Back to Journals » Therapeutics and Clinical Risk Management » Volume 19
Association of Oxygen Therapy with the Natural Disease Progression of Cystic Fibrosis: A Multi-State Model of the European Cystic Fibrosis Society Patient Registry
Authors Gambazza S , Orenti A, Pizzamiglio G, Zolin A, Colombo C, Laquintana D, Ambrogi F
Received 11 October 2022
Accepted for publication 12 February 2023
Published 13 March 2023 Volume 2023:19 Pages 255—267
DOI https://doi.org/10.2147/TCRM.S391476
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Simone Gambazza,1,2,* Annalisa Orenti,2,* Giovanna Pizzamiglio,3 Anna Zolin,2 Carla Colombo,4,5 Dario Laquintana,1 Federico Ambrogi2,6 On behalf of ECFSPR
1Healthcare Professions Department, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy; 2Department of Clinical Sciences and Community Health, Laboratory of Medical Statistics, Biometry and Epidemiology “G. A. Maccacaro”, Università degli Studi di Milano, Milan, Italy; 3Cystic Fibrosis Center – Adult Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy; 4Cystic Fibrosis Center, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy; 5Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milano, Italy; 6Scientific Directorate, IRCCS Policlinico San Donato, San Donato Milanese, MI, Italy
*These authors contributed equally to this work
Correspondence: Simone Gambazza, Healthcare Professions Department, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Via Francesco Sforza 35, Milan, 20122, Italy, Email [email protected]
Background: Association between dependence on oxygen therapy (OT) and natural disease progression in people with cystic fibrosis (pwCF) has not been estimated yet. The aim of this study is to understand the prognosis for pwCF on OT, evaluating how the transition probabilities from being alive without lung transplantation (LTx) to LTx and to death, and from being alive after LTx to death change in pwCF with and without OT.
Methods: We used 2008– 2017 data from the 35-country European CF Society Patient Registry. A multi-state model was fitted to assess the effects of individual risk factors on transition probabilities.
Results: We considered 48,343 pwCF aged from 6 to 50 years. OT (HR 5.78, 95% CI: 5.32– 6.29) and abnormal FEV1 (HR 6.41, 95% CI: 5.28– 7.79) were strongly associated with the probability of having LTx; chronic infection with Burkholderia cepacia complex (HR 3.19, 95% CI: 2.78– 3.67), abnormal FEV1 (HR 5.00, 95% CI: 4.11– 6.08) and the need for OT (HR 4.32, 95% CI: 3.93– 4.76) showed the greatest association with the probability of dying without LTx. Once pwCF received LTx, OT (HR 1.75, 95% CI: 1.41– 2.16) and abnormal FEV1 (HR 1.63, 95% CI: 1.18– 2.25) were the main factors associated with the probability of dying. An association of gross national income with the probability of receiving LTx and with the probability of dying without LTx was also found.
Conclusion: Oxygen therapy is associated with poor survival in pwCF with and without LTx; harmonization of CF care throughout European countries and minimization of the onset of pulmonary gas exchange abnormalities using all available means remains of paramount importance.
Keywords: cystic fibrosis, oxygen therapy, epidemiology, mortality, lung transplantation
Introduction
Improved diagnostics and treatment options have led to increased survival in people with cystic fibrosis (pwCF);1 however, respiratory failure still represents the primary cause of morbidity and mortality. Chronic and recurrent respiratory infections damage the lungs progressively, leading eventually to chronic hypoxemia. A reduction in oxygen saturation is most likely to occur either during sleep or during exercise and before it becomes apparent at rest during the day.2 Whether it is continuous, nocturnal or exertional, oxygen therapy (OT) is commonly prescribed in CF to normalize gas exchange, to relieve symptoms of dyspnea and fatigue and to delay the development of cor pulmonale.3 Published studies regarding the use of oxygen in CF are primarily about the effects of nocturnal and ambulatory oxygen.4 Despite the well-established benefit of supplemental oxygen in adults with severe hypoxemia who have chronic obstructive pulmonary disease (COPD),5 there is little evidence to correlate OT in pwCF with either a reduction in mortality or the alleviation of symptoms.4 Despite the clinical expectation that pwCF on OT are likely to have poor prognosis, to date, it remains unclear if OT is associated or not with the natural progression of the disease.4
The aim of this study was to evaluate how OT is associated with the course of CF disease, using a multi-state model which includes the transition probabilities from being alive without LTx to LTx and to death and from being alive after LTx to death. In addition, we also examined risk factors and comorbidities associated with these transitions.
Methods
Study Design
This observational cohort study used data from 2008 to 2017 from the European Cystic Fibrosis Society Patient Registry (ECFSPR), where each of the 35 member countries up to 2017 provided data under the existing ethical approval and data governance structures, in compliance with the Declaration of Helsinki. In 2017, the ECFSPR covered 35–99% of the CF population per country;6 nine countries had <80% coverage (Armenia, Bulgaria, Croatia, Lithuania, Norway, Poland, Romania, Spain, Turkey and Ukraine).6 The ECFSPR structure and operations have been previously described.7 Participating pwCF signed an informed consent, including consent to use their data for future research. Demographic and clinical characteristics of the complete patient population were extracted from the ECFSPR. Standard ECFSPR definitions applied to all variables.8 The study was approved by the ECFSPR Scientific Committee and by the ECFSPR Steering Committee (02/06/2019).
