Back to Journals » Journal of Blood Medicine » Volume 13
Baseline Thrombocytopenia and Disease Severity Among COVID-19 Patients, Tibebe Ghion Specialized Hospital COVID-19 Treatment Center, Northwest Ethiopia
Authors Asrie F, Tekle E , Gelaw Y , Dagnew M , Gelaw A, Negash M , Kassa E, Bizuneh S , Wudineh D
Received 15 March 2022
Accepted for publication 31 May 2022
Published 10 June 2022 Volume 2022:13 Pages 315—325
DOI https://doi.org/10.2147/JBM.S366478
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Martin H Bluth
Fikir Asrie,1 Esayas Tekle,2 Yemataw Gelaw,1 Mulat Dagnew,3 Aschalew Gelaw,3 Markos Negash,4 Eyuel Kassa,5 Segenet Bizuneh,6 Dessalew Wudineh7
1Department of Hematology and Immunohematology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 2Department of Medical and Laboratory Sciences, Institute of Health Sciences, Wollega University, Nekemte, Ethiopia; 3Department of Medical Microbiology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 4Department of Immunology and Molecular Biology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 5University of Gondar Comprehensive Specialized Hospital Laboratory, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 6Department of Internal Medicine, School of Medicine, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 7Department of Medical Laboratory Sciences, Institute of Health Sciences, Mizan Tepi University, Mizan Tepi, Ethiopia
Correspondence: Yemataw Gelaw, Email [email protected]
Background: Thrombocytopenia and platelet indices in COVID-19 patients were important for prompt treatment and management of the disease. Therefore, the main objective of this study was to assess the prevalence of thrombocytopenia, platelet indices, and its association with disease severity among COVID-19 patients at the Tibebe Ghion Specialized Hospital, COVID-19 treatment center, Northwest Ethiopia.
Methods: A cross-sectional study was conducted among 117 conveniently recruited COVID-19 patients from March to June 2021. Socio-demographic and clinical data were collected using a structured questionnaire and checklist, respectively. The platelet parameters were analyzed by the Mindray-BC 5800 automated hematological analyzer. ANOVA and Kruskal–Wallis tests were used to compare the difference between parametric and non-parametric continuous variables, respectively. Binary logistic regression was used to identify the factors associated with thrombocytopenia. A P-value < 0.05 was defined as statistically significant for all statistical tests.
Results: Among COVID-19 patients, 45, 43 and 29 were mild, moderate and severe, respectively. 65.8% of the patients were males and 34.2% were alcohol drinkers with a mean age of 50.6 ± 15.4. Moreover, 44.4% of the patients had co-morbidity. Thrombocytopenia was presented in 23.9% of the patients. It was 4.57 (95% CI: 1.30– 16.07) and 6.10 (95% CI: 1.54– 24.08) times more likely in the moderate and severe cases compared to mild cases, respectively. Disease severity was also associated with PDW (P-value = 0.001).
Conclusion: Even though thrombocytopenia was not presented in most moderate and severe COVID-19 patients, thrombocytopenia and PDW were associated with disease severity.
