levels and Osteoporosis in Postmenopausal Women
Received 18 January 2023
Accepted for publication 7 April 2023
Published 18 April 2023 Volume 2023:18 Pages 619—627
DOI https://doi.org/10.2147/CIA.S405317
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Zhi-Ying Wu
Dongmei Wang,1 Yimei Yang2
1Department of Radiology, Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China; 2Department of Obstetrics and Gynecology, Affiliated Hospital of Nantong University, Nantong, People’s Republic of China
Correspondence: Yimei Yang, Affiliated Hospital of Nantong University, #20 Xisi Road, Nantong, 226001, People’s Republic of China, Email [email protected]
Background: Vitamin D status is indicated by serum 25-hydroxyvitamin D [25(OH)D] levels, and the positive effects of high levels of vitamin D on bone mineral density (BMD) have not been ascertained. Therefore, we performed a study to analyze the association between serum 25(OH)D levels and osteoporosis in postmenopausal women.
Methods: We conducted a cross-sectional study using data from the National Health and Nutrition Examination Survey (NHANES). Multiple logistic regression was used to explore the relationship between serum 25(OH)D and osteoporosis of total femur, femoral neck and lumbar spine, with stratified analyses for age (< 65 and ≥ 65 years), BMI (< 25, 25 to < 30, ≥ 30 kg/m2) and survey months (winter months and summer months).
Results: In total, 2058 participants were enrolled in our study. In the fully adjusted model, compared with serum 25(OH)D levels < 50 nmol/L, the odds ratios (ORs) and 95% confidence intervals (CIs) of serum 25(OH)D 50–< 75 nmol/L and ≥ 75 nmol/L were 0.274 (0.138, 0.544) and 0.374 (0.202, 0.693) in osteoporosis of total femur, 0.537 (0.328, 0.879) and 0.583 (0.331, 1.026) in osteoporosis of femoral neck, and 0.614 (0.357, 1.055) and 0.627 (0.368, 1.067) in osteoporosis of lumbar spine, respectively. The protective effect of high 25(OH)D was observed at all three skeletal sites in those ≥ 65 years of age, whereas it was observed only in the total femur in those < 65 years of age.
Conclusion: In conclusion, adequate vitamin D may reduce the risk of osteoporosis in postmenopausal women in the United States, especially in those aged 65 years and older. More attention should be given to serum 25 (OH) D levels to prevent osteoporosis.
Keywords: 25-hydroxyvitamin D, osteoporosis, bone mineral density, postmenopausal women, NHANES
Background
Osteoporosis is a major public health problem because of the increased risk of fracture. More than 2 million Americans suffered fragility fractures in 2005, and more than 70% of these occur in women.1,2 Through dual-energy X-ray absorptiometry (DXA), bone mineral density (BMD) is measured to determine if bone loss (osteopenia or osteoporosis) has occurred.3 Fifty percent of white women have osteopenia or osteoporosis by age 60 years, and age is a crucial risk factor for bone loss.4 The prevalence of osteoporosis in women (10–17%) is higher than that in men (3–5%) over the age of 50.5
Vitamin D is important for bone health.6 Vitamin D status is indicated by serum 25-hydroxyvitamin D [25(OH)D] levels, and less than 30 ng/mL is considered insufficient. 25(OH)D insufficiency is prevalent among elderly women worldwide, with a prevalence of approximately 52% in North American postmenopausal women.7 Approximately 85.3% of the postmenopausal Moroccan women with osteoporotic vertebral fractures have vitamin D insufficiency.8 25(OH)D concentrations decrease significantly with age.4 Calcium and vitamin D supplementation is recommended to strengthen the bones, prevent and treat osteoporosis.9–11
Previous articles have investigated the relationship between serum 25(OH)D and BMD by race, gender, adult and elderly populations extensively.5,9,12 Some studies suppose that vitamin D levels significantly influence BMD and high 25(OH)D concentrations have positive associations with BMD.13,14 To achieve bone health in general population, the Institute of Medicine (IOM) guidelines and the Endocrine Society guidelines recommend 50 and 75 nmol/L respectively as sufficient levels of 25(OH)D.15,16 In a study of 1829 men and postmenopausal women older than 45 years, the highest BMD at lumbar spine and total hip correspond to higher 25(OH)D concentrations (53 and 75 nmol/L, respectively).17 Another study suggests that higher vitamin D levels maintain bone health and a greater drop in vitamin D results in a greater reduction in BMD of total hip and femoral neck.18 However, there is controversy regarding whether higher serum 25(OH)D has positive effects on BMD. The result of a cohort study, however, suggests that postmenopausal bone loss cannot be slowed by high serum 25(OH)D levels in Asian women.19 Another 3 years randomized controlled trial of 260 healthy black American women, 60 years and older, indicates that there is no difference in bone loss rate between serum 25(OH)D above 75 nmol/L and of 50 nmol/L.20 Therefore, we performed a study to analyze the association between serum 25(OH)D levels and osteoporosis in postmenopausal women.
