Back to Journals » Journal of Asthma and Allergy » Volume 15
Characteristics of Oral Corticosteroid Users Among Persons with Asthma on GINA Step 3 Therapy and Above: A Cross-Sectional Study in Portuguese Community Pharmacies
Authors Romão M , Bulhosa C, Mendes Z, Sousa C, Silva G, Pereira M, Bernardo F , Teixeira Rodrigues A, Fonseca JA, Correia de Sousa J
Received 9 June 2022
Accepted for publication 28 October 2022
Published 9 November 2022 Volume 2022:15 Pages 1579—1592
DOI https://doi.org/10.2147/JAA.S377896
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Amrita Dosanjh
Mariana Romão,1 Carolina Bulhosa,1 Zilda Mendes,1 Catarina Sousa,2 Graça Silva,2 Mariana Pereira,3 Filipa Bernardo,2 António Teixeira Rodrigues,1,4 João A Fonseca,3,5,6 Jaime Correia de Sousa4
1Centre for Health Evaluation & Research/Infosaude (CEFAR/IS), National Association of Pharmacies (ANF), Lisbon, Portugal; 2Medical Department, AstraZeneca, Barcarena, Portugal; 3MEDIDA – Medicina, Educação, Investigação, Desenvolvimento e Avaliação, Porto, Portugal; 4Life and Health Sciences Research Institute (ICVS), PT Government Associate Laboratory, School of Medicine, University of Minho, Braga, Portugal; 5Center for Health Technology and Services Research (CINTESIS), Faculty of Medicine, University of Porto, Porto, Portugal; 6CUF Allergy Unit, Porto Hospital and Institute, Porto, Portugal
Correspondence: Mariana Romão, Centre for Health Evaluation & Research/Infosaude (CEFAR/IS), National Association of Pharmacies (ANF), Rua Marechal Saldanha 1, Lisboa, 1249-069, Portugal, Email [email protected]
Purpose: Oral corticosteroids (OCS) are frequently used in asthma management but have an important risk-profile. The aim of the study is to characterize and compare the sociodemographic and clinical characteristics, treatment regimen and asthma control between OCS users and non-users among the population of asthma patients (≥ 18 years) at GINA step 3 and above treated with a fixed combination of an inhaled corticosteroid and a long-acting beta-agonist (ICS/LABA).
Methods: Cross-sectional study in Portuguese community pharmacies. Data was collected via paper-based interview delivered at the pharmacy (sociodemographic characteristics and asthma treatment regimen, namely ICS/LABA and OCS utilization), followed by a telephonic interview collecting smoking history, comorbidities, body mass index (BMI), history of exacerbations and asthma-related healthcare resource utilization (HCRU) in the previous 12 months, as well as asthma control using the Control of Allergic Rhinitis and Asthma Test (CARAT®).
Results: A total of 347 patients recruited in 98 pharmacies were included in the analysis. Of those, 328 had completed both questionnaires. A quarter of the individuals reported OCS use in the previous 12 months (OCS users), either as add-on therapy (6%) or exacerbation treatment (19%). Patients were mostly females (72%), with an average age of 59.5 years (SD=15.4). OCS users were significantly older and reported more frequently having conjunctivitis (25.9% vs 15.0%), osteoporosis (25.9% vs 13.4%), arthritis (14.6% vs 6.9%), and gastrointestinal disease (16.1% vs 8.1%). OCS users also reported greater urgent HCRU: unscheduled consultations (33.3% vs 9.3%) and emergency department (ED) visits (32.1% vs 12.1%). Both groups presented poor disease control (85.2% of OCS users vs 72.9% of non-OCS users).
Conclusion: These results highlight the burden of OCS therapy to asthma patients and the need to improve asthma management, by adopting OCS sparing strategies in this subgroup of patients.
Keywords: asthma, ICS/LABA, oral corticosteroids, asthma control, CARAT, COVID-19
Background
Asthma affects around 339 million people worldwide.1 Although death rates are higher in low and middle Socio-Demographic Index (SDI) countries, the prevalence of asthma is higher in high SDI countries.2 This condition is accountable for approximately 1% of the total disability-adjusted life years (DALYs), and 20.8% of total DALYs from chronic respiratory disease, globally.1 In Portugal, asthma estimated prevalence lays between 6.8% and 10.2%,3,4 ranking above the EU average.5 It is responsible for 1.3% of all-cause DALYs, which represents 26.3% of DALYs from chronic respiratory diseases in the country.2
Asthma symptoms and exacerbations limit patients’ activity and entail both high treatment costs and productivity losses.6 It also has a negative psychological and emotional impact on patients and their families, even if clinically controlled.7 Altogether, this translates into poor quality of life outcomes and a significant economic burden on patients and society in general.5
The Global Initiative for Asthma (GINA) recommendations state that patients on the higher treatment steps (steps 3, 4 and 5) should receive a combination of an inhaled corticosteroid and a long-acting beta-agonist (ICS/LABA) as controller therapy, increasing the dose according to the step.6 Evidence has shown that this treatment combination markedly reduces the frequency and severity of asthma symptoms and also reduces the risk of exacerbations and death.8–10 Asthma control depends on individual and disease factors, as well as on comorbidities, inhaler technique, smoking habits, and adherence to therapy. Patients with poor asthma control experience burdensome asthma symptoms and have more frequent and/or more serious exacerbation episodes. Such episodes are often unpredictable and very frightening for the patients and their families, sometimes requiring emergency department assistance and more or less prolonged treatment with oral corticosteroids (OCS).6 Additionally, patients that remain uncontrolled despite high doses of ICS/LABA may require add-on OCS to achieve disease control (GINA step 5). Thus, asthma patients are frequently exposed to treatment with systemic corticosteroids.11 Previously published literature indicates that, in the United States, Italy, France, Germany, or the United Kingdom, around 22.9% to 65% of patients with moderate or severe asthma (GINA steps 4 and 5) are treated with OCS (either as short- or long-term treatment).12,13
Oral corticosteroids have a significant side-effect profile, being associated with increased susceptibility to infections, osteoporosis, obesity, diabetes, heart failure, and other systemic corticosteroid-related morbidities, independent of the treatment pattern.14,15 Regular OCS use is not only associated with increased morbidity and mortality, but also represents a significant cost driver in asthma management.16,17
To our knowledge, there is still no published data about the frequency and the characteristics of OCS users among patients with severe asthma in Portugal. The results presented in this paper are part of a cross-sectional study conducted in Portuguese community pharmacies to characterize patients at GINA steps 3 and above. This paper aims to characterize the subgroup using OCS and compare them with those who do not use OCS. Additionally, the study explores the relationship between the different patient characteristics and the use of oral corticosteroids.
