Back to Journals » Journal of Asthma and Allergy » Volume 15
Assessment of Symptoms Control, Pulmonary Function and Related Quality of Life in Asthmatic Patients Treated with Extrafine Beclomethasone Dipropionate/Formoterol Fumarate 100/6 μg pMDI: Results of a Multicenter Observational Study in Romania (ALFRESCO Study)
Authors Ulmeanu R, Bloju S, Vittos O
Received 17 January 2022
Accepted for publication 27 May 2022
Published 8 July 2022 Volume 2022:15 Pages 919—933
DOI https://doi.org/10.2147/JAA.S358798
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Luis Garcia-Marcos
Ruxandra Ulmeanu,1 Sebastian Bloju,2 Oana Vittos3
1Postgraduate Training School of the Romanian Society of Pulmonology; Bronchoscopy Department - Institute of Pulmonology “Marius Nasta”, Bucharest, Romania; 2Medical Department, Chiesi Romania, Bucharest, Romania; 3Clinical Research Department, Medone Research, Bucharest, Romania
Correspondence: Oana Vittos, Clinical Research Department, Medone Research, 18, Alexandr Sergheevici Puskin Street, District 1, Bucharest, 011996, Romania, Tel +40 21 311 11 05, Fax +40 21 311 11 06, Email [email protected]
Introduction: Asthma treatment guidelines advocate the use of long-acting beta2-agonists (LABA) in addition to inhaled corticosteroids (ICS) in patients whose asthma is uncontrolled by ICS alone. This is the first study done in Romania, which collected the real-world data on the effects of Foster® (extrafine beclomethasone dipropionate/formoterol fumarate BDP/FF in a pressurized metered-dose inhaler pMDI 100/6 μg formulation) in adult asthmatic population.
Objective: We aimed to assess the asthma symptoms control, pulmonary function and quality of life parameters in a heterogeneous Romanian asthmatic adult outpatient population, treated with extrafine BDP/FF 100/6 μg pMDI.
Methods: This was a prospective, multicenter, observational study involving 30 pulmonologists randomly selected from the Romanian healthcare system, which did not declare any competing interests. Recruitment period was Oct 2018 - Feb 2019, while the patients’ observational period was 24 weeks. The study included poorly controlled and uncontrolled adult asthma outpatients treated with non-extrafine formulations medication, for which the treatment indication, according to Global Initiative for Asthma (GINA) 2018, was the use of an ICS-LABA combination. The study collected demographic data, smoking habits, comorbidities, data regarding asthma diagnosis, the evolution of asthma symptoms, spirometry, Asthma Control Questionnaire (ACQ-7) scoring test, current and concomitant treatment.
Results: Of 302 included patients, 290 completed the study. Pulmonary function parameters assessed during the trial (forced expiratory volume in one second - FEV1 and forced vital capacity - FVC) showed a significant improvement versus baseline (p< 0.001). ACQ-7 score decreased significantly from 3.09± 0.83 (visit 1) to 1.56± 0.89 (visit 2) and to 1.09± 0.81 (visit 3) (p< 0.001). At the end of the study, 127 (43.79%) patients were well controlled (ACQ-7 score < 0.75).
Conclusion: This observational study demonstrates the effectiveness and safety of extrafine fixed combination of BDP/FF (100/6 μg) pMDI in Romanian adult asthma patients uncontrolled with non-extrafine medication in a real-world setting, leading to clinically and statistically improvements in asthma control and pulmonary function.