Methods
Study Population
In this study, our data come from the National Health and Nutrition Examination Survey (NHANES) public database. The NHANES is a research project designed to assess the health and nutritional status of adults and children in the United States. Each participant provided written informed consent prior to participating in the survey, which was approved by the Ethics Review Board of the National Center for Health Statistics (NCHS). In addition, this study was based on a secondary analysis of the NHANES publicly available database and was therefore considered exempt by our own institution’s IRB.
Each consecutive 2 years in the NHANES database is a survey cycle. In this study, we included a total of 5 cycles (2005–2010, 2013–2014, 2017–2018). Femur and spine BMD data were not collected for the 2011–2012 and 2015–2016 cycles. In the five cycles included, there were 50,463 participants. Among 5526 postmenopausal women aged 50 years or older, participants with cancer (n=944) and missing BMD (n=2187), 25(OH)D (n=129), and covariates (n=208) were excluded. In total, 2058 participants were enrolled in our study (Figure 1).
 |
Figure 1 Flow chart of research sample selection. |
Study Variables
In this study, serum 25(OH)D was the independent variable. The DiaSorin Radioimmunoassay method was used for NHANES 2005–2006 to measure serum 25(OH)D concentrations. For NHANES 2007–2010, 2013–2014 and 2017–2018, the liquid chromatography-tandem mass spectrometry (LC-MS/MS) method was employed. To provide data consistent across cycles for analysis, serum 25(OH)D data in NHANES 2005–2006 have been converted by using regression to equivalent 25(OH)D measurements from a standardized LC-MS/MS method. According to the Endocrine Society, serum 25(OH)D levels of <50, 50–<75, and ≥75 nmol/L are considered deficient, insufficient, and sufficient, respectively.16
The dependent variables of this study were osteoporosis of total femur, femoral neck and lumbar spine. DXA scans of the lumbar spine and proximal femur have been administered in the NHANES mobile examination center (MEC) from 2005–2010, 2013–2014, and 2017–2018. The femur scans were obtained with Hologic QDR-4500A fan-beam densitometers (Hologic, Inc., Bedford, Massachusetts). The spine scans were acquired with Hologic QDR-4500A fan-beam densitometers in NHANES 2005–2010 and with Hologic Discovery model A densitometers (Hologic, Inc., Bedford, Massachusetts) in NHANES 2013–2014 and 2017–2018. In the 2005–2010 period, the femur scans were analyzed using Hologic Discovery v12.4 software, and spine scans were analyzed using Hologic APEX v3.0 software. APEX v4.0 was used to analyze femur and spine scans acquired in 2013–2014 and 2017–2018. Further details of the DXA examination protocol are provided on the NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm). Osteoporosis was defined as a BMD 2.5 or more standard deviations below the young normal (T-score ≤ −2.5).21
The covariates included in our study were age, race, education level, family poverty income ratio (PIR), body mass index (BMI), total calcium, serum phosphorus, smoking behavior, alcohol consumption, moderate activities, survey months, and years since menopause. Among these variables, age, family PIR, BMI, total calcium, serum phosphorus, and years since menopause were used as continuous variables, and the rest were categorical variables.