Methods
The current paper follows the STROBE guidelines for reporting of cross-sectional studies.18 This was an observational, cross-sectional, multicenter study conducted in Portuguese community pharmacies affiliated to the National Association of Pharmacies (ANF) (94% of all Portuguese community pharmacies). Each enrolled pharmacy assigned at least one pharmacist to receive adequate training to ensure the compliance with the study procedures at the pharmacy.
Pharmacies recruited adult (≥18 years) asthma patients without any cognition impairment receiving one of the following treatment regimens for asthma: (1) a high-dose inhaled corticosteroid combined with long-acting beta-agonist (ICS/LABA); (2) a medium-dose ICS/LABA plus another controller treatment; or (3) an ICS (irrespectively of the dose) and an oral corticosteroid (OCS) for asthma. The total daily ICS doses according to international nonproprietary name (INN) as well as controller treatments considered for patient screening and data analysis are described in Tables S1 and S2. A flowchart was developed to assist the pharmacist in the screening process, including a step to perform the calculation of the daily dose of inhaled corticosteroids.
A pop-up triggered by the eligible drugs was implemented on the pharmacy software to remind the pharmacist to check the eligibility criteria in the context of a pharmacy dispense. Pharmacists could also identify eligible patients by consulting the patient records or invite patients they knew to be current users of such therapy.
Eligible patients were invited to participate in the study and asked to sign an informed consent form. If the patient declined to participate, a refusal form collecting basic information (gender, age group, inclusion criteria, OCS utilization and reason for declining) was filled in by the pharmacist, to allow a comparison between participants and non-participants.
Data Collection
Data were collected in two parts. The first consisted of a paper-based interview delivered by a trained pharmacist on the enrolment day, collecting information referring to patient’s treatment regimen and adherence to ICS/LABA. Patients were asked if they had used OCS anytime in the previous 12 months. If yes, the pattern of OCS use was assessed (long-term use = using OCS every day for at least 29 consecutive days; short-term use = using OCS for periods ≤28 days) and the information regarding the INN, dosage, posology, duration, and indication (asthma/other disease) was collected. For short-term OCS use, the time since the last episode was also collected. The second part was a telephonic interview (scheduled to 24 to 48 hours after the enrolment day) regarding patient’s clinical and anthropometric characteristics (comorbidities, smoking status, BMI), history of exacerbations in the previous year (unscheduled medical appointments, emergency department visits, and hospital admissions), healthcare resource utilization in the previous year (scheduled medical appointments and doctor’s medical specialty), and asthma symptom control in the previous 4 weeks (telephone version of the Control of Allergic Rhinitis and Asthma Test – CARAT®).19
Sample Size
The study was powered to allow proportion estimates with a 95% confidence interval and a maximum absolute error of 5% in the characterization of the included patients. A 20% attrition rate between the enrollment and the phone interview was also considered, resulting in an estimated sample size of 461 patients. Based on the literature,14 we expected that, among these, there would be at least 96 patients treated with both ICS/LABA and OCS, which would allow the characterization of the OCS use with a maximum absolute error of 10% for 95% confidence interval.
Statistical Analysis
Recruiting pharmacies were characterized by geographic distribution and compared with the national distribution of pharmacies, using Chi-square test for adjustment. Missing at random assumption was tested by comparing sociodemographic variables of respondents vs non-respondent patients. A descriptive analysis of all variables collected was performed. Categorical variables were summarized by absolute and relative frequencies. Continuous variables were summarized using central tendency and dispersion measurements. Proportions were calculated excluding missing values.
All corticosteroid doses were converted to prednisolone equivalent, as previously done by other authors.11,15,16,20 The conversion table is available in Table S3. For long-term users, the OCS exposure – expressed in mg of prednisolone/day/year – was calculated using the converted daily dose, multiplied by the number of days using OCS in the previous year and divided by 365 days. For patients using OCS for >1 year, OCS exposure equals the converted daily dose. For short-term users, since we only collected the INN and posology data for the most recent OCS episode, those were assumed as a proxy for the dose of all the episodes reported by the patient for the previous year. Hence, the OCS exposure was estimated multiplying this dose by the number of episodes and the average number of days per episode (patient-reported), dividing by 365. OCS users were then classified into low-dose users (<2.5mg prednisolone/day/year) and high-dose users (≥2.5mg prednisolone/day/year).21,22
For subgroup analysis (OCS users vs non-OCS users), the Chi-square test for independence or Fisher exact test were used for categorical variables, as appropriate, and non-parametric test such as Mann–Whitney Wilcoxon test was used for continuous variables (after rejecting the normality and homoscedasticity assumption for parametric tests). OCS use was defined as having used OCS at least once in the previous 12 months.