Keywords: asthma, ACQ-7, symptoms, ICS/LABA, maintenance and reliever therapy, MART
Introduction
Asthma is one of the most common chronic diseases worldwide in all age groups, with more than 300 million affected individuals and its prevalence keeps increasing in certain countries,1 being at the same time a major public health problem worldwide, which might potentially negatively impact patient’s quality of life.2
The primary aim of asthma treatment is to achieve and maintain overall asthma control by reducing the severity of current symptoms and minimizing future risk regarding asthma-related mortality, exacerbations, persistent airflow limitation and side-effects.2
Asthma treatment guidelines advocate the use of long-acting beta2-agonists (LABA) in addition to inhaled corticosteroids (ICS), either separately or as a fixed-dose formulation, in patients whose asthma is uncontrolled by ICS alone.2,3 For ICS/formoterol fixed combination, the recommendation is the use as maintenance only or as maintenance and reliever therapy (MART).2
The extrafine formulation of beclomethasone dipropionate/formoterol fumarate (BDP/FF) 100/6 µg in a pressurized metered-dose inhaler (pMDI) is known to produce high and homogeneous lung deposition, allowing at the same time a uniform treatment for the bronchoconstriction, as well as inflammation within the entire bronchial tree.4–6
Also, the efficacy of BDP/FF 100/6 µg pMDI in patients with moderate to severe asthma was demonstrated in several randomized clinical trials (RCTs);7,8 nevertheless, it should be emphasized that the effectiveness of the treatment is dependent also on other factors as the medical device used, the inhalation technique, patient’s treatment adherence. Those factors are playing an important role in drug delivery and distribution along the bronchial tree, including the peripheral airways. Observational studies could add significant information to the finding of RCTs, by recording effectiveness and safety data in real-life situations.
This is the first study done in Romania collecting real-life data regarding the efficacy and safety of BDP/FF, an extrafine pMDI fixed-dose formulation, used as maintenance only or as MART in adult asthmatic patients.
Materials and Methods
Study Design
This is a prospective, multicenter, observational study that took place in Romania, involving 30 pulmonologists, randomly selected from the national healthcare system, which did not declare any competing interests. Each investigator planned to recruit ten (10) asthmatic patients visiting his office, fulfilling the protocol criteria.
Recruitment took place between October 2018 - Feb 2019, while the patient’s observation period was 24 weeks, including three visits: visit 1 (V1) - inclusion visit; visit 2 (V2) - 12-weeks follow-up visit and visit 3 (V3) - 24-weeks follow-up visit.
The study included poorly controlled and uncontrolled adult asthma outpatients (males and females) being treated with non-extrafine formulations medication, if they had a positive bronchodilator reversibility test (increase in forced expiratory volume in 1st second (FEV1) of >12% and >200 mL from baseline, 10–15 minutes after 200–400 mcg salbutamol or equivalent) and for which the treatment indication, according to Global Initiative for Asthma (GINA) 2018 guidelines, was the use of an ICS-LABA combination.2
The investigator, based on own medical judgement and daily practice regarding the management of asthma patients, unconditionally by the participation of the patient in the study, prescribed an extrafine formulation of BDP/FF 100/6 µg pMDI, in accordance with its approved Summary of Product Characteristics (SmPC). The dose of extrafine ICS/formoterol was adjusted by the investigators in accordance to her/his decision, based on her/his medical experience and SmPC. The investigator was fully responsible for his/her decision in the patient’s interest and was not influenced by the study. The study excluded patients which met any of the BDP/FF 100/6 µg pMDI SmPC contraindications, limitations, restrictions or if they were participating in another clinical trial. The medication was bought by each patient from public pharmacies.
Data regarding patient baseline characteristics (age, gender, height, weight, education, smoking habits, concomitant diseases, number of years of asthma diagnosis and previous asthma medical treatment) were collected in the Case Report Form (CRF). Asthma symptoms (cough, wheezing reported by subject, wheezing observed during the clinical examination, thoracic constriction, dyspnea at rest and exertional dyspnea), data regarding pulmonary function tests (FEV1 morning pre-dose, forced vital capacity (FVC) morning pre-dose and FEV1/FVC % morning pre-dose), Asthma Control Questionnaire-7 (ACQ-7) score (Figure 1), use of medication, adherence to treatment, asthma symptomatology evolution and adverse drug reactions (ADRs) were collected during each study visit.