Keywords: COVID-19, Ethiopia, risk stratification, severity, thrombocytopenia
Background
Severe acquired respiratory syndrome corona virus-2 (SARS-CoV-2) occurred in Wuhan, China in December 2019.1 The virus has genomic similarity to the severe acute respiratory syndrome-related coronavirus and is named SARS-CoV-2 which was finally dubbed coronavirus disease 2019 (COVID-19) by the World Health Organization.2,3
Hemostasis is a complex process that keeps blood in a fluid state. It includes the clot formation and fibrinolysis.4 The imbalance of hemostasis cascades cause excessive clot formation or bleeding. In the majority of COVID-19 patients, abnormal hemostasis is common and associated disease severity and prognosis.5–7 Hypercoagulability is a usual pathobiological presentation in COVID-19 patients.8 Therefore, it is essential to assess the hemostasis system including the platelets (PLT) parameter in COVID-19 patients for proper disease management.9
Thrombocytopenia in COVID-19 patients is caused by a variety of mechanisms. One of the postulated mechanisms is that coronaviruses may infect bone marrow cells, resulting in abnormal hematopoiesis and finally causes a low platelet production. The other possible mechanism may be due to viral infection and inflammation that results in lung damage. This leads to activating platelets in the lungs, resulting in aggregation and formation of micro-thrombi, which increases platelet consumption. Autoimmunity may be another potential player that induces antiphospholipid antibody production which was verified in various viral infections. COVID-19 may raise levels of auto-antibodies and immune complexes, causing the immune system to specifically destroy platelets.10
The management of critically ill patients has been incredibly hard because of the complexity and the insufficient evidence on the pathogenesis of COVID-19.11 Accordingly, laboratory data like PLT parameters are needed. They play an important role in the clinical decision, treatment, and management of the diseases to improve the clinical care and ensure prompt treatment.12,13. It gives medical professionals the evidence-based data. Therefore, the main objective of the study was to assess the prevalence of thrombocytopenia and its association with COVID-19 severity.
Materials and Methods
Study Design, Setting and Population
A prospective cross-sectional study was conducted from March 2021 to June 2021 on COVID-19 patients attending Tibebe Ghion Specialized Hospital COVID-19 treatment center, northwest, Ethiopia. The Hospital is found in Bahir Dar town which is 565 km away from Addis Ababa, the capital city of Ethiopia. The hospital is providing different medical services to more than 5 million people in the region.14 All real-time reverse transcription polymerization chain reaction (RT-qPCR) confirmed COVID-19 patients were the source of populations and the study populations were all RT-qPCR confirmed COVID-19 patients during the study period. But, COVID-19 patients those unable to give biological samples, those who had a predetermined platelet abnormality and those on antithrombotic therapy before the baseline platelet determination were excluded from the study.
Sample Size Determination and Sampling Technique
The sample size was determined by using a census method.15 Therefore, the entire COVID-19 cases that fulfilled the inclusion criteria within the study period were recruited. A total of 117 COVID-19 patients (45 milds, 43 moderates, and 29 severe cases) were included by the convenient sampling technique.
Operational Definitions
Thrombocytopenia
Thrombocytopenia was defined as a platelet count < 150 x 103cells/µL.16
Alcohol Drinkers
Men who consume more than 4 drinks/day or more than 14 drinks/week or women who consume more than 3 drinks/day or more than 7 drinks/week.17,18
Mild Cases
Mild clinical symptoms and no pneumonia manifestation found on imaging.19
Moderate Cases
Patients have symptoms like fever and respiratory tract symptoms and pneumonia manifestation can be seen in imaging.19
Severe Cases
Include those with any of the following; respiratory distress, respiratory rate ≥30 breaths/min; oxygen saturation ≤ 93% at rest; and partial pressure of oxygen ≤300. Patients with greater than 50% lesion progression within 24 to 48 hours in pulmonary imaging should be treated as severe cases if a respiratory failure occurs and mechanical ventilation is required, shock, and complications from another organ failure that require monitoring and treatment in the ICU.19
Data Collection Procedures
Socio-Demographic and Clinical Data Collection
A pretested structured questionnaire was used as a tool for the collection of socio-demographic data. Relevant socio-demographic data (gender, age, residence, current marital status, educational status, occupation, alcohol use) and past and current medical history, including the history of chronic disease, were collected by trained nurses. A clinical data and clinical examination of patient’s vital signs like body temperature, blood pressure, pulse rate, respiratory rate, and oxygen saturation percentage were also measured and recorded on a data collection checklist.
To assure the quality of the data, the e questionnaire was prepared in English, translated to the Amharic language, and then translated back to English to check for consistency. It was also pre-tested on 5% of COVID-19 patients at Debre-Markos University’s COVID-19 treatment center for its accuracy before actual data collection. The appropriate one-day training was given to all data collectors about the objective of the study, confidentiality issues, study participants’ rights, consenting, and techniques of the interview before data collection. Socio-demographic and clinical data were collected by trained and experienced nurses under the close supervision of investigators. Vital sign measurements (blood pressure, temperature, pulse rate and respiratory rate were measured two times and the mean was taken as the actual measurement. Every collected data was checked for its completeness daily by the investigators.