Statistics
To express the degree of lung damage, we use the forced expiratory volume in the first second (FEV1) calculated using z-score from the Global Lung Initiative (GLI) reference equations;9 information on ethnicity is not yet collected by the ECFSPR and for the purposes of the study we assumed that the pwCF were all Caucasian. FEV1 was considered in the normal range when above the lower limit of normal (LLN), corresponding to a z-score of −1.64. Body mass index (BMI) was categorized according to the nutritional goal for CF, that is a BMI at or above 22 kg/m2 (females) and 23 kg/m2 (males) for those >18 years of age.10 For pwCF ≤18 years of age we used BMI z-score computed on the CDC references,11 with nutritional goal at or above 0 z-score.10 Countries were classified into two groups (low versus high income) based on gross national income (GNI) per capita in 2017, obtained from the World Bank tables.12
We interpreted the course of CF disease as characterized by different types of events occurring after diagnosis. When considering CF from the perspective of respiratory disease, pwCF can die without having had LTx or can receive LTx and then die and we therefore used the multi-state model to estimate the transition probabilities of patients between these events (otherwise called states).13 As summarized in Figure 1, pwCF could have been enrolled in the ECFSPR alive and without LTx, eventually being in a successive state (eg, alive with LTx, death without LTx), or they could have been enrolled alive with LTx, eventually transitioning to death with LTx. Furthermore, to assess the effect of individual risk factors on the transition probabilities from one state to the other, a multi-state regression model was fitted using age as timescale. The explanatory variables included in this model are: exposure to oxygen therapy, FEV1 z-score (categorized as above or below the LLN, as recommended14); age at diagnosis (categorized as <1 year, 1–18 years, >18 years); sex, CFTR genotype (categorized as severe – with both alleles in class 1, 2 or 3, mild – with at least one allele in class 4 or 5, unknown); BMI nutritional goal; presence of diabetes treated with daily insulin; chronic Pseudomonas aeruginosa (PSA); chronic Burkholderia cepacia complex (BCC); use of pancreatic enzymes; history of pneumothorax; history of hemoptysis >250mL; GNI (low and high income countries).
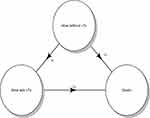 |
Figure 1 Multi-state model for pwCF: arrows denote direction of transition probabilities (T): from alive without LTx to alive with LTx (T1) or to death (T2); and from alive with LTx to death (T3). |
Presence of oxygen supplementation is collected by the ECFSPR as oxygen therapy during the year of follow-up; this variable was included in the model as a time-dependent variable since a subject can enter the registry and not be on oxygen therapy and then begin oxygen therapy during follow-up; this change is taken into account in the model. On the other hand, all other variables are included in the model considering their value at baseline, that is the time of entry in the registry.
The results of the regression model are provided for each transition in terms of Hazard Ratio (HR), with 95% confidence intervals (95% CI) and p-value. Regarding missing data, we performed a case-wise complete analysis. Categorical data are presented as number and percentage (computed excluding missing data). Analyses were done with the open-source software R Core Team, version 3.6.2 with the mstate15 package added.
Results
For the years 2008–2017, the ECFSPR collected information on 62,367 pwCF from 35 countries. For the purpose of this study, we considered 48,343 individuals between the ages of 6 and 50 with verified lung transplantation status. Of these, 46,951 were alive without LTx and 1392 were alive with LTx at enrollment in the ECFSPR. During follow-up 2392 received LTx, 2234 died without LTx and 753 died after LTx. Overall, median follow-up time for those alive without LTx to LTx is 10.9 (95% CI: 10.8 to 11) years and 11 (95% CI: 10.8 to 11) years for pwCF who died without LTx. Follow-up time for those who received LTx and died is 12.8 (95% CI: 10.6 to 16) years.
As summarized in Table 1, pwCF on OT had more severe features of CF disease: 59% had been diagnosed with CF within the first year of life; 34% and 7% were diagnosed between 1–18 and >18 years old, respectively. We report an uneven distribution of pwCF on OT between countries with high versus low incomes (Figure 2), with the odds of being in OT at baseline reduced by 19% (OR 0.81, 95% CI: 0.72 to 0.91) for pwCF living in countries with low income.
 |
Table 1 Main Demographic and Clinical Characteristics of the 48,343 Considered pwCF |
Low-income nations registered higher use of OT from 2011, which may be attributable to additional nations joining the ECFSPR, namely Ukraine, Romania, North Macedonia and Lithuania. In particular, for pwCF aged 12–24 and 25–34 living in low-income countries, consistently higher oxygen utilization was reported than for pwCF in high-income countries.
By adjusting the multi-state model for all of the features of CF disease mentioned above, pwCF on oxygen therapy showed worse transition probabilities (Figure 3). For example, the probability of receiving LTx by 50 years old is 17.5 (95% CI: 10.5–29.2)% and the chance of dying by age 50 without LTx is 81.1 (95% CI: 72.6–90.6)% for those individuals who start OT at 20 years of age. On the other hand, pwCF without oxygen supplementation at 20 years old have 20.3 (95% CI: 15.1–27.2)% and 34.7 (95% CI: 29.2–41.1)% probability, respectively. Even after LTx, pwCF in OT at 20 years old show 87.7 (95% CI: 80.3–95.7)% probability of dying by age 50 compared to 69.8 (95% CI: 58.6–83.2)% for pwCF not on OT at 20 years old (Figure 3, lower panels).