 |
Figure 1 Asthma Control Questionnaire-7 items. Abbreviations: FEV 1, forced expiratory volume in 1st second; FEV 1%; forced expiratory volume in 1st second/forced vital capacity. Note: Reproduced with permission from QOL Technologies Ltd, Professor Elizabeth F Juniper for Chiesi. Available from: http://www.qoltech.co.uk/index.htm. |
Pulmonary function was assessed using spirometry (performed before drug administration) as per routine practice and according to guidelines. Moreover, the morning dose intake treatment was done at site after performing the pulmonary function test. This procedure was performed in order to collect the real data regarding lung function, as well as assessing the patients’ inhalation technique and providing correction instructions, where needed.
The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The study was approved by the Romanian National Bioethics Committee for Medicine and Medical Devices (NBCMMD) and Romanian National Agency for Medicines and Medical Devices (NAMMD). All included patients provided written Informed Consent Form (ICF).
Statistics
Due to observational nature of the study, without comparative design, it was decided that each site, out of the 30 pneumologists randomly selected from Romanian healthcare system, will recruit 10 patients in order to obtain the most representative data set. Since no statistical hypothesis test was planned, the study sample size was not based on a formal power calculation, but estimated on recruitment capabilities of the sites.
The statistical analysis was essentially descriptive, using Student’s t test, Fisher’s test and Chi-Square test. Scale variables were reported as mean ± standard deviation (SD) and summarized categorical variables using frequencies and percentages. For all tests, a p-value <0.05 was considered statistically significant. SPSS Statistics (version 19.0, Chicago, Illinois, USA) was used.
Results
A total of 302 asthma adult outpatients were included, of which 290 patients were eligible, completed the study and were part of the statistical analysis. Most of the 290 patients were females (62.07%), with a mean age of 55.94±14.68 years. Mean body mass index (BMI) was 28.89±2.83 kg/m2, without significant statistical gender differences. Most patients were non-smokers (73.4%) and had common comorbidities such as: high blood pressure (45.9%), allergic rhinitis (28.6%) and ischemic heart disease (21%) (Table 1). If measured by the investigators, vital sign measurements (systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), and respiratory rate) were recorded at study inclusion (Table 1).
 |
Table 1 Baseline Characteristics |
At inclusion, symptoms reported by most patients were exertional dyspnea (93.8%) and cough (92.4%). Symptoms were most frequently reported to occur during the morning (77.6%) (Table 2). Spirometry at inclusion showed reduced values, both for male and female groups: the mean FEV1 value was 1.72±0.49 L, FVC 2.47±0.6 L and FEV1/FVC 70.96±14.07%, respectively. Mean ACQ-7 score at study inclusion was 3.09±0.83 (Table 2). The mean duration for asthma diagnosis was 11.12±10.71 years (Table 1), while the most frequent maintenance treatment used prior to study inclusion was a combination of ICS-LABA (65.2%), followed by ICS alone (27.9%) (Table 3). All patients received prescription of extrafine BDP/FF 100/6 μg pMDI, used as maintenance only [(33 pts (11.38%)] or as MART [257 pts (88.62%)].
 |
Table 2 Asthma Symptoms and Pulmonary Function at Inclusion (V1) |
 |
Table 3 Maintenance Medication/Active Substances Classes Used for Asthma Control Prior to Study Inclusion |
Pulmonary function and asthma symptoms improved throughout the study. FEV1 mean value increased from 1.72±0.61 L (V1) to 2.25±0.77 L (V3) (p<0.001), FVC mean value increased from 2.47±0.83 L (V1) to 2.89±0.83 L (V3) (p<0.001) (Table 4). The mean ACQ-7 score for all 290 patients decreased significantly throughout the study, from the mean value of 3.09±0.83 (V1) to 1.09±0.81 (V3) (p<0.001) (Table 4 and Figure 2).