Blood Sample Collection and Analysis
After getting written informed consent from the study participants, 3 mL venous blood was collected aseptically from the patients by an experienced medical laboratory technologist from their antecubital vein by using a vacutainer collection system in an ethylene diamine tetraacetic acid anti-coagulated test tube (BD Vacutainer® K2-EDTA tube, Plymouth, England). The blood specimen and anticoagulant were mixed gently. Then the collected blood was checked for proper labeling, hemolysis, and clotting pre-analytically.
Then after the PLT count was determined at Amhara Public Health Institute Laboratory by using the Mindray BC-5800 automated hematological analyzer (Mindray, China). The hematological analyzer uses the impedance principle in which a constant electric current is passed through an isotonic solution. Then it measures the changes in electrical resistance that occur when blood cells pass through the detection aperture.20
To maintain the quality of the laboratory results, pre-analytical, analytical, and post-analytical precautions of quality and the standard operating procedure (SOP) were strictly followed. All manufacturer instructions and SOP were strictly followed. Every specimen collection procedure was performed aseptically to avoid contamination for both the patient and the specimen, and the collected blood sample was checked for appropriate labeling, hemolysis and clotting pre-analytically. Preventive maintenance of the instruments was done daily, weekly, and monthly by laboratory personnel. All reagents and materials were checked for their expiration date, leakage, and signs of deterioration. The quality of the complete blood count (CBC) results were maintained by running commercially prepared three-level quality control (low, normal, and high) reagents before running the patient’s sample. The quality control result was compared with a specific limit or range of quality control material. The patient specimen was run after the quality control result passed. The quality was also checked by background reading.
Safety Management
Data collectors were provided training on COVID-19 prevention, including when to get tested, use of personal protective equipment, and cleaning. Conditions of entry were displayed for any customers or visitors on entry points. Hand sanitizer was provided at multiple locations throughout the workplace. Detergent/ disinfectant surface wipes were provided to clean workstations and equipment used. Bathrooms were stocked with hand soap and paper towels and had posters with instructions on how to wash your hands. There was frequent cleaning of touched areas and surfaces.
Disinfectant solutions were maintained at an appropriate strength and used per the manufacturer’s instructions. Data collectors were wearing gloves when cleaning and washing hands thoroughly with soap and water before and after each activity. In general, the use of the required personal protective equipment like a particulate respirator, mask, gown and gloves was ensured when contacting the patient and blood sample collection from the patient and also whenever it is required in the process. All intervention procedures were done by sterilized and safe maneuvers.
Data Management and Statistical Analysis
The data were checked for completeness, cleaned, sorted, and categorized daily before being entered into EpiData version 4.6 and exported to IBM SPSS version 25 for analysis. Socio-demographic and clinical characteristics of study participants were summarized by using descriptive statistics and then presented in tables and text. Categorical variables were given as frequency rates and percentages; continuous variables were expressed as mean and standard deviation (SD) (parametric data) or median and Interquartile range (IQR) (non-parametric data). The Shapiro–Wilk test was used to verify the normality of the distribution of continuous variables. Moreover, binary logistic regression was used to identify the factors associated with thrombocytopenia. In all statistical tests, a p-value < 0.05 was considered statistically significant.
Ethical Considerations
The study was conducted after it was reviewed and approved by the Ethical Review Committee of the School of Biomedical and Laboratory Sciences, the University of Gondar with a reference number of SBMLS/2743/13. A permission letter to conduct the study was also obtained from Tibebe Ghion Specialized Referral Hospital’s chief executive officer before the commencement of the study, and the study was as per the principles of the declaration of Helsinki II. Written informed consent was also obtained from each study participant and any participant who was not willing to participate in the study was not forced to participate. They were also informed that all the data obtained would be kept confidential by using codes instead of any personal identifiers and used only for the study purpose. Moreover, all laboratory test results were communicated to their physicians for prompt patient management.