Univariable logistic regression was used to select candidate variables (p-value ≤ 0.25) to enter a multivariable logistic regression model to identify significant factors (among sociodemographic, clinical, health, and therapeutic characteristics) associated with OCS use. The final model was obtained by a stepwise logistic regression analysis of the variables selected in the univariate analysis. Odds ratios (OR) and 95% confidence intervals (CI) were calculated. Data analysis was performed with SAS® Enterprise Guide® 4.1 using a p-value cut-off of 0.05.
Methodological Adaptations in the Context of the COVID-19 Pandemic
The COVID-19 pandemic largely affected the functioning of community pharmacies and the daily routines of pharmacy users, especially respiratory patients, posing significant challenges to the recruitment. Thus, there was the need to adopt a pragmatic approach to the protocol and implement mitigation measures to increase the study sample: (1) The recruitment period was extended (from 4 to 7.5 months); (2) the recruitment rate was closely monitored and promoted by reinforcing eligibility criteria and study procedures among pharmacy teams (via emails and phone calls); (3) Pharmacists were incentivized to actively search for eligible patients in the pharmacy records; (4) a second training session was carried out in March 2021 to review the study procedures and eligibility criteria, as well as sharing successful experiences of pharmacists participating in the study, aiming to boost the recruitment after the lockdown period; In the data analysis phase, (5) the thresholds for daily corticosteroid doses were adjusted so that the superior limit of each category would be included in the category above and the list of eligible controller treatments was extended; (6) patients with a 48 h+ interval between the two parts of the questionnaire were also included. The revised criteria are displayed in Table S2.
Results
Among the 1221 pharmacies invited to participate in the study, 181 (15%) accepted the invitation and received the proper training to ensure compliance with the study procedures (Figure 1). From the 3rd of November 2020 to the 14th of June 2021, 106 pharmacies invited a total of 455 patients, of which 410 accepted to be part of the study (participation rate 90.1%). Patients who did not meet the eligibility criteria (n=53) or whose eligibility could not be verified (n=10) were excluded, resulting in 347 patients with the first part of the questionnaire complete, recruited in 98 pharmacies. Of these, 328 (95.5%) also completed the phone interview. This sample size allows proportion estimates with 95% confidence interval and a maximum error of 5% for ICS/LABA users and 11% for the subgroup of OCS users. The median time between the enrollment and telephone interview was 4 days.
 |
Figure 1 Study flowchart. |
There were no statistically significant differences between the regional distribution of pharmacies with recruited patients and the global universe of pharmacies. The proportion of men was higher among those who declined to participate in the study (51.1%) than among those who accepted (28.5%) (p-value=0.0021), and the age distribution of these two groups also differed (p-value=0.0046). We found no statistically significant differences regarding OCS utilization between participants and non-participants (p>0.05). Among those who accepted to participate, no statistically significant differences were found between the complete cases and the 19 patients who did not complete the telephone interview, regarding gender and age.
Most of the individuals in the study were female (71.5%). The average age was 59.5 years (SD=15.4) and about 40% of the patients were above 65 years old (Table 1). Two thirds of the sample (66.8%) were above the normal BMI: 38.4% were overweight and 28.4% were obese. There were 10.0% smokers and 23.8% ex-smokers. Four in every five patients (79.9%) reported having at least another disease other than asthma. On average, each patient had 3.1 (SD=2.3) comorbidities. Anxiety was the most common comorbidity (49.4%) in the total sample, followed by rhinitis (47.0%), hypertension (40.2%), and sinusitis (37.5%). Depression was present in one in five studied patients (21.3%). Approximately three out of four patients reported poor disease control (75.9%), according to the total CARAT® score, that assesses the control of rhinitis and asthma symptoms over the preceding 4 weeks (average score=19.4, SD=6.5). Patients were mostly at step 4 (47.8%) and step 5 (51.9%), as defined by GINA 2021. At the time of study inslusion, most patients were already using the ICS/LABA (96.5%) and reported using it every day (93.6%).
 |
Table 1 Characteristics of Adult Asthma Patients Receiving GINA Step 3 Therapy and Above, Overall and by History of OCS Use in the Previous 12 Months |
OCS Users and Non-Users
A total of 86 patients (24.8% of total sample) reported to have used OCS in the previous year (Table 1). Patients using OCS were mostly females (70.9%), with an average age of 56.3 years (SD=16.2). This subgroup was significantly younger, compared to non-OCS users (average age of 60.5 years, SD=15.0; p<0.05). No statistically significant differences were found regarding sex, BMI, smoking status, and ICS/LABA utilization (current/new user, time since using ICS/LABA, and adherence) (p>0.05). Although there were no differences between groups regarding the proportion of smokers, patients in the OCS group smoked significantly less cigarettes per day – nearly half the average number reported by non-OCS users (6.4 vs 11.1, respectively; p<0.05).
The average number of comorbidities was similar between both groups (3.0, SD=2.2 for non-OCS; and 3.3, SD=2.6 for OS users), but significantly higher proportions of OCS-users reported conjunctivitis (25.9% vs 15.0%), osteoporosis (25.9% vs 13.4%), gastrointestinal disease (16.1% vs 8.1%), and arthritis (14.6% vs 6.9%), compared to non-OCS users (p<0.05).
Healthcare Resource Utilization and Asthma Control
In the 12 months prior to the study, 59.2% of the participants had at least one routine medical appointment (64.2% of OCS users and 57.5% of non-users, p>0.05) (Table 2). A greater proportion of OCS patients reported having at least one unscheduled consultation (33.3% vs 9.3%) or emergency department (ED) visit (32.1% vs 12.1%) due to asthma in that same period, compared to non-OCS users (p<0.0001). Analyzing unscheduled consultations and ED visits together, OCS users reported greater urgent healthcare resource use (49.4% vs 19.0%, p<0.0001).
 |
Table 2 Healthcare Resource Utilization and Measures of Asthma Control, Overall and by History of OCS Use in the Previous 12 Months |
Among those who recurred to the ED, 17.9% (3% of the total sample) were admitted to the hospital for at least one night (20.0% among non-OCS users and 15.4% among OCS users). Notably, about 30.2% of the patients did not have any type of medical appointment (whether planned or not) in the 12 months prior to the study. This proportion is lower among the OCS users than non-users (18.5% and 34.0%, respectively, p=0.008).