Statistical Analysis
Because of the complex sample design of NHANES, we applied appropriate sample weights when analyzing the data. In the description of baseline data, continuous and categorical variables were expressed as the weighted mean (standard error, SE) and numbers (weighted percentage), respectively. Between different groups of serum 25(OH)D concentrations, for continuous and categorical variables, P values were calculated using the weighted linear regression and chi-square test, respectively. Multiple logistic regression was used to explore the relationship between serum 25(OH)D and osteoporosis of total femur, femoral neck and lumbar spine, with stratified analyses for age (<65 and ≥65 years), BMI (<25, 25 to <30, ≥30 kg/m2) and survey months (winter months and summer months). Full adjustment model was adjusted for age, race, education level, family PIR, BMI, total calcium, serum phosphorus, smoking behavior, alcohol consumption, moderate activities, survey months, and years since menopause. The analyses were conducted using Stata/BE V17 (StataCorp LLC, USA), R software (version 4.2.0) and EmpowerStats software (X&Y Solutions, Boston, MA), and P<0.05 was considered statistically significant.
Results
After excluding the missing data of serum 25(OH)D, BMD of total femur, femoral neck and lumbar spine, and covariates, the final analysis sample was 2058 (Figure 1). Table 1 presents the characteristics of the participants in the study. Race, education level, family PIR, BMI, smoking behavior, alcohol consumption, moderate activities, and survey months differed significantly among the three serum 25(OH)D groups (<50, 50–<75, ≥75 nmol/L).
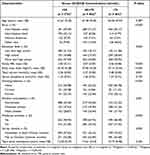 |
Table 1 Weighted Characteristics of the Study Participants Based on Serum 25-Hydroxyvitamin D [25(OH)D] Concentrations |
Table 2 shows the associations between serum 25(OH)D and osteoporosis of total femur, femoral neck and lumbar spine. In the unadjusted model, statistical significance was observed only in the total femur. After adjusting for age, race, and BMI, with the increase in serum 25(OH)D concentrations, the risk of osteoporosis in the total femur, femoral neck, and lumbar spine decreased. In the fully adjusted model, compared with serum 25(OH)D levels <50 nmol/L, the odds ratios (ORs) and 95% confidence intervals (CIs) of serum 25(OH)D 50–<75 and ≥75 nmol/L were 0.274 (0.138, 0.544) and 0.374 (0.202, 0.693) in osteoporosis of total femur, 0.537 (0.328, 0.879) and 0.583 (0.331, 1.026) in osteoporosis of femoral neck, and 0.614 (0.357, 1.055) and 0.627 (0.368, 1.067) in osteoporosis of lumbar spine, respectively.
 |
Table 2 Associations Between Serum 25(OH)D and Osteoporosis of Total Femur, Femoral Neck and Lumbar Spine |
Figures 2-4 show the results of the regression analysis after stratification by age, BMI and survey months. As shown in the figure, the protective effect of high 25(OH)D was observed at all three skeletal sites in those ≥65 years of age, whereas it was observed only in the total femur in those <65 years of age (Figure 2). In addition, the protective effect of high 25(OH)D on total femur was observed at BMI ≥25 kg/m2, and this relationship was also seen in the femoral neck at BMI 25 to <30 kg/m2 (Figure 3). After stratification by survey months, a decreased risk of osteoporosis in the total femur and lumbar spine was observed in the winter months with increasing serum 25(OH)D concentrations (Figure 4).