Results
Socio-Demographic and Behavioral Characteristics
A total of 117 COVID-19 patients were enrolled in this study. Of the included participants, 77 (65.8%) were male and 94 (80.3%) were urban residents. Education-wise, 49 (41.9%) have attained university or college, while the majority of the participants were government employees, 42 (35.9%), followed by merchants, 27 (23.1%). Of the study participants, 102 (87.2%) were married and 40 (34.2%) had alcohol consumption habits. Most of the patients were between the age of 41 and 60 years old (36.8%) followed by the age of < 40 years old (34.2%) with a mean age of 50.6 ±15.4 (Table 1).
 |
Table 1 Socio-Demographic and Behavioral Characteristics of Study Participants Attending at the Tibebe Ghion Specialized Hospital, COVID-19 Treatment Center 2021 (N=117) |
Clinical Characteristics
Of the total study population, 52 (44.4%) had co-morbidity. Co-morbidity was significantly higher in moderate and severe COVID-19 patients compared to mild COVID-19 patients (24.4%, 53.5% and 62.1% of mild, moderate and severe patients, respectively) (Chi-Square p-vale = 0.002). The most common co-morbidities were diabetic Mellitus (17.1%) and hypertension (14.5%) and the most common clinical presentations (signs and symptoms) were fever (57.26%), headache (59.83%), breathing difficulty (51.28%), cough (44.44%), and myalgia (51.28%) (Table 2).
 |
Table 2 Clinical Characteristics of the Study Participants Attending the Tibebe Ghion Specialized Hospital, COVID-19 Treatment Center 2021 (N=117) |
Platelet Parameters of COVID-19 Patients
In the current study, 28 (23.9%) of the COVID-19 patients had thrombocytopenia. The median PLT count was 198 x 109/L (IQR; 123) with a mean platelet volume (MPV) of 9.1fl (IQR; 1.5). They also had 63.03 x 109/L platelet large cell count (P-LCC) (SD; 26.1 x 109/L). PLT count was lower in moderate and severe cases. But, it was statistically insignificant (P-value = 0.114). Mean platelet volume also had no significant differences between mild, moderate and severe groups (P-value = 0.891). Moreover, the PCT (p-value = 0.081), P-LCC (P-value = 0.178) and P-LCC% (P-value = 0.237) had no significant differences between mild, moderate and severe groups. However, Platelet distribution width (PDW) (P-value = 0.001) and thrombocytopenia (P-value 0.008) had significant differences between mild, moderate and severe groups (Tables 3 and 4).
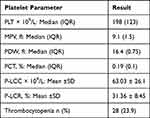 |
Table 3 Platelet Parameters of COVID-19 Patients Attending the Tibebe Ghion Specialized Hospital, COVID-19 Treatment Center (N = 117) |
 |
Table 4 Comparison of Baseline Platelet Parameters with the Clinical Stage of COVID-19 Patients Attending the Tibebe Ghion Specialized Hospital, COVID-19 Treatment Center (N = 117) |
Factors Associated with Thrombocytopenia
The prevalence of thrombocytopenia was higher in females and older age groups. It was also higher in COVID-19 patients who had co-morbidity and alcohol consumption habits. However, all had no significant association with thrombocytopenia. On the other hand, the clinical stage of the COVID-19 patient had a significant association with thrombocytopenia. The odds of thrombocytopenia were 4.57 (1.30–16.07) and 6.10 (1.54–24.08) times more likely among moderate and severe COVID-19 patients compared to mild COVID-19 patients, respectively (Table 5).