Regarding disease control, the CARAT® test revealed a high proportion of patients with poorly controlled disease (score <25) in both groups, although significantly higher in the OCS users’ group (85.2% vs 72.9% in the non-OCS group, p<0.05), as shown in Figure 2.
Patient Characteristics Associated with OCS Use
The logistic regression analysis included 328 patients. The remaining 19 patients were excluded due to having at least one missing variable. Four variables were significantly associated with greater odds of using OCS: age ≤65 years (OR=1.999; CI: [1.087; 3.676]), along with having conjunctivitis (OR=2.178; CI: [1.110; 4.274]) or osteoporosis (OR=2.612; CI: [1.272; 5.363]), and using at least one urgent healthcare resource in the previous 12 months (OR=3.617; CI: [2.018; 6.483]) (Figure 3). On the other hand, the odds of using OCS were slightly lower among those with better disease control in the previous 4 weeks (ie, higher CARAT score), compared to those with poorer disease control (OR=0.954; CI: [0.914; 0.996]).
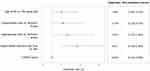 |
Figure 3 Patient characteristics significantly associated with the use of oral corticosteroids (p<0.05). |
OCS Utilization Profile
Of the 86 patients who reported to have used OCS in the previous 12 months, 21 (24.4%) were using it as add-on controller therapy (long-term use), and 65 (75.6%) were prescribed with OCS for exacerbation treatment (short-term use).
Long-term OCS users had an average OCS exposure of 12.6mg prednisolone/day/year (SD=11.9) (n=16). On average, these patients had been using OCS for 7.2 years (SD=6.7), and 57.1% used it for asthma only, 14.3% for asthma and other condition (unspecified) and 28.6% for a pathology other than asthma (unspecified).
Short-term OCS users reported an average of 2.1 episodes (SD=2.9) of OCS use in the previous year, with an average length of 8.5 days (SD=5.7). The average OCS exposure was equivalent to 1.6mg prednisolone/day/year (SD=2.3mg) (n=49). Only 8.2% of the patients in this subgroup were being treated for an exacerbation at the time of the study. Less than 10% of the short-term OCS users received this therapy for a disease other than asthma (unspecified), while 12.3% received it for asthma and other condition (unspecified) and 78.5% received it specifically for asthma.
The OCS exposure was estimated for 65 patients (the remaining had missing information that precluded the estimation), concluding that 23 patients (35.4%) were high-dose users. Table 3 shows the distribution of the patient characteristics associated with OCS use (identified in the logistic regression model) for each of these subgroups (low- and high-dose users). The complete table appears on Table S4. A higher proportion of patients above 65 years old, as well as a higher prevalence of osteoporosis was observed among high-dose OCS users.
 |
Table 3 Patient Characteristics Associated with OCS Use, According to the Exposure to OCS |
Discussion
This was an observational, cross-sectional, multicentre study conducted in Portuguese community pharmacies to characterize asthma patients at GINA’s higher treatment steps and the subgroup additionally using oral corticosteroids. Overall, the characteristics of the patients included match those described in the literature for similar populations.23–26 It is important to highlight that patient recruitment and data collection took place during the COVID-19 pandemic, and therefore most of the recall period overlapped with the first year of the public health crisis.
One in four patients (24.8%) reported to have used OCS in the previous year, either as short- or long-term therapy. A study including several European countries estimated that 22.9% of German patients at GINA steps 4 and 5 attending a General Practitioners office and 27.9% of the patients treated by pulmonologists were prescribed with OCS.12 The same study found higher prevalence of OCS use in Italy, United Kingdom and France: 42.5%, 46.2%, and 48.8%, respectively. A systematic literature review revealed that 33.2% to 65% of patients with moderate or severe asthma were prescribed with OCS.13 The proportion of OCS users found in our study sits considerably lower than the other countries’ estimates, apart from Germany. There could be several explanations for this finding. One is that, as we will further discuss, the lower rates of severe asthma exacerbations, as well as the reduced number of contacts with prescribers due to the pandemic, probably decreased the need and limited the access to OCS prescriptions. Another potential reason is the avoidance of OCS prescription or use (either as short- or long-term therapy) due to fear of immunosuppression facing the SARS-CoV-2 pandemic. Ultimately, our results may reflect a low baseline level of oral corticosteroid utilization among patients at GINA step 3 and above in Portugal.
Three out of ten patients reported not having used any healthcare resource in the year prior to the study, and a considerable proportion of patients (40.8%) did not attend to any routine medical appointment in the same period, which goes against the asthma management recommendations that advocate each patient should have an asthma review at least once a year.27 Unscheduled medical appointments,28–30 Emergency Department visits (particularly among non-OCS users),28,30,31 and hospitalizations29,30,32 were also less frequent than reported by other studies. In fact, only 18% of the participants visited the ED due to asthma in the previous year, a lower proportion than that reported by step 3+ patients using a short-acting beta agonist (SABA) inhaler (45%) in a previous study conducted in Portugal.33 Literature shows reduced Emergency Department utilization by respiratory patients in the context of the COVID-19 pandemic, compared to previous years.34–36 This could be explained by the various measures and behaviors adopted in the face of the COVID-19 crisis, such as lockdowns, mask use, improving the ventilation of spaces, as well as the decrease in air pollution levels, which may have had a protective role, helping to decrease the frequency or severity of exacerbations.34,35,37–42 On the other hand, during most of the recall period, there were considerable limitations in access to healthcare,43,44 and the fear of getting infected with SARS-CoV-2 may also have discouraged some patients from seeking medical attention during that period, as some authors have suggested.34,36,45 Notwithstanding, healthcare resource utilization (HCRU) was significantly higher among OCS users, which is consistent with the literature.17,46
The frequency of short-term OCS use, along with the proportion of patients with poorly controlled disease (75.9%) suggest low effectiveness of the inhaled controller therapy, either due to insufficient dose, poor inhaler technique or poor adherence.20 However, almost all patients reported full adherence to ICS/LABA (“I use it every day”) (93.6%). Even though patients may have actually increased adherence to controller therapy fearing the outcomes of an infection with SARS-CoV-2,47 it is likely that the self-reported adherence to ICS/LABA was much overestimated due to social-desirability bias, as the literature shows lower levels of adherence to ICS/LABA in asthma patients,48–50 as well as a low correspondence between patient- and physician-assessed adherence.51 As discussed before, the lack of adequate symptom assessment and subsequent treatment adjustment (usually provided in routine medical appointments) may help to explain the low effectiveness of the inhaled controller and the high proportion of patients with poor disease control.