 |
Table 4 Pulmonary Function and ACQ-7 |
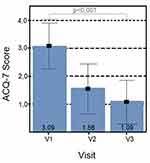 |
Figure 2 ACQ-7 score evolution. Abbreviations: ACQ-7, Asthma Control Questionnaire-7 items; V1, visit 1; V2, visit 2; V3, visit 3. |
The frequency of the most reported symptoms decreased; exertional dyspnea from 93.8% (V1) to 53.4% (V3) (p<0.001) and cough from 92.4% (V1) to 32.8% (V3) (p<0.001). Patients were stratified based on every symptom presented and the ACQ-7 reported value was calculated for each group presenting a certain symptom. A significant decrease in ACQ-7 score was observed (p<0.001) for all groups. For the most frequently reported symptoms, exertional dyspnea and cough, the ACQ-7 mean value decreased as follows: from 3.11±0.83 (V1) to 1.3±0.84 (V3) and from 3.1±0.82 (V1) to 1.54±0.77 (V3) (p<0.001), respectively (Table 5). We noticed that during the treatment with BDP/FF 100/6 μg pMDI, the improvement of asthma symptomatology, defined as frequency of symptoms reported, as well as the asthma control measured by ACQ-7, was significant throughout the study (p<0.001), irrespective to the prior non-extrafine ICS-LABA treatment received by the subjects before the study inclusion (Tables 6–8). For patients treated with different ICS-LABA combinations (non-extrafine formulations) prior study inclusion, after the change in treatment to BDP/FF 100/6 μg pMDI, the ACQ-7 value decreased significantly from 3.16±0.82 (V1) to 1.18±0.77 (V3) (p<0.001) (Table 4).
 |
Table 5 Asthma Symptoms Evolution During the Observational Study |
 |
Table 6 ACQ-7 Score and Asthma Symptoms for Patients Treated with Non-Extrafine BUD/F Prior V1, Followed by Extrafine BDP/FF 100/6 μg pMDI |
 |
Table 7 ACQ-7 Score and Asthma Symptoms for Patients Treated with FLU/SAL Prior V1, Followed by Extrafine BDP/FF 100/6 μg pMDI |
 |
Table 8 ACQ-7 Score and Asthma Symptoms for Patients Treated with Non-Extra Fine ICS-LABA Prior V1, Followed by Extrafine BDP/FF100/6 μg pMDI |
Use of extrafine BDP/FF 100/6 μg pMDI improved the ACQ-7 score from V1 to V3, for all patients and the improvement was significant regardless of the therapy mode applied, maintenance only or MART (p<0.001) (Table 9).
 |
Table 9 ACQ-7 for Different Extrafine BDP/FF 100/6 μg pMDI Treatment Regimens |
The ACQ-7 variation during the study was analyzed for patients in relation to their treatment adherence and it was observed that adherence decreased when improved asthma control was obtained (Table 10). During the study duration, the patients skipped maintenance medication for a median interval of 5.25 days. At V3, 127 (43.79%) of patients reached well-controlled asthma status, defined by ACQ-7 score <0.75 (Table 11).
 |
Table 10 ACQ-7 Score and Treatment Adherence |
 |
Table 11 ACQ-7 Score Evolution |
The frequency of symptoms decreased from V1 [77.79% (in the morning), 68.28% (in the night) and 48.28% (in the evening)] to V3 [30.00% (in the morning), 10.00% (in the night) and 14.14% (in the evening), respectively] (p<0.001) (Table 12). In similar manner, the reliever use decreased throughout the study, reaching at V3 a percentage of 67.93% of subjects not requiring reliever use, during interrogated period (Figure 3).
 |
Table 12 Asthma Symptomatology Worsening Chronology |
 |
Figure 3 The average number of puffs of reliever medication used every day. Abbreviations: V1, visit 1; V2, visit 2; V3, visit 3. |
There were 4 ADRs reported (1.37% of patients) and no Serious Adverse Events (SAE) or hospitalization due to asthma worsening were reported during the study.