 |
Table 5 Factors Associated with Thrombocytopenia Among COVID-19 Patients (N = 117) |
Discussion
Thrombocytopenia was the most common manifestation of the early epidemic of viral infection. It was observed in 20–55% of the SARS epidemic.21 Among COVID-19 patients, thrombotic complications become a major concern.22,23 These complications may cause thrombocytopenia which intern may associate with disease severity. Thrombocytopenia can prolong hospitalization, increase the need of ventilation, and increase patient mortality.24
In the present study, thrombocytopenia at admission was presented in 23.9% of the COVID-19 patients (95% CI: 17.1–32.5%). This might be due to COVID-19 patients having a higher level of P-selectin expression and activated platelets, elevated circulating platelet-leukocyte aggregates, increased aggregation, and thromboxane generation. This leads to platelet consumption.25,26 At admission, P-selectin expression was significantly increased in severe COVID-19 patients.27 Rampotas Alexandros and Pavord Sue report that PLTs were aggregated in COVID-19 patients that showed the important role of platelets in thrombotic complications or respiratory failure in COVID-19 patients.28 The direct attack on hematopoietic stem/progenitor cells by the virus, lung injury, and platelet destruction by auto-antibodies may also cause thrombocytopenia.29 Following the viral infection, the cytokine storm destroys bone marrow progenitor cells and leads to a decrease in PLT production. The virus also causes lung damage, resulting in PLT aggregation and micro-thrombi formation in the lungs which causes platelet consumption. Production of autoantibody against PLT glycoproteins also destructs autoantibody-coated PLTs by the reticuloendothelial system leading to thrombocytopenia.30 A similar findings were reported in Turkey (25.1%),31 China (17.8%),32 Iran (30%)33 and USA (20%).26
Early identification of severe patients will improve patient outcomes and reduce mortality. The current study revealed that the assessment baseline thrombocytopenia had significant value in the stratification of COVID-19 clinical stage at admission. It was significantly higher in moderate and severe COVID-19 patients compared to mild cases. It was 4.57 and 6.10 times more likely in moderate and severe COVID-19 patients respectively. The presence of thrombocytopenia may indicate the presence of some forms of coagulopathy with an increased risk of thrombotic events.34 The risk of the thrombotic event was increased as the disease prognosis is worsening. A higher prevalence of thrombotic events was found in ICU COVID-19 compared to non-ICU COVID-19 patients.35–38 Other studies also supported this finding. Jin et al reported that PLT was correlated with COVID-19 disease severity.39 The Sayad et al study was also reported that thrombocytopenia could be important indicator of severe COVID-19 which might associate with mortality.33 According to the systematic review and Meta-analysis reported by Lippi et al thrombocytopenia increased the risk of disease severity more than five times more likely.40 Moreover, a study in the USA by Barrett et al showed that COVID-19 disease severity was 1.4 times more likely among patients with thrombocytopenia compared to those without thrombocytopenia.26
Contrarily, there was another study disagreed with this finding. A study in the USA by Hana et al showed that platelet count at admission had no a significant difference between severe disease versus non-severe disease.41 The difference might attribute to the difference in the comparison method. In the current study, the comparison of platelet count and disease severities was made by grouping the patients with thrombocytopenia and without thrombocytopenia. On the other hand, the study in the USA used the mean platelet count which is affected by extreme values. This was also demonstrated by the present study. The average PLT count, expressed by median (IQR), had no significant association with disease severity (Kruskal–Wallis P-value = 0.114). This indicated that grouping COVID-19 patients as thrombocytopenia and without thrombocytopenia might have a better clinical significance value than comparing them by the average PLT count. Indeed, there was a study that showed the average PLT count was significantly lower in severe COVID-19 patients than in non-severe cases.42
The current study also showed that PDW was significantly associated with disease severity (P-value = 0.001). Platelet distribution width reflects the variation in the size of PLTs. It increases when PLT destruction increases and when the new large immature platelet production raises for compensation. A study conducted by Bhandary et al also showed that PDW was significantly associated with disease severity and it was higher in severe cases.43 Moreover, Aydınyılmaz et al showed that PDW had a statistically significant change between severe (admitted at ICU) and non-severe COVID-19 patients (ward patients).44 Tire et al also revealed that PDW had a statistical difference between outpatients, patients who received standard treatment in the hospital and patients who had to be connected to a mechanical ventilator (ICU patients).45
However, other studies revealed that PDW had no significant association with disease severity.31,46,47 The difference might be due to the comparison groups. The studies, which were done by Gulu et al and Chaudhary et al, compared the moderate and severe groups while in the current study the comparison was done between mild, moderate and severe groups. On the other hand, Yardimci et al compared the ward and ICU COVID-19 patients.