In our study, patients taking OCS were younger than non-OCS users (average age: 56.3 and 60.5 years, respectively), while in other published research, OCS users were found to be older.17,52,53 During the study period, older populations were especially shielded for fear of the Covid-19 pandemic, which may have prevented severe asthma exacerbations and resulted in lower OCS use by these patients.
Conjunctivitis, arthritis, osteoporosis, and gastrointestinal disease were almost twice as prevalent in the OCS users’ group compared to non-OCS users. Previously published literature states that patients with conjunctivitis and arthritis are often exposed to OCS therapy.54,55 On the other hand, osteoporosis and gastrointestinal diseases are frequently described as OCS side-effects.16 OCS users were more likely to have an unscheduled consultation and/or recur to an ED visit, which is expected, not only because those are usually the circumstances of OCS prescription (for short-term therapy), but it has also been described by other studies.21 We found no statistically significant differences regarding sex, BMI, and smoking status.
OCS users reported lower levels of disease control than non-users.56 OCSs are used as treatment for asthma exacerbations. In this sense, poorly controlled patients are more likely to use OCSs than controlled patients.57 Patients with difficult-to-treat asthma are also more likely to be exposed to OCS as maintenance therapy. In any case, the side-effects associated with OCS use, whether as short- or long-term therapy, are extensively documented and should not be ignored.14,15 Long-term OCS users represented about a quarter of all OCS users in the study. The average daily dose (12.6 mg prednisolone/day/year) was equivalent to a high dose of prednisolone (≥2.5 mg prednisolone/day/year). Also, these patients had been using OCS chronically for over 7 years, on average. Considering the life expectancy of asthma patients, this anticipates worrying levels of systemic corticosteroid exposure over a lifetime, if no other treatment regimen is implemented. This reinforces the need for different disease management strategies and therapeutic approaches, such as the adoption of corticosteroid-sparing strategies.11,15,16 Randomized, controlled trials with biologics showed a reduction in exacerbation rates, thereby reducing the need for OCS bursts and lowering cumulative OCS exposure.58 A multifaceted approach to systematically diagnose and characterize severe asthma, identify and treat risk factors and comorbidities and target patients most likely to benefit from advanced therapies, such as biologicals, is crucial to reduce the overall steroid load. PONENTE study demonstrated that it is possible to apply a personalized oral corticosteroid reduction algorithm, including adrenal insufficiency assessment, for patients with severe asthma to allow the rapid and safe reduction of oral corticosteroids.59
The multivariable logistic regression revealed a positive association of patients’ characteristics like age (≤65 years), conjunctivitis, osteoporosis, and urgent healthcare resources utilization with OCS use. Conversely, a higher CARAT score was associated with a lower chance of using OCS. When adjusting to the remaining variables, arthritis and gastrointestinal disease were not associated with increased odds of OCS use. Although OCS users were younger than non-users in this study, when drilling down according to the OCS exposure, the proportion of older participants (>65 years) was higher among the high-dose group compared to low-dose OCS users (52.2% vs 21.4%). Furthermore, there was a greater prevalence of osteoporosis among those exposed to higher OCS doses. This finding may be confounded by the older age of the patients subjected to high-dose treatment (since osteoporosis increases with age and after menopause), but one cannot exclude it as a possible side-effect of the systemic corticosteroids. In any case, it is still worrying that, among all patients exposed to OCS, high-dose users were mostly above 65 years old.
Strengths and Limitations
This study uses real-world data to describe the patterns of OCS utilization among asthma patients treated at GINA step 3 and above and to estimate said population’s level of asthma control. By collecting drug utilization data through pharmacies, the study aims to achieve a representative sample of the Portuguese asthma population. Moreover, this methodology ensures better quality data about the therapeutic regimen, compared to self-reported alone or to prescription-based analyses, which do not account for the drug dispense. This provides a better picture of the medicines’ utilization patterns in the usual practice in the Portuguese context.
Potential sources of bias were addressed in the methodology: pharmacists received training and periodic reminders about eligibility criteria and standardized study procedures; the pop-up installed in the pharmacy software aimed to decrease selection bias; and the refusal form provided basic information about patients who declined to participate, allowing to assess the degree of selection bias.
Notwithstanding, there are some limitations inherent to this study. First, some degree of selection bias might have influenced the results, since the sex and age distribution were different between those who accepted and who declined to participate in the study. However, as the declining rate was only 9.9%, we believe that the recruited patients are a robust sample of the interest population (adult asthma patients on GINA’s highest treatment steps).