Discussion
The results of our study showed that postmenopausal women with higher serum 25(OH)D levels are less likely to suffer from osteoporosis. Both serum 25(OH)D levels of 50–<75 nmol/L and ≥75 nmol/L were protective against osteoporosis, compared with serum 25(OH)D levels <50 nmol/L. Specifically, high serum 25(OH)D levels had a significant protective effect on total femur and femoral neck, but not on lumbar spine. After age stratification, the protective effect of high 25(OH)D levels was observed at all three skeletal sites in elderly women aged ≥65 years.
Deficiency of vitamin D in elderly postmenopausal women is common, and it is considered a key determinant for bone health.7 Vitamin D deficiency leads to lower BMD and osteoporosis.12,22 Several studies have shown that higher serum 25(OH)D levels improve bone health, and thus reduce the risk of osteoporosis and fragility fractures.23,24 However, the relationship between 25(OH)D levels and BMD at different bone sites in postmenopausal women is controversial. A study of postmenopausal Chinese women showed that serum 25(OH)D predicted femoral neck BMD, but not lumbar spine BMD.25 According to another study, higher levels of 25(OH)D in postmenopausal Japanese women (≥70 nmol/L) were associated with a greater BMD in femoral neck.24 Furthermore, Yoshimura et al26 discovered that osteoporosis at the femoral neck, but not at the L2-4, may be prevented by higher serum 25(OH)D levels. In contrast, Garnero et al27 noted that 25(OH)D levels were not related to hip BMD. However, Pasco et al13 reported that lumbar and hip BMD were more likely normal in women with high serum 25(OH)D levels than in those with low levels. According to our findings, high levels of 25(OH)D protected bone health in the total femur and femoral neck, but not the lumbar spine.
Vitamin D deficiency causes an increase in parathyroid hormone (PTH) secretion, which results in high bone turnover and increases bone resorption in postmenopausal women.28 Vitamin D deficiency leads to bone loss, which occurs from the cortical bone and thus contributes to the pathogenesis of osteoporosis.29 In studies of vitamin D supplementation, one study30 reported that vitamin D supplementation can delay bone loss. Another study23 indicated that vitamin D supplementation can improve bone mass in people with vitamin D deficiency. Burger et al31 showed that femoral neck BMD increased significantly with vitamin D supplementation, while lumbar BMD did not change. Given vitamin D promotes calcium absorption and may have a positive effect on cortical BMD. Because of this, femoral necks with a significant amount of cortical bone may be more susceptible than lumbar spines. Our results are consistent with this principle.
In our study, we found that high vitamin D status has significant protective effects at the three bone sites in elderly women aged ≥65 years, but not in women aged <65 years. There was an inconsistency according to the relationship between 25(OH)D levels and BMD changes in several studies in elderly men and women. The negative effect of low 25(OH)D levels on hip BMD varied by age, with an association among older men (aged ≥75 years) but not younger men.32 In a 4-year follow-up study of older subjects (mean age, 74 years), there was no association with 25(OH)D levels and lumbar or hip BMD changes.33 Furthermore, Kitamura et al19 showed that high serum 25(OH)D levels do not retard bone loss at lumbar spine or femoral neck in Japanese women (mean age, 63.1 years). The reason for the inconsistency is unclear. It is assumed that the age and vitamin D status of populations in different studies are responsible for the various results.
In the present study, the mean value of serum 25(OH)D was 76.65 nmol/L in summer months, which was significantly higher than that of 70.99 nmol/L in winter months (P = 0.015). The results were consistent with the previous study.34 Seasonal changes in BMD had also been reported, and BMD in winter was lower than that in summer.35 The changes of BMD were affected by the variations of vitamin D levels.18 Our results suggested that sufficient vitamin D in winter has a stronger protective effect on osteoporosis in elderly postmenopausal women. It might be more important for postmenopausal women to maintain higher concentrations of serum 25(OH)D in winter.