Strengths and Limitations
Determining the severity of the disease with a simple and inexpensive test might have social benefits and might help the scientific community for future studies. However, the current study had limitations. First, this study was a single-center study. Second, the sample size was relatively low. Therefore, a multicenter study with larger sample size is needed to verify the current findings.
Conclusions and Recommendations
Thrombocytopenia was presented in 23.9% of COVID-19 patients. It was significantly associated with the clinical stage of the disease. However, still, most moderate and severe patients did not have thrombocytopenia (69.8% and 62.1%, respectively). PDW was also associated with disease severity. Therefore, Thrombocytopenia and PDW can be used as risk stratification markers in COVID-19 patients. Furthermore, a multicenter study with larger sample size is needed to verify the current findings.
Abbreviations
CBC, Complete Blood Count; COVID-19, Corona Virus Disease 2019; IQR, Inter Quartile Range; MPV, Mean Platelet Volume; PLT, Platelet; PCT, Plateletcrit; PDW, Platelet Distribution Width; P-LCC, Platelet Large Cell Count; P-LCCR, Platelet Large Cell Count Ratio; RT-qPCR, Real-Time Reverse Transcriptase-Polymerase Chain Reaction; SARS-CoV-2, Severe Acute Respiratory Syndrome Corona Virus-2; SOP, Standard Operating Procedures.
Data Sharing Statement
All data supporting these findings is contained within the manuscript.
Consent for Publication
Not applicable. This manuscript does not contain any individual persons’ data.
Acknowledgment
We would like to thank the Department of Hematology and Immunohematology, School of Biomedical and Laboratory Sciences, the University of Gondar. We also wish to extend our sincere thanks and gratitude to the Tibebe Ghion Specialized Hospital staff for their willingness and support during data collection and the Amhara Public Health Institute Laboratory staff who supported CBC laboratory analysis. Finally, many thanks were given to all study participants for their willingness to participate and for providing the necessary information during the data collection.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors received no specific funding for this work.
Disclosure
The authors declare that there is no conflict of interest regarding the publication of this manuscript.
References
1. UNICEF. Improving Child Nutrition: The Achievable Imperative for Global Progress. New York: UNICEF; 2013:1–14.
2. Wang L, Wang Y, Ye D, Liu Q. Review of the 2019 novel coronavirus (SARS-CoV-2) based on current evidence. Int J Antimicrob Agents. 2020;55(6):879. doi:10.1016/j.ijantimicag.2020.105948
3. King AM, Lefkowitz EJ, Mushegian AR, et al. Changes to taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2018). Arch Virol. 2018;163(9):2601–2631. doi:10.1007/s00705-018-3847-1
4. Rodak BF, Keohane EM, Fritsma GA. Hematology-E-Book: Clinical Principles and Applications. Elsevier Health Sciences; 2013.
5. Friedrich MS, Studt J-D, Braun J, Spahn DR, Kaserer A. Coronavirus-induced coagulopathy during the course of disease. PLoS One. 2020;15(12):e0243409. doi:10.1371/journal.pone.0243409
6. Mezgebe M, Jacobson BF, Mayne ES, Louw S. Change in platelet indices in patients with Coronavirus disease‐2019 (COVID‐19): a reflection of platelet activation and contribution to immunothrombosis? Int J Lab Hematol. 2022;44(1):e46–e8. doi:10.1111/ijlh.13705
7. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi:10.1016/S0140-6736(20)30211-7
8. Cao W, Li T. COVID-19: towards understanding of pathogenesis. Cell Res. 2020;30(5):367–369. doi:10.1038/s41422-020-0327-4
9. Getu S, Tegenaw Tiruneh HA, Hailemichael W, Kiros T, Belay DM, Kiros M. Coagulopathy in SARS-CoV-2 infected patients: implication for the management of COVID-19. J Blood Med. 2021;12:635. doi:10.2147/JBM.S304783
10. Xu P, Zhou Q, Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann Hematol. 2020;99(6):1205–1208. doi:10.1007/s00277-020-04019-0
11. Elshazli RM, Toraih EA, Elgaml A, et al. Diagnostic and prognostic value of hematological and immunological markers in COVID-19 infection: a meta-analysis of 6320 patients. PLoS One. 2020;15(8):1–20. doi:10.1371/journal.pone.0238160
12. Yousif NG, Altimimi AN, Al-amran FG, et al. Hematological changes among Corona virus-19 patients: a longitudinal study. Sys Rev Pharm. 2020;11(5):862–866.