Table 2 summarizes the strength of association between selected covariates and transitions from one state to another. FEV1< LLN (HR 6.41, 95% CI: 5.28–7.79) (Supplemental Figure 1) and oxygen therapy (HR 5.78, 95% CI: 5.32–6.29) (Figure 3) are strongly associated with the probability of undergoing LTx, whereas males (HR 0.81, 95% CI: 0.74–0.87) and people living in a low-income country (HR 0.63, 95% CI: 0.52–0.76) are less likely to receive LTx. This can be seen further in Figure 4, where cumulative probabilities for pwCF living in high and low-income countries are consistently separated, whether they had OT or not at age 20. Transition probabilities stratified by GNI are identical when assessing the risk of death after receiving LTx, which is higher for those who were on OT.
 |
Table 2 Adjusted Hazard Ratio (HR) for the Transition Probabilities from the Multi-State Model |
There was evidence for a strong association between FEV1 < LLN and being on oxygen therapy and the transition probability of dying without LTx (Table 2). Chronic infection with BCC represented a risk factor for the survival probability (HR 3.19, 95% CI: 2.78–3.67), whereas male sex showed 17% lower risk of death (HR 0.83, 95% CI: 0.75–0.91) compared to females (Supplemental Figure 2). Individuals who were diagnosed with CF after the first year of life had 21% lower risk of death (HR 0.79, 95% CI: 0.72–0.88).
The fitted multi-state model also shows that after LTx, oxygen therapy (HR 1.75, 95% CI: 1.41–2.16) and FEV1<LLN (HR 1.63, 95% CI: 1.18–2.25) almost doubled the risk of death. Additionally, severe disease, characterized by CF-related diabetes (HR 1.39, 95% CI: 1.15–1.67), pancreatic insufficiency (HR 2.44, 95% CI: 1.26–4.70) and a history of hemoptysis (>250mL) (HR 1.44, 95% CI: 1.06–1.96), increases the risk of death (Table 2). On the other hand, a late CF diagnosis, between 1 and 18 years of age, is associated with a significantly reduced probability of dying in pwCF who had LTx (HR 0.82, 95% CI: 0.67–0.99). There is no evidence for an association between BMI (Supplemental Figure 3) and Pseudomonas aeruginosa infection (Supplemental Figure 4) and the risk of death after LTx.
We also found evidence of differences between HRs for OT among transition probabilities (Table 2): when pwCF were alive without LTx the HR of OT for death was lower than the HR of OT for LTx (P<0.001); for pwCF alive with LTx, the HR of OT for death was lower compared to the HR of OT for death in pwCF alive without LTx (P<0.001).
Discussion
Cystic fibrosis is a multisystem disease. Although there have been steady and significant improvements in median survival over the past decades,16–18 most of the comorbidities and premature mortality in pwCF are due to chronic respiratory infection and subsequent respiratory failure; approximately, 20% of individuals develop severe lung disease by the age of 30.19 Our findings show that 6.1% of pwCF in the present study are on OT, a key factor associated with transition probabilities between states in pwCF either with or without LTx. Oxygen treatment for CF exacerbations is similar to the initial oxygen treatment for COPD exacerbations,20 whereas chronic oxygen therapy is prescribed in pwCF to relieve symptoms of dyspnea and fatigue and to delay the development of right heart failure.3 The last systematic review on OT for CF reported that OT should be reserved for those individuals with objective evidence of hypoxemia, either at rest while awake or during either exercise or sleep.4 To date, there are no published studies that have been able to establish whether OT alleviates symptoms or, most importantly, is associated with natural disease progression. In the ECFSPR, from 2008 to 2017, oxygen therapy was recorded as any use of oxygen in the year; therefore, in this study it was considered to be a proxy of a significant clinical event that marked not only a hypoxemic episode but also the initial failure of the respiratory system to cope with the higher metabolic demands imposed by the disease.
Recently, the Cystic Fibrosis Foundation (CFF) identified supplemental oxygen requirement as a clinical milestone for LTx referral since it was found to be repeatedly associated with death without LTx in pwCF.21 Supplemental oxygen was a predictor of death without LTx (HR 2.1, 95% CI: 1.7–2.6) in 3340 adult patients with CF with FEV1<30% in the United States of America (US).22 An earlier Canadian study of 673 individuals who were followed between 1977 and 1989 identified PaO2 lower than 55mmHg as a risk factor for death within two years.23 Dependence on supplemental oxygen seemed to indicate decreased survival also in children with CF selected for LTx in the US during the period from 1992 to 2002.24 Adult patients requiring supplemental oxygen were identified as having an increased risk of death while on the waiting list for LTx (1.07, 95% CI: 1.00–1.14) in one US study conducted between 2005 and 2013,25 and a study from the French CF Registry identified long-term oxygen therapy as a prognostic factor associated with death or LTx within 4 years in adults.26 Overall, our study demonstrates how oxygen therapy is associated not only with the probability of dying without LTx, conceived as a competitive event against the probability of receiving LTx, but also with the probability of dying after LTx. This outcome indicates that a high degree of attention should be devoted to pwCF immediately after they show signs of respiratory system failure in order to maintain adequate gas exchange, independent of age or severity of lung disease. It is well documented that respiratory failure does not only occur in end-stage lung disease; before becoming chronic, respiratory failure is episodic, occurring during exercise or during sleep, even in stable clinical conditions. Abnormalities in arterial blood gas pressure are more manifest during exercise and sleep but can also occur during daytime activities when symptoms of lung disease are exacerbated. Therefore, oxygen supplementation becomes a marker of the severity of the disease and does affect survival, even after LTx, as shown in the present study. This indicates that, rather than early referral of pwCF to LTx centers, which are not specialized CF centers, multi-disciplinary teams (MDT) at CF clinics should optimize diagnostic methods for the detection of early markers of alterations in pulmonary gas exchange and intervene therapeutically. At the very minimum, MDTs should implement potentiating therapies as soon as oxygen supplementation is required and before respiratory failure becomes chronic.