Importantly, the distribution of patients by GINA treatment steps is not representative of the proportions found in the real-world asthma population because patients in step 3 were only eligible if they reported to have used OCS in the previous year. Moreover, the active search for eligible patients in the pharmacy records may have introduced some unbalance in the relative proportions of the GINA steps, because pharmacists could have paid more attention to a certain subgroup when going through the records. As we did not collect any data about the method of recruitment of each patient, it is not possible to conduct a scenario analysis that excluded these patients.
The study relies on self-reported, retrospective data. Thus, it may be subjected to information and response bias in general. Given the long recall period (12 months), the healthcare resource utilization and OCS utilization (especially short-term) data may be affected by recall bias. The self-reported adherence to ICS/LABA may as well be affected by social desirability bias, ie, patients may have reported higher levels of adherence compared to the reality to avoid judgement or comments from the pharmacist.
Conclusion
The findings of this study indicate that one in four asthma patients treated at GINA step 3 and above required treatment with oral corticosteroids (either long- or short-term use). Compared to non-OCS users, this subgroup is younger, revealed poorer disease control and reported a greater use of healthcare resources in the previous year. Moreover, a higher proportion of patients with diseases associated with systemic corticosteroid exposure (arthritis and osteoporosis) was found for OCS users. These results highlight the burden of OCS therapy to asthma patients and should help raise awareness for the need to improve asthma management and adopt OCS sparing strategies in this subgroup of patients with poor asthma outcomes despite heavy treatment.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
This study was performed in accordance with ethical principles that are consistent with the Declaration of Helsinki, ICH GCPs, GPP and the applicable legislation on Non-Interventional Studies and/or Observational Studies. The protocol was reviewed by the Institute of Bioethics of the Catholic University of Porto. All participants provided signed informed consent.
Consent for Publication
All authors reviewed the manuscript and consent with the publication of its content, including images and text.
Acknowledgments
The authors are grateful to all community pharmacy staff who participated in patient recruitment and data collection, as well as all participants who voluntarily agreed to complete the survey. We would also like to acknowledge Katerina Maslova and Chris Brooks (AstraZeneca) for reviewing and commenting on the manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was sponsored and funded by AstraZeneca, Portugal.
Disclosure
MR, CB, MP, ATR, and ZM report no conflicts of interest in this work. CS, GS and FB are employees of AstraZeneca. JAF reports personal fees from AstraZeneca, Viatris, and GSK; grants and personal fees from Novartis and from Mundipharma, outside the submitted work and is co-founder of MEDIDA, Lda that provided project management services for the study. JCS reports Advisory Board from Boehringer Ingelheim, personal fees and Advisory Board from GSK, grants, personal fees and Advisory Board from AstraZeneca, personal fees and Advisory Board from Bial, non-financial support from Mundipharma, personal fees from Sanofi, Advisory Board from Novartis, outside the submitted work.
References
1. Global Asthma Network. The global asthma report 2018. Auckland, New Zealand; 2018. Available from: http://globalasthmareport.org/resources/Global_Asthma_Report_2018.pdf. Accessed November 8, 2022.
2. Institute for Health Metrics and Evaluation (IHME). GBD 2019 Cause and Risk Summary: [Asthma]. Seattle, USA: IHME; 2020.
3. Sa-Sousa A, Morais-Almeida M, Azevedo LF, et al. Prevalence of asthma in Portugal - The Portuguese National Asthma Survey. Clin Transl Allergy. 2012;2(1):1–12. doi:10.1186/2045-7022-2-15
4. De Sousa JC, Santo ME, Colaço T, Almada-Lobo F, Yaphe J. Asthma in an urban population in Portugal: a prevalence study. BMC Public Health. 2011;11. doi:10.1186/1471-2458-11-347
5. Eurostat - Statistics explained. Respiratory diseases statistics - self-reporting of respiratory diseases (figure 1 - asthma); 2021. Available from: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Respiratory_diseases_statistics&stable=0&redirect=no.
6. Global Initiative for Asthma (GINA). Pocket guide for asthma management and prevention (2021 update); 2021. Available from: https://ginasthma.org/wp-content/uploads/2021/05/GINA-Pocket-Guide-2021-V2-WMS.pdf.
7. Cunha ÂGJ, Mendes AZ, Carvalho FDW, Paula MAR, Brasil TG. The impact of asthma on quality of life and anxiety: a pilot study. J Asthma. 2018;56:680–685.
8. Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med. 2000;343:332–336. doi:10.1056/NEJM200008033430504
9. Adams NP, Bestall JC, Malouf R, Lasserson TJ, Jones P. Beclomethasone versus placebo for chronic asthma (review). Cochrane Database Syst Rev. 2008;(1). doi:10.1002/14651858.CD003135.pub4
10. Juniper EF, Kline PA, Vanzieleghem MA, Ramsdale EH, Byrne PMO, Hargreave FE. Effect of long-term treatment with an inhaled corticosteroid (budesonide) on airway hyperresponsiveness and clinical asthma in nonsteroid-dependent asthmatics. Am Rev Respir Dis. 1990;142(4):832–836. doi:10.1164/ajrccm/142.4.832
11. Ekström M, Nwaru BI, Hasvold P, Wiklund F, Telg G, Janson C. Oral corticosteroid use, morbidity and mortality in asthma: a nationwide prospective cohort study in Sweden. Allergy. 2019;68:1–10. doi:10.15036/arerugi.68.1
12. Tran TN, King E, Sarkar R, et al. Oral corticosteroid prescription patterns for asthma in France, Germany, Italy and the UK. Eur Respir J. 2020;55(6):1902363. doi:10.1183/13993003.02363-2019
13. Bleecker ER, Menzies-Gow AN, Price DB, et al. Systematic literature review of systemic corticosteroid use for asthma management; 2020. Available from: www.atsjournals.org.