We assessed the association of serum 25(OH)D levels and osteoporosis at multiple bone sites in a representative sample of the US population, and adjusted for exhaustive covariates. There are several limitations. First, this is a cross-sectional study, and a causal role for higher vitamin D levels in reducing the risk of osteoporosis cannot be determined. Second, some variables were self-reported by participants, which may have recall bias. Longitudinal studies with larger sample sizes are needed in the future to further investigate the association of serum 25(OH)D concentrations and osteoporosis.
Conclusion
In conclusion, adequate vitamin D may reduce the risk of osteoporosis in postmenopausal women in the United States, especially in those aged 65 years and older. More attention should be given to serum 25(OH)D levels to prevent osteoporosis.
Abbreviations
25(OH)D, 25-hydroxyvitamin D; NHANES, National Health and Nutrition Examination Survey; ORs, odds ratios; CIs, confidence intervals; DXA, dual-energy X-ray absorptiometry; BMD, bone mineral density; NCHS, National Center for Health Statistics; LC-MS/MS, liquid chromatography-tandem mass spectrometry; MEC, mobile examination center; PIR, poverty income ratio; BMI, body mass index; PTH, parathyroid hormone.
Data Sharing Statement
The data analyzed in this study are available at the NHANES website (https://www.cdc.gov/nchs/nhanes/).
Acknowledgments
We thank all the staff of NHANES for their hard work. Thanks also to the participants who provided the data.
Funding
This study was supported by the Nantong Technology Project (Program No. MSZ2022086).
Disclosure
Dongmei Wang and Yimei Yang declare that they have no conflict of interest.
References
1. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465–475. doi:10.1359/jbmr.061113
2. Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520–2526.
3. Kanis JA, Melton LJ, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9:1137–1141.
4. Looker AC, Orwoll ES, Johnston CC, et al. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res. 1997;12:1761–1768.
5. Looker AC, Sarafrazi Isfahani N, Fan B, Shepherd JA. Trends in osteoporosis and low bone mass in older US adults, 2005-2006 through 2013-2014. Osteoporos Int. 2017;28:1979–1988.
6. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281.
7. Holick MF, Siris ES, Binkley N, et al. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90:3215–3224.
8. El Maghraoui A, Ouzzif Z, Mounach A, et al. Hypovitaminosis D and prevalent asymptomatic vertebral fractures in Moroccan postmenopausal women. BMC Womens Health. 2012;12:11.
9. Bischoff-Ferrari HA, Kiel DP, Dawson-Hughes B, et al. Dietary calcium and serum 25-hydroxyvitamin D status in relation to BMD among U.S. adults. J Bone Miner Res. 2009;24:935–942.
10. Reid IR. Short-term and long-term effects of osteoporosis therapies. Nat Rev Endocrinol. 2015;11:418–428.
11. Chiang CM, Ismaeel A, Griffis RB, Weems S. Effects of Vitamin D Supplementation on Muscle Strength in Athletes: a Systematic Review. J Strength Cond Res. 2017;31:566–574.
12. Gutiérrez OM, Farwell WR, Kermah D, Taylor EN. Racial differences in the relationship between vitamin D, bone mineral density, and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos Int. 2011;22:1745–1753.
13. Pasco JA, Henry MJ, Nicholson GC, Brennan SL, Kotowicz MA. Behavioural and physical characteristics associated with vitamin D status in women. Bone. 2009;44:1085–1091.
14. Sadat-Ali M, Al Elq AH, Al-Turki HA, Al-Mulhim FA, Al-Ali AK. Influence of vitamin D levels on bone mineral density and osteoporosis. Ann Saudi Med. 2011;31:602–608.
15. Ross AC, Manson JE, Abrams SA, et al. The 2011 dietary reference intakes for calcium and vitamin D: what dietetics practitioners need to know. J Am Diet Assoc. 2011;111:524–527.
16. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930.