Our findings reinforce the importance of FEV1 as a marker of CF disease across the three transition probabilities, even if z-score is used instead of percent of predicted. The idea that pwCF are at risk just because FEV1 falls to below an arbitrary cut-off point of percent of predicted is increasingly being questioned. The CFF recently recommended initiating discussions on LTx when pwCF present with a FEV1 below 50% of predicted,21 since the previously accepted indicator of FEV1 <30% predicted as a marker of shortened survival seems no longer appropriate,22,23,27–29 especially when considering that some reference equations used before the implementation of GLI may overestimate lung function.30 On the whole, FEV1 z-score below −1.64 should indicate to the MDT that they need to address the issue with urgency and intervene with more frequent follow-up and optimization of therapy, including regular physical activity and exercise which have been demonstrated to increase survival.31,32 Striving to maintain the FEV1 of pwCF within normal ranges should be a common goal for all MDTs.
Regarding the role of other covariates in the transition probabilities between the different states, we found evidence of an association between chronic BCC infection and the probability of dying without LTx. This association is possibly inflated by BCC being listed so far as a contraindication for LTx in most countries. Until now, BCC has been considered a significant barrier to the success of LTx in pwCF:21 data from the Canadian CF Registry and the US CFF Patient Registry showed that the risk of death after first lung transplantation increased if pwCF were infected with BCC before transplantation (HR 1.40, 95% CI: 1.03–1.91),33 but this was not found in our data (HR 0.90, 95% CI: 0.54–1.50).
A significant difference in survival between the sexes is however apparent in our study. For females with CF, there is an increased risk of dying without LTx and also after LTx. As reported elsewhere,21 this survival difference disappears in those alive with LTx who later die. With regard to age at diagnosis, pwCF with a late diagnosis have significant lower hazards of death, before or after LTx, which is likely due to a mild course of CF disease.
Our study also investigated whether GNI was associated with the probability of survival with or without LTx in pwCF. In a recent study, survival across Europe was found to be influenced by socioeconomic factors.34 It is therefore relevant that we also found a statistical association between GNI and the probability of receiving LTx, which is lower for pwCF living in low-income European countries, and a statistical association between GNI and the probability of dying without LTx, which is higher for pwCF living in low-income European countries. This finding underpins the inequalities in CF healthcare across Europe. How CF standards of care are implemented differs across Europe, as does how OT is considered and managed. OT for pwCF is reimbursed in most European countries except for Bulgaria, Moldova, Serbia, Russia and Ukraine. In Latvia, the therapy is only reimbursed for adults.6 There is further disparity around access to transplant programs and in some countries there is no access at all.21 As reported by the US, socioeconomic status influences outcomes for patients awaiting LTx; however, social disparities might affect LTx outcomes only before transplantation.25 Our findings did not demonstrate that GNI is associated with death after LTx either. Overall, clinical features associated with a poor outcome after LTx remain previous exposure to OT, FEV1<LLN, the presence of cystic fibrosis-related diabetes (CFRD), use of pancreatic enzymes and history of hemoptysis.
Future actions are needed to ensure equal access to recommended standards of care for pwCF in European countries and, in particular, reimbursement of OT and access to advanced therapeutic regimes such as LTx. Harmonization of how OT is prescribed for pwCF is of utmost importance; it is currently based on evidence from people with COPD which means our understanding of the true significance of the variable oxygen therapy during the year of follow-up collected by ECFSPR is unknown. Redefining how this variable is collected may also help us to understand more about the effect of oxygen usage, whether it be administered intermittently, such as during pulmonary exacerbation, overnight or during exercise, or on a long-term basis. Gathering additional information about OT in the future, for the 2935 pwCF included in the present study, could also shed more light onto how and for what OT has been prescribed.
Altogether, our results bring to the literature that OT and FEV1 below LLN are associated with a worse transition probability towards death, with or without LTx. However, bearing in mind that FEV1 is just one indication of CF lung disease it could be appropriate to model survival probabilities by exploring other, more sensitive markers of lung disease, such as lung clearance index (LCI).35–37 The decision to include LCI as a variable in the ECFSPR from 2018 onwards is therefore rather appropriate since it can help to diagnose earlier stage lung disease than if only a decrease in FEV1 is considered; this has promising implications for future prognostication in CF, as recently presented elsewhere.38 We hope it is, or will become, available and reimbursed in as many countries as possible.