14. Ramsahai JM, Wark PA. Appropriate use of oral corticosteroids for severe asthma. Med J Aust. 2018;209(S2):S18–21. doi:10.5694/mja18.00134
15. Sweeney J, Patterson CC, Menzies-Gow A, et al. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the optimum patient care research database and the British thoracic difficult asthma registry. Thorax. 2016;71(4):339–346. doi:10.1136/thoraxjnl-2015-207630
16. Volmer T, Effenberger T, Trautner C, Buhl R. Consequences of long-term oral corticosteroid therapy and its side-effects in severe asthma in adults: a focused review of the impact data in the literature. Eur Respir J. 2018;52(4):1800703. doi:10.1183/13993003.00703-2018
17. Janson C, Lisspers K, Ställberg B, et al. Health care resource utilization and cost for asthma patients regularly treated with oral corticosteroids - A Swedish observational cohort study (PACEHR). Respir Res. 2018;19(1). doi:10.1186/s12931-018-0855-3
18. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement guidelines for reporting observational studies. Ann Intern Med. 2007;18(6):800–804.
19. Fonseca JA, Azevedo L, Morais-Almeida M. Validation of a questionnaire (CARAT10) to assess rhinitis and asthma in patients with asthma. Allergy. 2010;65:1042–1048. doi:10.1111/j.1398-9995.2009.02310.x
20. Sousa AS, Almeida R, Vicente R, et al. High oral corticosteroid exposure and overuse of short-acting beta-2-agonists were associated with insufficient prescribing of controller medication: a nationwide electronic prescribing and dispensing database analysis. Clin Transl Allergy. 2019;9:1–10. doi:10.1186/s13601-019-0243-1
21. Zeiger RS, Schatz M, Li Q, Chen W, Khatry DB, Tran TN. Burden of chronic oral corticosteroid use by adults with persistent asthma. J Allergy Clin Immunol. 2017;5(4):1050–1060.e9. doi:10.1016/j.jaip.2016.12.023
22. Sadatsafavi M, Khakban A, Tavakoli H, Ehteshami-Afshar S, Lynd LD, FitzGerald JM. Trends in oral corticosteroids use in severe asthma: a 14-year population-based study. Respir Res. 2021;22(1). doi:10.1186/s12931-021-01696-x
23. Bugalho de Almeida A, Todo-bom A, Fonseca JA, et al. INASma - Sumário do Inquérito Nacional de Controlo da Asma. DGS; 2010.
24. Tanosaki T, Kabata H, Matsusaka M, et al. Clinical characteristics of patients with not well-controlled severe asthma in Japan: analysis of the Keio Severe Asthma Research Program in Japanese population (KEIO-SARP) registry. Allergol Int. 2021;70(1):61–67. doi:10.1016/j.alit.2020.06.002
25. Wang E, Wechsler ME, Tran TN, Heaney LG, Jones RC. Characterization of severe asthma worldwide data from the international severe asthma registry. Chest. 2020;157:790–804. doi:10.1016/j.chest.2019.10.053
26. Kritikos V, Price D, Papi A, et al. The burden of self-reported rhinitis and associated risk for exacerbations with moderate-severe asthma in primary care patients. J Asthma Allergy. 2020;13:415–428. doi:10.2147/JAA.S266204
27. Global Initiative for Asthma. Global strategy for asthma management and prevention, 2021; 2021.
28. Trevor J, Lugogo N, Carr W, et al. Severe asthma exacerbations in the United States: incidence, characteristics, predictors, and effects of biologic treatments. Ann Allergy Asthma Immunol. 2021;127:579–587.e1. doi:10.1016/j.anai.2021.07.010
29. Baptist AP, Hao W, Song PX, Carpenter L, Steinberg J, Cardozo LJ. A behavioral intervention can decrease asthma exacerbations in older adults. Ann Allergy Asthma Immunol. 2020;124:1.
30. Czira A, Turner M, Martin A, et al. A systematic literature review of burden of illness in adults with uncontrolled moderate/severe asthma. Respir Med. 2022;191:106670. doi:10.1016/j.rmed.2021.106670
31. Ren Tay T, Siang Wong H, Ihsan R, et al. Comparison of the proportion and healthcare utilisation of adult patients with uncontrolled severe asthma versus non-severe asthma seen in a southeast asian hospital-based respiratory specialist clinic. Ann Acad Med Singapore. 2017;46(6):1.
32. Chiner E, Hernández C, Blanco-Aparicio M, Funenga-Fitas E, Jiménez-Ruiz C. Patient perspectives of the influence of severe and non-severe asthma on their quality of life: a national survey of asthma patients in Spain. Clin Respir J. 2021;16:130–141.
33. Romão M, Godinho AR, Teixeira PM, et al. Characteristics of reliever inhaler users and asthma control: a cross-sectional multicenter study in Portuguese community pharmacies. J Asthma Allergy. 2021;2021:14–943. doi:10.2147/JAA.S315678
34. de Boer G, Braunstahl GJ, Hendriks R, Tramper-Stranders G. Asthma exacerbation prevalence during the COVID-19 lockdown in a moderate-severe asthma cohort. BMJ Open Respir Res. 2021;8(1):e000758. doi:10.1136/bmjresp-2020-000758
35. Wee LE, Conceicao EP, Tan JY, Ying Sim JX, Venkatachalam I. Reduction in asthma admissions during the COVID-19 pandemic: consequence of public health measures in Singapore. Eur Respir J. 2021;57(4):2004493. doi:10.1183/13993003.04493-2020
36. Kyriakopoulos C, Gogali A, Exarchos K, et al. Reduction in hospitalizations for respiratory diseases during the first COVID-19 wave in Greece. Respiration. 2021;100(7):588–593. doi:10.1159/000515323
37. Dezman ZDW, Stryckman B, Zachrison KS, et al. Masking for COVID-19 is associated with decreased emergency department utilization for non-COVID viral illnesses and respiratory conditions in Maryland. Am J Med. 2021;134(10):1247–1251. doi:10.1016/j.amjmed.2021.06.008
38. Chan KPF, Kwok WC, Ma TF, et al. Territory-wide study on hospital admissions for asthma exacerbations in the COVID-19 pandemic. Ann Am Thorac Soc. 2021;18(10):1624–1633. doi:10.1513/AnnalsATS.202010-1247OC
39. Sigala Ι, Giannakas T, Giannakoulis VG, et al. Effect of COVID-19-related lockdown on hospital admissions for asthma and COPD exacerbations: associations with air pollution and patient characteristics. J Pers Med. 2021;11(9):867. doi:10.3390/jpm11090867
40. Salciccioli JD, She L, Tulchinsky A, Rockhold F, Carlos Cardet J, Israel E. Effect of COVID-19 on asthma exacerbation. J Allergy Clin Immunol Pract. 2021;9(7):2896–2899.e1. doi:10.1016/j.jaip.2021.04.038
41. Shah SA, Quint JK, Nwaru BI, Sheikh A Impact of COVID-19 national lockdown on asthma exacerbations: interrupted time-series analysis of English primary care data. Available from: http://thorax.bmj.com/.