13. Henry BM, De Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58(7):1021–1028. doi:10.1515/cclm-2020-0369
14. Wassihun B, Deribe L, Worede N, Gultie T. Prevalence of disrespect and abuse of women during child birth and associated factors in Bahir Dar town, Ethiopia. Epidemiology and Health. 2018;40:e2018029. doi:10.4178/epih.e2018029
15. Israel GD. Determining Sample Size. Israel GD; 1992.
16. Liu H, Wang Z, Sun H, et al. Thrombosis and coagulopathy in COVID-19: current understanding and implications for antithrombotic treatment in patients treated with percutaneous coronary intervention. Front Cardiovascular Med. 2021;2:378.
17. Control CfD, Prevention. Alcohol use and your health. Available from: https://wwwcdcgov/alcohol/pdfs/alcoholyourhealthpdf.
18. Bidira K, Tamiru D, Belachew T. Anthropometric failures and its associated factors among preschool-aged children in a rural community in southwest Ethiopia. PLoS One. 2021;16(11):e0260368. doi:10.1371/journal.pone.0260368
19. National Health Commission of the People’s Republic of China. Chinese Management Guideline for COVID-19 (Version 6.0). National Health Commission of the People’s Republic of China; 2014.
20. Addis Ababa University. T. A. Performance Evaluation of Cell-Dyn 1800 and Sysmex KX-21 Hematology Analyzers at ST. Paul’s Hospital Millennium Medical College. Addis Ababa, Ethiopia: Addis Ababa University; 2015.
21. Wool GD, Miller JL. The impact of COVID-19 disease on platelets and coagulation. Pathobiology. 2021;88(1):15–27. doi:10.1159/000512007
22. Lee SG, Fralick M, Sholzberg M. Coagulopathy associated with COVID-19. Canadian Med Assoc J. 2020;192(21):E583. doi:10.1503/cmaj.200685
23. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi:10.1056/NEJMoa2002032
24. Delshad M, Safaroghli-Azar A, Pourbagheri-Sigaroodi A, Poopak B, Shokouhi S, Bashash D. Platelets in the perspective of COVID-19; pathophysiology of thrombocytopenia and its implication as prognostic and therapeutic opportunity. Int Immunopharmacol. 2021;99:107995. doi:10.1016/j.intimp.2021.107995
25. Daniels SA, Wei H, Denning DW. Platelet size as a predictor for severity and mortality in COVID-19 patients: a systematic review and meta-analysis. medRxiv. 2021.
26. Barrett TJ, Bilaloglu S, Cornwell M, et al. Platelets contribute to disease severity in COVID‐19. J Thrombosis Haemostasis. 2021;19(12):3139–3153. doi:10.1111/jth.15534
27. Yatim N, Boussier J, Chocron R, et al. Platelet activation in critically ill COVID-19 patients. Ann Intensive Care. 2021;11(1):1–12. doi:10.1186/s13613-021-00899-1
28. Rampotas A, Pavord S. Platelet aggregates, a marker of severe COVID-19 disease. J Clin Pathol. 2021;74(11):750–751. doi:10.1136/jclinpath-2020-206933
29. Nigam JS, Kumar A, Sinha R, et al. Association of Peripheral Blood Parameters With Outcomes of COVID-19 Infection in a Tertiary Care Setting of Eastern India: an Institute-Based Study. Cureus. 2021;13:12.