Despite the Copernican revolution that has been ignited by highly effective CFTR modulator therapies, many pwCF still do not have, and are unlikely to have in the near future, access to these new drugs, either because of their genotype or due to country-specific health policies.39 It is therefore imperative that CF MDTs recognize early-stage pulmonary disease using the latest and most sensitive diagnostic methods and technology available, and treat it as soon as symptoms are manifest.
Limitations and Strengths
This study has limitations: it is limited to the variables included in the ECFSPR database; data were collected before 2018, the year in which the ECFSPR started to record data about CFTR modulator therapies (though we can estimate, looking at data from 201840 that less than 16% of pwCF included in the present analysis took one of the CFTR modulator therapies before 2018 and thus the bias on the overall probabilities is small); we considered OT as present from its first occurrence onwards, thus assuming that respiratory failure was irreversible. There is no way, however, given how this variable was collected by the ECFSPR, to understand how pwCF were exposed to OT or the rationale behind its administration and we therefore acknowledge that we made a strong assumption. We have considered the need for oxygen supplementation, even just once, as an indicator that the respiratory system is profoundly compromised by the disease. On the other hand, this could be deemed to be one of the strengths of the study since we demonstrate that the prescription of OT can be regarded as a red flag and turning point, for the worse, in the course of CF disease.
Another strong point of this study is that it is based on high-quality data41,42 from a large population of pwCF from 35 countries and data were analyzed using a technique that allowed us to model the transition from one condition to another as an extension of competing risk models. In addition, our findings corroborate the relevance of using FEV1 z-score based on the GLI equations as a marker of CF-disease severity. These equations eliminate age, sex and height bias and provide a more standardized approach to measuring lung function than the traditional method of percent of predicted.
Conclusion
With the statistical evidence presented in this study, we have demonstrated that treatment with oxygen therapy, abnormal FEV1, presence of CFRD and use of pancreatic enzymes are all features of CF that consistently affect the transition probabilities of surviving, with or without LTx. Since prognosis is also determined by economic disparities at a country level, which in turn affect standards of care and the availability of diagnostic and therapeutic instruments, we strongly recommend that the harmonization of CF care throughout the countries in Europe remains of paramount importance to the CF community, to prevent by all possible means, as much as possible, the onset of respiratory failure.
Abbreviation
pwCF, people with Cystic Fibrosis; OT, Oxygen therapy; COPD, Chronic Obstructive Pulmonary Disease; LTx, Lung Transplantation; ECFSPR, European Cystic Fibrosis Society Patient Registry; CF, Cystic Fibrosis; FEV1, Forced Expiratory Volume in the first second; GLI, Global Lung Function; LLN, Lower Limit of Normal; BMI, Body Mass Index; GNI, Gross National Income; CFTR, Cystic Fibrosis Transmembrane conductance Regulator; PSA, Pseudomonas aeruginosa; BCC, Burkholderia cepacia complex; HR, Hazard Ratio; IQR, Interquartile Range; CFF, Cystic Fibrosis Foundation; MDTs, Multi-Disciplinary Teams; CFRD, Cystic Fibrosis-related Diabetes; LCI, lung clearance index.
Acknowledgments
The present findings were presented at the 43rd ECFS Conference in Lyon (FR) in 2020 as a poster (S. Gambazza, F. Ambrogi, A. Zolin, A. Orenti. “Lung transplantation and mortality in patients with cystic fibrosis under oxygen therapy”. Journal of Cystic Fibrosis 2020 Vol. 19 S106).
We thank the people with CF, and their families, for consenting to the inclusion of their data in the ECFSPR. We thank the centers and individual country representatives for allowing the use of the data, and the ECFSPR for providing access to pseudonymized patient data. We thank Dr. Alice Fox for critically revising the manuscript.