Discussion
This is the first real-world study of the use of the extrafine BDP/FF 100/6 µg pMDI, both as maintenance only or MART, for adult asthmatic outpatients, done in Romania, aiming to assess asthma symptoms control, evolution of pulmonary function and quality of life parameters.
In this study, the use of the extrafine BDP/FF 100/6 µg pMDI showed utility in the management of asthma in poorly controlled or uncontrolled adult outpatients in real-life environment.
The patient population included in the study is considered representative for Romanian asthmatic population in terms of age (± 55.9 years), gender distribution, asthma duration (±11 years), BMI (± 28 kg/m2), smoking status (current smokers 10%, former smokers 16.6%), similar to other recent published data.9
The study demonstrated that the use of the extrafine BDP/FF 100/6 µg pMDI, regardless of prior study medication used by the patients, lead to clinically and statistically improvements during the 24-week observational period in pulmonary function [FEV1 mean value increased from 1.72±0.61 L (V1) to 2.25±0.77 L (V3) (p<0.001), FVC mean value increased from 2.47±0.83 L (V1) to 2.89±0.83 L (V3) (p<0.001)], decrease of asthma symptoms frequency (p<0.001) and improvement in asthma control, evaluated by ACQ-7 [ACQ-7 score decreased from the mean value of 3.09±0.83 (V1) to 1.09±0.81 (V3) (p<0.001)]. The improvement from baseline in pulmonary function measurements proves that included subjects had room for improvement during treatment. At the end of the study 43.79% of the included subjects were well controlled (ACQ-7<0.75). These findings are similar to other real-world evidences that have indicated that only 50% of patients with asthma meet the criteria for well-controlled asthma, at best.10–15
At inclusion, out of the 290 patients who completed the study, 33.8% presented all six assessed asthma symptoms, while at end of the study, only 1.7% reported all symptoms. As predicted, there were no symptom-free patients at inclusion, while at the end of study this increased to 33.79%. Symptoms improved significantly during the study, demonstrating the efficacy of extrafine BDP/FF 100/6 µg pMDI treatment. The reliever use decreased throughout the study, reaching at V3 a percentage of 67.93% of subjects not requiring reliever use, during interrogated period (Figure 3). Those data should be interpreted with caution as long as data regarding reliever use are based on patient’s declarations. Approximately one quarter (24.6%) of patients skipped days of maintenance treatment since they did not feel the need to take their maintenance therapy medication every day. These findings are similar to those of the INSPIRE study.10
The extrafine BDP/FF 100/6 µg pMDI was well tolerated and safety profile was in line with the known effects of this type of medication. This could be explained by the ultra-micronized structure of particles that are transported directly to lungs, with minimal systemic absorption.6 The extrafine pressurized metered-dose inhaler formulation could increase the pulmonary absorption despite the use of a reduced nominal dose of corticosteroid. Moreover, the systemic exposure is lower and lung deposition is higher when using an extra fine pMDI.6
As per existing guidelines when study recruitment took place (2018–2019), as well as current guidelines, the asthma control is described by the frequency of asthma symptoms, frequency of reliever medication use, pulmonary functional tests and physical activity limitation, while future risks are characterized by longer-term factors such as decline in pulmonary function, frequency of asthma exacerbations and adverse effects of anti-asthmatic medication.2,5,6,16,17 Asthma severity has traditionally been defined using clinical features present only in the absence of therapy, which is why the quantitative ACQ was developed and validated by Juniper et al.18 This questionnaire assesses the level of asthma control independent of asthma severity level.19,20 Current practice guidelines, GINA 2022, define ACQ scores as it follows: <0.75 indicates a high probability of “well-controlled” asthma, 0.75–1.5 is a “grey zone”, and >1.5 indicates a high probability of “poor-controlled” asthma and states that the minimum clinically important difference between measurements is 0.5.16 In the current observational study, it could be noticed that such difference exists between ACQ-7 scores from V1 to V3.