17. Aleteng Q, Zhao L, Lin H, et al. Optimal vitamin D Status in a middle-aged and elderly population residing in Shanghai, China. Med Sci Monit. 2017;23:6001–6011.
18. Zhu K, Hunter M, Hui J, et al. Longitudinal stability of vitamin D status and its association with bone mineral density in middle-aged Australians. J Endocr Soc. 2022;7:bvac187.
19. Kitamura K, Nakamura K, Saito T, et al. High serum 25-hydroxyvitamin D levels do not retard postmenopausal bone loss in Japanese women: the Yokogoshi study. Arch Osteoporos. 2013;8:153.
20. Aloia J, Fazzari M, Islam S, et al. Vitamin D supplementation in elderly Black women does not prevent bone loss: a randomized controlled trial. J Bone Miner Res. 2018;33:1916–1922.
21. Dimai HP. Use of dual-energy X-ray absorptiometry (DXA) for diagnosis and fracture risk assessment; WHO-criteria, T- and Z-score, and reference databases. Bone. 2017;104:39–43.
22. Çolak Y, Afzal S, Nordestgaard BG. 25-Hydroxyvitamin D and risk of osteoporotic fractures: Mendelian randomization analysis in 2 large population-based cohorts. Clin Chem. 2020;66:676–685.
23. Winzenberg T, Powell S, Shaw KA, Jones G. Effects of vitamin D supplementation on bone density in healthy children: systematic review and meta-analysis. BMJ. 2011;342:c7254.
24. Nakamura K, Tsugawa N, Saito T, et al. Vitamin D status, bone mass, and bone metabolism in home-dwelling postmenopausal Japanese women: yokogoshi Study. Bone. 2008;42:271–277.
25. Deng WM, Wei QS, Tan X, Shao Y, Chen XH, Sun WS. Relation of serum 25 hydroxyvitamin D levels to bone mineral density in southern Chinese postmenopausal women: a preliminary study. Indian J Med Res. 2015;142:430–437.
26. Yoshimura N, Muraki S, Oka H, et al. Serum levels of 25-hydroxyvitamin D and the occurrence of musculoskeletal diseases: a 3-year follow-up to the road study. Osteoporos Int. 2015;26:151–161.
27. Garnero P, Munoz F, Sornay-Rendu E, Delmas PD. Associations of vitamin D status with bone mineral density, bone turnover, bone loss and fracture risk in healthy postmenopausal women. The OFELY study. Bone. 2007;40:716–722.
28. Gao C, Qiao J, Li SS, et al. The levels of bone turnover markers 25(OH)D and PTH and their relationship with bone mineral density in postmenopausal women in a suburban district in China. Osteoporos Int. 2017;28:211–218.
29. Lips P. Vitamin D physiology. Prog Biophys Mol Biol. 2006;92:4–8.
30. Lee DY, Jee JH, Cho YY, et al. Serum 25-hydroxyvitamin D cutoffs for functional bone measures in postmenopausal osteoporosis. Osteoporos Int. 2017;28:1377–1384.
31. Burger H, van Daele PL, Algra D, et al. The association between age and bone mineral density in men and women aged 55 years and over: the Rotterdam Study. Bone Miner. 1994;25:1–13.
32. Ensrud KE, Taylor BC, Paudel ML, et al. Serum 25-hydroxyvitamin D levels and rate of Hip bone loss in older men. J Clin Endocrinol Metab. 2009;94:2773–2780.
33. Hannan MT, Felson DT, Dawson-Hughes B, et al. Risk factors for longitudinal bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res. 2000;15:710–720.
34. MacDonald D, Swaminathan R. Seasonal variation in 25-OH vitamin D in plasma of Hong Kong Chinese. Clin Chem. 1988;34:2375.
35. Rosen CJ, Morrison A, Zhou H, et al. Elderly women in northern New England exhibit seasonal changes in bone mineral density and calciotropic hormones. Bone Miner. 1994;25:83–92.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