The ECFSPR contributors list consists of the representatives of the countries whose data is used in this article, and the members* of the Scientific Committee who reviewed the initial data application and the final manuscript:
Irena Kasmi (Department of Paediatrics, “Mother Thereza” Hospital Center, Tirana, Albania); Satenik Harutyunyan (Yerevan University CF Centre, Muratsan Hospital, Yerevan, Armenia); Andreas Pfleger (Department of Paediatrics and Adolescent Medicine, Division of Paediatric Pulmonology and Allergology, Medical University of Graz, Graz, Austria);
Géraldine Daneau (Sciensano, Epidemiology and public health, Health services research, Brussels, Belgium); Elise Lammertijn* (Cystic Fibrosis Europe, Brussels; Association Muco A.S.B.L. – Mucovereniging V.Z.W., Brussels, Belgium); Guergana Petrova (Paediatric Clinic, Alexandrovska University Hospital, Sofia, Bulgaria); Duška Tješić-Drinković (Cystic Fibrosis Centre - Paediatrics and Adults, University Hospital Centre Zagreb, Zagreb, Croatia); Pavel Drevinek (Department of Medical Microbiology, Second Faculty of Medicine, Charles University and Motol University Hospital, Prague, Czech Republic); Milan Macek Jr* (Department of Biology and Medical Genetics, Second Faculty of Medicine, Charles University and Motol University Hospital, Prague, Czech Republic); Hanne Vebert Olesen (Department of Pediatrics and Adolescent Medicine, Cystic Fibrosis Center, Aarhus University Hospital, Aarhus, Denmark); Pierre-Régis Burgel* (Respiratory Medicine and National Cystic Fibrosis Reference Center, Cochin Hospital, Assistance Publique-Hôpitaux de Paris, Université de Paris, Institut Cochin, INSERM U1016, Paris, France); Lydie Lemonnier-Videau (Vaincre la Mucoviscidose, Paris, France); Lutz Naehrlich (German Center of Lung Research, Universities of Giessen and Marburg Lung Center, Justus-Liebig-University Giessen, Giessen, Germany); Elpis Hatziagorou* (Cystic Fibrosis Unit, Hippokration General Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece); Andrea Párniczky (Heim Pál National Pediatric Institute, Budapest, Hungary); Godfrey Fletcher (The Cystic Fibrosis Registry of Ireland, Dublin, Ireland); Meir Mei-Zahav (Pulmonary Institute, Schneider Children’s Medical Center of Israel, Petah Tikva, Israel; Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel); Rita Padoan (Department of Pediatrics, Cystic Fibrosis Regional Support Centre, University of Brescia, Brescia; Scientific Board of Italian CF Registry, Rome, Italy); Elina Aleksejeva (Department of Pneumology, Children’s Clinical University Hospital, Rīga Stradinš University, Riga, Latvia); Kestutis Malakauskas (Department of Pulmonology, Adult Cystic Fibrosis center, Lithuanian University of Health Sciences, Kaunas, Lithuania); Anna-Maria Charatsi (Paediatric Department, Centre hospitalier Luxembourg, Luxembourg); Oxana Turcu (Department of Paediatrics, Ambulatory Cystic Fibrosis and Other Rare Diseases Center, Institute for Maternal and Child Healthcare, State University of Medicine and Pharmacy “Nicolae Testemitanu”, Chisinau, Republic of Moldova); Vincent Gulmans (Dutch Cystic Fibrosis Foundation (NCFS), Baarn, The Netherlands); Stojka Fustik (Centre for Cystic Fibrosis, University Children’s Hospital, Skopje, North Macedonia); Ivana Arnaudova Danevskai (Centre for Cystic Fibrosis, Children and adults, Institute for respiratory diseases in children, Kozle, North Macedonia); Egil Bakkeheim (Dep. of Paediatrics, Norwegian Cystic Fibrosis Registry, Oslo University Hospital, Oslo, Norway); Lukasz Woźniacki (Dziekanow Paediatric Hospital, Cystic Fibrosis Centre, Institute of Mother and Child, Warsaw, Poland); Luísa Pereira (Centre for Cystic Fibrosis, Hospital de Santa Maria, Lisbon, Portugal); Liviu Pop (Victor Babes University of Medicine and Pharmacy Timisoara, National Cystic Fibrosis Centre Timisoara, Romania); Elena Kondratyeva (Research Centre for Medical Genetics, Moscow, Russian Federation); Milan Rodić (National Centre for Cystic Fibrosis, Mother and Child Health Institute of Serbia “Dr Vukan Čupić”, Belgrade, Serbia); Hana Kayserová (Cystic Fibrosis Centre, University Hospital of Bratislava, Bratislava, Slovakia); Uroš Krivec (Department of Paediatric Pulmonology, University Children’s Hospital, Ljubljana University Medical Centre, Ljubljana, Slovenia); Maria Dolores Pastor-Vivero (Paediatric Pneumology and Cystic Fibrosis Unit, Osakidetza, Hospital Universitario Cruces, Bizkaia, Spain); Isabelle de Monestrol (Stockholm CF centre, Karolinska University Hospital, Karolinska Institutet, Stockholm, Sweden); Anders Lindblad* (Gothenburg CF Centre, Queen Silvia Children’s Hospital, The Sahlgrenska Academy at the University of Gothenburg, Gothenburg, Sweden); Andreas Jung (Paediatric Pulmonology, University Children`s Hospital Zurich, Zurich, Switzerland); Deniz Dogru (Cystic Fibrosis Registry of Turkey, Ankara, Turkey); Halyna Makukh (Institute of Hereditary Pathology Ukrainian National Academy of Medical Sciences, Lviv, Ukraine); Siobhán B. Carr* (Department of Respiratory Paediatrics, Royal Brompton Hospital; NHLI, Imperial College, London, UK); Rebecca Cosgriff (Cystic Fibrosis Trust, London, UK).