30. Delshad M, Safaroghli-Azar A, Pourbagheri-Sigaroodi A, Poopak B, Shokouhi S, Bashash D. Platelets in the perspective of COVID-19; pathophysiology of thrombocytopenia and its implication as prognostic and therapeutic opportunity. Int Immunopharmacol. 2021;99:107995.
31. Güçlü E, Kocayiğit H, Okan HD, et al. Effect of COVID-19 on platelet count and its indices. Revista da Associação Médica Brasileira. 2020;66:1122–1127. doi:10.1590/1806-9282.66.8.1122
32. Liu Y, Sun W, Guo Y, et al. Association between platelet parameters and mortality in coronavirus disease 2019: retrospective cohort study. Platelets. 2020;31(4):490–496. doi:10.1080/09537104.2020.1754383
33. Sayad B, Rahimi Z. Blood coagulation parameters in patients with severe COVID-19 from Kermanshah Province, Islamic Republic of Iran. Eastern Mediterranean Health J. 2020;26(9):999–1004. doi:10.26719/emhj.20.105
34. Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(23):2950–2973. doi:10.1016/j.jacc.2020.04.031
35. Zanza C, Racca F, Longhitano Y, et al. Risk management and treatment of coagulation disorders related to COVID-19 infection. Int J Environ Res Public Health. 2021;18(3):1268. doi:10.3390/ijerph18031268
36. Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi:10.1016/j.thromres.2020.04.024
37. Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thrombosis Haemostasis. 2020;18(6):1421–1424. doi:10.1111/jth.14830
38. Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi:10.1007/s00134-020-06062-x
39. Jin X, Duan Y, Bao T, et al. The values of coagulation function in COVID-19 patients. PLoS One. 2020;15(10):e0241329. doi:10.1371/journal.pone.0241329
40. Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clinica chimica acta. 2020;506:145–148. doi:10.1016/j.cca.2020.03.022
41. Hana C, Aboulenain S, Dewaswala N, Narendran V. Does Thrombocytopenia Truly Correlate with COVID-19 Severity? Blood. 2020;136:39–40. doi:10.1182/blood-2020-142440
42. Bao C, Tao X, Cui W, et al. SARS-CoV-2 induced thrombocytopenia as an important biomarker significantly correlated with abnormal coagulation function, increased intravascular blood clot risk and mortality in COVID-19 patients. Exp Hematol Oncol. 2020;9(1):1–8. doi:10.1186/s40164-020-00172-4
43. Bhandary C, Agarkar P, Manivannan P, Kar R, Basu D. Hematological parameters in covid-19 illness: emphasis on platelet indices as a marker of severity of disease. Indian J Hematol Blood Transfusion. 2020;1:S128.
44. Aydınyılmaz F, Aksakal E, Pamukcu HE, et al. Significance of MPV, RDW and PDW with the Severity and Mortality of COVID-19 and Effects of Acetylsalicylic Acid Use. Clin Applied Thrombosis Hemostasis. 2021;27:10760296211048808. doi:10.1177/10760296211048808
45. Tire Y, Yazar MA, Erdem SS. Can changes in platelet count, mean platelet volume, and platelet distribution width be used to determine the severity of COVID-19? Med Sci Discovery. 2021;8(10):581–585. doi:10.36472/msd.v8i10.611
46. Yardımcı AC, Yıldız S, Ergen E, et al. Association between platelet indices and the severity of the disease and mortality in patients with COVID-19. Eur Rev Med Pharmacol Sci. 2021;25(21):6731–6740. doi:10.26355/eurrev_202111_27118
47. Chaudhary N, Raju K, Kamarthi P. Profile of Platelet Parameters in Coronavirus Disease–Positive Cases—An Observational Study. Biomed Res Therapy. 2021;8(10):4649–4654. doi:10.15419/bmrat.v8i10.700
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