The approach to the management of asthma using ICS/formoterol combination as MART is facilitating a simplified asthma management for the patients requiring only a single inhaler. In this manner, when used as reliever, patients receive a standard dose of ICS in addition to LABA, which is targeting the inflammation associated with symptoms increase.21
In the current 24-week period observational real-world data study, same approach as the one recommended by GINA was followed, namely using extrafine BDP/FF 100/6 µg pMDI as MART for most of the patients (88.62%) and it shown utility in the management of asthma in poorly controlled or uncontrolled adult outpatients, by achieving current asthma control and reducing future risk, similar to RCTs using BDP/FF extrafine formulation7 or other ICS/formoterol combination as MART regimen.22–24
The improvement noticed in asthma evolution could be explained by the drug extrafine particles, which allow a better deposition in the lungs, together with a uniform treatment throughout the entire bronchial tree4–6,25 without inducing systemic load.8,26 In addition, other studies demonstrated that the BDP/FF 100/6 µg combination has a synergistically bronchorelaxant effect on medium bronchi and small airways.27
It is known that observational studies could add important real-life findings regarding effectiveness and safety from the daily clinical practice, which complement the knowledge driven from RCTs, mainly due to the fact that observational studies do not exclude patients with different comorbidities.25,28,29
There are also certain limitations of the observational studies that should be mentioned, for the current observational study as well, where some variables were missing due to chance and patient’s inhalation technique is variable, despite of investigator’s assessment and advise.
In other real-life studies was observed that patients treated with extrafine BDP/FF formulation, compared to ICS-LABA larger particle size combinations, achieved a greater level of asthma control,30 improved the quality of life31 and this fact might be explained by higher deposition of extrafine particles in the small airways. Similar to the current study findings, the real-life effectiveness and safety of BDP/FF were also demonstrated by other study in adult asthma patients including smokers.25
Due to observational study design, we cannot make definitive statements regarding causal relationship of observed findings within our study.
Conclusion
The results of this observational study demonstrate the effectiveness and safety of extrafine fixed combination of BDP/FF (100/6 μg) pMDI in Romanian adult asthma patients uncontrolled with non-extrafine medication in a real-world setting, leading to clinically and statistically improvements in asthma control and pulmonary function.
Abbreviations
ACQ-7, Asthma Control Questionnaire-7 items; ADR, adverse drug reaction; BDP/FF, beclomethasone dipropionate/formoterol fumarate; BMI, body mass index; BUD/F, budesonide/formoterol; CRF, case report form; DBP, diastolic blood pressure; FEV1, forced expiratory volume in 1st second; FEV1/FVC, forced expiratory volume in 1st second/forced vital capacity; FVC, forced vital capacity; FLU/SAL, fluticasone/salmeterol; GINA, global initiative for asthma; HR, heart rate; ICF, informed consent form; ICS, inhaled corticosteroid; LABA, long-acting beta-2-agonist; LAMA, long-acting muscarinic antagonist; LTRA, leukotriene receptor antagonists; MART, maintenance and reliever therapy; NAMMDR, National Agency for Medicines and Medical Devices of Romania; NBCMMD, National Bioethics Committee for Medicine and Medical Devices; pMDI, pressurized metered-dose inhaler; SAE, serious adverse event; SABA, short-acting beta-2-agonist; SBP, systolic blood pressure; SD, standard deviation; SmPC, summary of product characteristics; V (1, 2, 3), visit (1, 2, 3).
Data Sharing Statement
The datasets generated and/or analyzed during the current study are not publicly available due to the fact that they belong to Chiesi, as Sponsor of the study, but are available from the corresponding author on reasonable request and with prior permission of Chiesi.
Ethics Approval and Informed Consent
The observational study was approved by the NBCMMD and NAMMD. All subjects have signed the ICF approved by NBCMMD.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The observational study was sponsored by Chiesi Romania.