Simone Gambazza and Annalisa Orenti are co-first authors for this study.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
The study was partially funded by Italian Ministry of Health – Current research IRCCS.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Stephenson AL, Stanojevic S, Sykes J, Burgel PR. The changing epidemiology and demography of cystic fibrosis. Presse Med. 2017;46(6 Pt 2):e87–e95. doi:10.1016/j.lpm.2017.04.012
2. Katz ES. Cystic fibrosis and sleep. Clin Chest Med. 2014;35(3):495–504. doi:10.1016/j.ccm.2014.06.005
3. Schidlow DV, Taussig LM, Knowles MR. Cystic fibrosis foundation consensus conference report on pulmonary complications of cystic fibrosis. Pediatr Pulmonol. 1993;15(3):187–198. doi:10.1002/ppul.1950150311
4. Elphick HE, Mallory G. Oxygen therapy for cystic fibrosis. Cochrane Database Syst Rev. 2009;1:CD003884. doi:10.1002/14651858.CD003884.pub3
5. Hopkinson NS, Molyneux A, Pink J, Harrisingh MC. Chronic obstructive pulmonary disease: diagnosis and management: summary of updated NICE guidance. BMJ. 2019;366(July):1–7. doi:10.1136/bmj.l4486
6. Zolin A, Orenti A, Naehrlich L, Jung A, van Rens J. ECFS patient registry annual report 2018; 2020. Available from: https://www.ecfs.eu/projects/ecfs-patient-registry/annual-reports.
7. Viviani L, Zolin A, Mehta A, Olesen HV. The European cystic fibrosis society patient registry: valuable lessons learned on how to sustain a disease registry. Orphanet J Rare Dis. 2014;9(1):1–14. doi:10.1186/1750-1172-9-81
8. Zolin A, Orenti A, Naehrlich L, van Rens J. ECFSPR annual report 2017; 2019.
9. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi:10.1183/09031936.00080312
10. Turck D, Braegger CP, Colombo C, et al. ESPEN-ESPGHAN-ECFS guidelines on nutrition care for infants, children, and adults with cystic fibrosis. Clin Nutr. 2016;35(3):557–577. doi:10.1016/j.clnu.2016.03.004
11. Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11. 2002;246:1–190.
12. Gross National Income per Capita. Atlas method and PPP; 2019. Available from: https://databank.worldbank.org/home.
13. Meira-Machado LF, de Uña-álvarez J, Cadarso-Suárez C, Andersen PK. Multi-state models for the analysis of time-to-event data. Stat Methods Med Res. 2009;18(2):195–222. doi:10.1177/0962280208092301
14. Stanojevic S, Kaminsky DA, Miller M, et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J. 2021:2101499. doi:10.1183/13993003.01499-2021
15. de Wreede LC, Fiocco M, Putter H. The mstate package for estimation and prediction in non- and semi-parametric multi-state and competing risks models. Comput Methods Programs Biomed. 2010;99(3):261–274. doi:10.1016/j.cmpb.2010.01.001
16. Keogh RH, Szczesniak R, Taylor-Robinson D, Bilton D. Up-to-date and projected estimates of survival for people with cystic fibrosis using baseline characteristics: a longitudinal study using UK patient registry data. J Cyst Fibros. 2018;17(2):218–227. doi:10.1016/j.jcf.2017.11.019
17. Stephenson AL, Sykes J, Stanojevic S, et al. Survival comparison of patients with cystic fibrosis in Canada and the United States. Ann Intern Med. 2017;166(8):537. doi:10.7326/M16-0858
18. Burgel PR, Bellis G, Olesen HV, et al. Future trends in cystic fibrosis demography in 34 European countries. Eur Respir J. 2015;46(1):133–141. doi:10.1183/09031936.00196314
19. Cystic Fibrosis Foundation. Patient Registry Annual Data Report. Cystic Fibrosis Foundation; 2019.
20. O’Driscoll BR, Howard LS, Earis J, et al. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017;72(6):i1–i90. doi:10.1136/thoraxjnl-2016-209729
21. Ramos KJ, Smith PJ, McKone EF, et al. Lung transplant referral for individuals with cystic fibrosis: cystic fibrosis foundation consensus guidelines. J Cyst Fibros. 2019;18(3):321–333. doi:10.1016/j.jcf.2019.03.002
22. Ramos KJ, Quon BS, Heltshe SL, et al. Heterogeneity in survival in adult patients with cystic fibrosis with FEV1 < 30% of predicted in the United States. Chest. 2017;151(6):1320–1328. doi:10.1016/j.chest.2017.01.019
23. Kerem E, Reisman J, Corey M, Canny GJ, Levison H. Prediction of mortality in patients with cystic fibrosis. N Engl J Med. 1992;326(18):1187–1191. doi:10.1056/NEJM199204303261804
24. Liou TG, Adler FR, Cox DR, Cahill BC. Lung transplantation and survival in children with cystic fibrosis. N Engl J Med. 2007;357(21):2143–2152. doi:10.1056/NEJMoa066359
25. Krivchenia K, Tumin D, Tobias JD, Hayes D. Increased mortality in adult cystic fibrosis patients with medicaid insurance awaiting lung transplantation. Lung. 2016;194(5):799–806. doi:10.1007/s00408-016-9927-7
26. Nkam L, Lambert J, Latouche A, Bellis G, Burgel PR, Hocine MN. A 3-year prognostic score for adults with cystic fibrosis. J Cyst Fibros. 2017;16(6):702–708. doi:10.1016/j.jcf.2017.03.004