42. Davies GA, Alsallakh MA, Sivakumaran S, et al. Impact of COVID-19 lockdown on emergency asthma admissions and deaths: national interrupted time series analyses for Scotland and Wales. Thorax. 2021;76(9):867–873. doi:10.1136/thoraxjnl-2020-216380
43. MOAI Consulting. Impacto da pandemia COVID- 19 na prestação de cuidados de saúde em Portugal [Impact of the COVID-19 pandemic in healthcare delivery in Portugal - In Portuguese Language]. Available from: https://www.saudeemdia.pt/dl/202007_ApresentacaoSaudeemDia_v2.pdf.
44. GFK Metris. Estudo à População: acesso a cuidados de saúde em tempos de pandemia [Population survey: access to healthcare during the COVID-19 pandemic - In Portuguese Language]; 2020. Available from: https://www.saudeemdia.pt/dl/estudo_a_populacao.pdf.
45. Mantica G, Riccardi N, Terrone C, Gratarola A. Non-COVID-19 visits to emergency departments during the pandemic: the impact of fear. Public Health. 2020;183:40. doi:10.1016/j.puhe.2020.04.046
46. Voorham J, Xu X, Price DB, et al. Healthcare resource utilization and costs associated with incremental systemic corticosteroid exposure in asthma. Allergy. 2019;74(2):273–283. doi:10.1111/all.13556
47. Gao Dong Y, Ding M, Dong X, et al. Risk factors for severe and critically ill COVID-19 patients: a review. Allergy. 2021;76(2):428–455. doi:10.1111/all.14657
48. Cochrane MG, Bala MV, Downs KE, Mauskopf J, Ben-Joseph RH. Inhaled corticosteroids for asthma therapy: patient compliance, devices, and inhalation technique. Chest. 2000;117(2):542–550. doi:10.1378/chest.117.2.542
49. Bårnes CB, Suppli C, Dmsc U. Asthma and Adherence to Inhaled Corticosteroids: current Status and Future Perspectives. Respir Care. 2015;60:455–468. doi:10.4187/respcare.03200
50. Wu AC, Butler MG, Li L, et al. Primary adherence to controller medications for asthma is poor. Ann Am Thorac Soc. 2011;2011(7):161–166.
51. Jácome C, Pereira AM, Almeida R, et al. Patient-physician discordance in assessment of adherence to inhaled controller medication: a cross-sectional analysis of two cohorts. BMJ Open. 2019;9(11):e031732. doi:10.1136/bmjopen-2019-031732
52. Sá-Sousa A, Almeida R, Vicente R, et al. High oral corticosteroid exposure and overuse of short-acting beta-2-agonists were associated with insufficient prescribing of controller medication: a nationwide electronic prescribing and dispensing database analysis. Clin Transl Allergy. 2019;9(1). doi:10.1186/s13601-019-0286-3
53. Taube C, Bramlage P, Hofer A, Anderson D. Prevalence of oral corticosteroid use in the German severe asthma population. ERJ Open Research. 2019;5(4):00092–2019. doi:10.1183/23120541.00092-2019
54. Price D, Castro M, Bourdin A, Fucile S, Altman P. Short-course systemic corticosteroids in asthma: striking the balance between efficacy and safety. Eur Respir Rev. 2020;29:190151. doi:10.1183/16000617.0151-2019
55. Hua C, Buttgereit F, Combe B. Glucocorticoids in rheumatoid arthritis: current status and future studies. RMD Open. 2020;6(1):e000536. doi:10.1136/rmdopen-2017-000536
56. Sullivan PW, Ghushchyan VH, Globe G, Schatz M. Oral corticosteroid exposure and adverse effects in asthmatic patients. J Allergy Clin Immunol. 2018;141(1):110–116.e7. doi:10.1016/j.jaci.2017.04.009
57. Graff S, Vanwynsberghe S, Brusselle G, et al. Chronic oral corticosteroids use and persistent eosinophilia in severe asthmatics from the Belgian severe asthma registry. Respir Res. 2020;21(1):1–11. doi:10.1186/s12931-020-01460-7
58. Chung LP, Upham JW, Bardin PG, Hew M. Rational oral corticosteroid use in adult severe asthma: a narrative review. Respirology. 2020;25(2):161–172. doi:10.1111/resp.13730
59. Menzies-Gow A, Gurnell M, Heaney LG, et al. Oral corticosteroid elimination via a personalised reduction algorithm in adults with severe, eosinophilic asthma treated with benralizumab (PONENTE): a multicentre, open-label, single-arm study. Lancet Respir Med. 2022;10(1):47–58. doi:10.1016/S2213-2600(21)00352-0
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