Disclosure
RU reports speakers fees, congress fees, and clinical studies from Chiesi Romania, and Novartis Pharma Services Romania; speakers fees and congress fees from Angelini Pharmaceuticals, AstraZeneca Pharma, Boehringer Ingelheim, SERVIER PHARMA SRL, and Pfizer Romania SRL; speakers fees from Roche Romania and Lilly Oncology; clinical studies from GSK, Parexel, Mundipharma, Bayer, Aradigm, Sanofi, and Ab Science, outside the submitted work. OV is an employee of Medone Research (CRO) and its existing contract service includes fees for Clinical Research Services from Chiesi Romania SRL and Servier Pharma SRL. SB is an employee of Chiesi Romania. The authors report no other conflicts of interest in this work.
References
1. Oladunni E, Sumita S. The global impact of asthma in adult populations. Ann Glob Health. 2019;85(1):2.
2. Global Initiative for Asthma. Global strategy for asthma management and prevention; 2018. Available from: www.ginasthma.org.
3. Ducharme FM, Ni Chroinin M, Greenstone I, Lasserson TJ. Addition of long-acting beta2-agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma. Cochrane Database Syst Rev. 2010;4. doi:10.1002/14651858.CD005533.pub2
4. Bousquet J, Poli G, Acerbi D, et al. Systemic exposure and implications for lung deposition with an extra-fine hydrofluoroalkane beclometasone dipropionate/formoterol fixed combination. Clin Pharmacokinet. 2009;48(6):347–358. doi:10.2165/00003088-200948060-00001
5. De Backer W, Devolder A, Poli G, et al. Lung deposition of BDP/formoterol HFA pMDI in healthy volunteers, asthmatic, and COPD patients. J Aerosol Med Pulm Drug Deliv. 2010;23(3):137–148. doi:10.1089/jamp.2009.0772
6. Scichilone N, Battaglia S, Sorino C, et al. Effects of extra-fine inhaled beclomethasone/formoterol on both large and small airways in asthma. Eur J Allergy Clin Immunol. 2010;65(7):897–902. doi:10.1111/j.1398-9995.2009.02306.x
7. Papi A, Corradi M, Pigeon-Francisco C, et al. Beclomethasone–formoterol as maintenance and reliever treatment in patients with asthma: a double-blind, randomized controlled trial. Respir Med. 2013;1(1):23–31.
8. Huchon G, Magnussen H, Chuchalin A, Dymek L, Gonod FB, Bousquet J. Lung function and asthma control with beclomethasone and formoterol in a single inhaler. Respir Med. 2009;103(1):41–49. doi:10.1016/j.rmed.2008.09.002
9. Bumbacea D, Panaitescu C, Bumbacea RS. Patient and physician perspectives on asthma and its therapy in Romania: results of a multicenter survey. Medicina. 2021;57(10):1089. doi:10.3390/medicina57101089
10. Partridge MR, van der Molen T, Myrseth SE, Busse WW. Attitudes and actions of asthma patients on regular maintenance therapy: the INSPIRE study. BMC Pulm Med. 2006;6(1):1–9. doi:10.1186/1471-2466-6-13
11. Tuomisto LE, Ilmarinen P, Niemelä O, Haanpää J, Kankaanranta T, Kankaanranta H. A 12-year prognosis of adult-onset asthma: Seinäjoki adult asthma study. Respir Med. 2016;117:223–229. doi:10.1016/j.rmed.2016.06.017
12. Price D, Fletcher M, Van Der Molen T. Asthma control and management in 8000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med. 2014;24(1):1–10. doi:10.1038/npjpcrm.2014.9
13. Olaguibel JM, Quirce S, Juliá B, et al. Measurement of asthma control according to global initiative for asthma guidelines: a comparison with the asthma control questionnaire. Respir Res. 2012;13(1):1–10. doi:10.1186/1465-9921-13-50
14. Vermeire PA, Rabe KF, Soriano JB, Maier WC. Asthma control and differences in management practices across seven European countries. Respir Med. 2002;96(3):142–149. doi:10.1053/rmed.2001.1241
15. Backer V, Bornemann M, Knudsen D, Ommen H. Scheduled asthma management in general practice generally improve asthma control in those who attend. Respir Med. 2012;106(5):635–641. doi:10.1016/j.rmed.2012.01.005
16. Global Initiative for Asthma. Global strategy for asthma management and prevention; 2022. Available from: www.ginasthma.org.