27. Robinson PD, Latzin P, Ramsey KA, et al. Preschool multiple-breath washout testing. An official American thoracic society technical statement. Am J Respir Crit Care Med. 2018;197(5):e1–e19. doi:10.1164/rccm.201801-0074ST
28. Milla CE, Warwick WJ. Risk of death in cystic fibrosis patients with severely compromised lung function. Chest. 1998;113(5):1230–1234. doi:10.1378/chest.113.5.1230
29. Doershuk CF, Stern RC. Timing of referral for lung transplantation for cystic fibrosis: overemphasis on FEV1 may adversely affect overall survival. Chest. 1999;115(3):782–787. doi:10.1378/chest.115.3.782
30. Stanojevic S, Stocks J, Bountziouka V, et al. The impact of switching to the new global lung function initiative equations on spirometry results in the UK CF registry. J Cyst Fibros. 2014;13(3):319–327. doi:10.1016/j.jcf.2013.11.006
31. Hebestreit H, Hulzebos EHJ, Schneiderman JE, et al. Cardiopulmonary exercise testing provides additional prognostic information in cystic fibrosis. Am J Respir Crit Care Med. 2019;199(8):987–995. doi:10.1164/rccm.201806-1110OC
32. Nixon PA, Orenstein DM, Kelsey SF, Doershuk CF. The prognostic value of exercise testing in patients with cystic fibrosis. N Engl J Med. 1992;327(25):1785–1788. doi:10.1056/NEJM199212173272504
33. Stephenson AL, Ramos KJ, Sykes J, et al. Bridging the survival gap in cystic fibrosis: an investigation of lung transplant outcomes in Canada and the United States. J Heart Lung Transplant. 2021;40(3):201–209. doi:10.1016/j.healun.2020.12.001
34. McKone EF, Ariti C, Jackson A, et al. Survival estimates in European cystic fibrosis patients and the impact of socioeconomic factors: a retrospective registry cohort study. Eur Respir J. 2021;58(3):2002288. doi:10.1183/13993003.02288-2020
35. Davies JC, Sermet-Gaudelus I, Naehrlich L, et al. A Phase 3, double-blind, parallel-group study to evaluate the efficacy and safety of tezacaftor in combination with ivacaftor in participants 6 through 11 years of age with cystic fibrosis homozygous for F508del or heterozygous for the F508del-CFTR mutati. J Cyst Fibros. 2021;20(1):68–77. doi:10.1016/j.jcf.2020.07.023
36. Milla CE, Ratjen F, Marigowda G, Liu F, Waltz D, Rosenfeld M. Lumacaftor/ivacaftor in patients aged 6–11 years with cystic fibrosis and homozygous for F508del-CFTR. Am J Respir Crit Care Med. 2017;195(7):912–920. doi:10.1164/rccm.201608-1754OC
37. Frauchiger BS, Binggeli S, Yammine S, et al. Longitudinal course of clinical lung clearance index in children with cystic fibrosis. Eur Respir J. 2021;58(1):2002686. doi:10.1183/13993003.02686-2020
38. Kurz JM, Ramsey KA, Rodriguez R, et al. Association of lung clearance index with survival in individuals with cystic fibrosis. Eur Respir J. 2021. doi:10.1183/13993003.00432-2021
39. Barry PJ, Taylor-Cousar JL. Triple combination cystic fibrosis transmembrane conductance regulator modulator therapy in the real world - opportunities and challenges. Curr Opin Pulm Med. 2021;27(6):554–566. doi:10.1097/MCP.0000000000000819
40. Zolin A, Orenti A, Naehrlich L, Jung A, van Rens J. ECFSPR annual report 2018; 2020.
41. EMA/CHMP/SAWP/622564/2018. Qualification opinion on the European Cystic Fibrosis Society Patient Registry (ECFSPR) and CF pharmaco-epidemiology studies. Available from: https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/qualification-opinion-european-cystic-fibrosis-society-patient-registry-ecfspr-cf-pharmaco_en.pdf.
42. Naehrlich L, Fox A, Krasynk M, Orenti A, Zolin A, van Rens J. P080 the European Cystic Fibrosis Society Patient Registry (ECFSPR) data validation programme: accuracy and consistency of data. J Cyst Fibros. 2019;18:S80. doi:10.1016/S1569-1993(19)30374-1
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Short-Term Oxygen Therapy Outcomes in COPD
Soumagne T, Maltais F, Corbeil F, Paradis B, Baltzan M, Simão P, Abad Fernández A, Lecours R, Bernard S, Lacasse Y
International Journal of Chronic Obstructive Pulmonary Disease 2022, 17:1685-1693
Published Date: 28 July 2022
Characteristics of Elderly Hip Fracture Patients in Jordan: A Multicenter Epidemiological Study
Dawod MS, Alisi MS, Saber YO, Abdel-Hay QA, Al-Aktam BM, Alfaouri Y, Alfraihat LB, Albadaineh AA, Abuqudiri AZ, Odeh RM, Altamimi AAR, Alrawashdeh MA, Alebbini MM, Abu-Dhaim OA, Al-Omari AA, Alaqrabawi I, Alswerki MN, Abuawad A, Al Nawaiseh MR, Hammad Y, Al-Ajlouni J
International Journal of General Medicine 2022, 15:6591-6598
Published Date: 13 August 2022
Ceftazidime/Avibactam for the Treatment of Carbapenem-Resistant Pseudomonas aeruginosa Infection in Lung Transplant Recipients
Chen J, Liang Q, Ding S, Xu Y, Hu Y, Chen J, Huang M
Infection and Drug Resistance 2023, 16:2237-2246
Published Date: 15 April 2023