17. Bateman ED, Reddel HK, Eriksson G, et al. Overall asthma control: the relationship between current control and future risk. J Allergy Clin Immunol. 2010;125(3):600–608. doi:10.1016/j.jaci.2009.11.033
18. Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14(4):902–907. doi:10.1034/j.1399-3003.1999.14d29.x
19. Juniper EF, Bousquet J, Abetz L, Bateman ED; GOAL Committee. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the asthma control questionnaire. Respir Med. 2006;100(4):616–621. doi:10.1016/j.rmed.2005.08.012
20. Cockroft DW, Swystun VA. Asthma control versus asthma severity. J Allergy Clin Immunol. 1996;98(6):1016–1018. doi:10.1016/S0091-6749(96)80185-0
21. Pavord ID, Jeffery PK, Qiu Y, et al. Airway inflammation in patients with asthma with high-fixed or low-fixed plus as-needed budesonide/formoterol. J Allergy Clin Immunol. 2009;123(5):1083–1089. doi:10.1016/j.jaci.2009.02.034
22. Rabe KF, Pizzichini E, Stallberg B, et al. Budesonide/formoterol in a single inhaler for maintenance and relief in mild-to-moderate asthma: a randomized, double-blind trial. Chest. 2006;129(2):246–256. doi:10.1378/chest.129.2.246
23. O’Byrne PM, Bisgaard H, Godard PP, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir Crit Care Med. 2005;171(2):129–136. doi:10.1164/rccm.200407-884OC
24. Kuna P, Peters MJ, Manjra AI, et al. Effect of budesonide/formoterol maintenance and reliever therapy on asthma exacerbations. Int J Clin Pract. 2007;61(5):725–736. doi:10.1111/j.1742-1241.2007.01338.x
25. Brusselle G, Peché R, Van den Brande P, Verhulst A, Hollanders W, Bruhwyler J. Real-life effectiveness of extrafine beclometasone dipropionate/formoterol in adults with persistent asthma according to smoking status. Respir Med. 2012;106(6):811–819. doi:10.1016/j.rmed.2012.01.010
26. Paggiaro P. New pharmacologic perspectives in pneumology: beclomethasone-formoterol extrafine. Open Respir Med J. 2009;3(1):38–42. doi:10.2174/1874306400903010038
27. Calzetta L, Matera MG, Facciolo F, et al. Beclomethasone dipropionate and formoterol fumarate synergistically interact in hyperresponsive medium bronchi and small airways. Respir Res. 2018;19(1):65. doi:10.1186/s12931-018-0770-7
28. Silverman SL. From randomized controlled trials to observational studies. Am J Med. 2009;122(2):114–120. doi:10.1016/j.amjmed.2008.09.030
29. Ware JH, Hamel MB. Pragmatic trials–guides to better patient care? N Engl J Med. 2011;364(18):1685–1687. doi:10.1056/NEJMp1103502
30. Muller V, Galffy G, Eszes N, et al. Asthma control in patients receiving inhaled corticosteroid and long-acting beta2-agonist fixed combinations. A real-life study comparing dry powder inhalers and a pressurized metered dose inhaler extrafine formulation. BMC Pulm Med. 2011;11(1):40. doi:10.1186/1471-2466-11-40
31. Allegra L, Cremonesi G, Girbino G, et al. Real-life prospective study on asthma control in Italy: cross-sectional phase results. Respir Med. 2012;106(2):205–214. doi:10.1016/j.rmed.2011.10.001
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
