Back to Journals » International Journal of General Medicine » Volume 16
Association of Body Composition and Handgrip Strength with Interleukin-6 (IL-6) and Vitamin D Level in Cancer Patients
Authors Sutandyo N , Cintakaweni DMW, Setiawan L, Hariani R, Utami N
Received 30 September 2022
Accepted for publication 28 April 2023
Published 23 May 2023 Volume 2023:16 Pages 1995—2001
DOI https://doi.org/10.2147/IJGM.S388457
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Noorwati Sutandyo,1 Della Manik Worowerdi Cintakaweni,2 Lyana Setiawan,3 Ririn Hariani,2 Nani Utami2
1Department of Hematology and Medical Oncology, Dharmais National Cancer Center Hospital, Jakarta, Indonesia; 2Department of Clinical Nutrition, Dharmais National Cancer Center Hospital, Jakarta, Indonesia; 3Department of Integrated Laboratory, Dharmais National Cancer Center Hospital, Jakarta, Indonesia
Correspondence: Noorwati Sutandyo, Email [email protected]
Introduction: Cachexia is prevalent in cancer and is associated with poorer prognosis. We aimed to investigate the association of interleukin-6 (IL-6) and vitamin D levels with cachexia in cancer patients. We also assessed the relationship between body composition profile and cachexia, IL-6, and vitamin D levels.
Methods: A cross-sectional study was conducted at Dharmais National Cancer Hospital. The study included patients with newly diagnosed biopsy-proven nasopharyngeal cancer, lung cancer, breast cancer, cervical cancer, or non-Hodgkin lymphoma. Blood samples, anthropometrics, and body composition were measured.
Results: A total of 150 cancer patients were included in the study, with a median age of 52 years, and 64% (n = 96) are female. The prevalence of cachexia was 57%. Cancer patients with cachexia had higher IL-6 levels (P = 0.025). No association between cachexia and vitamin D levels was found (P = 0.787). Patients with cachexia had lower body composition components than those without cachexia (P < 0.05). Vitamin D levels were positively correlated with muscle mass, visceral fat, and handgrip strength (P < 0.05), while no association between IL-6 and body composition was found.
Conclusion: Cancer-associated cachexia is associated with a higher level of IL-6, lower BMI, lower fat mass index, and lower visceral fat. Vitamin D levels, but not IL-6, are correlated with muscle mass, muscle strength, and visceral fat in cancer patients.
Keywords: interleukin-6, vitamin D, cachexia, handgrip strength, cancer, nutrition
Introduction
Cachexia is one of the comorbidity in cancer, characterized by progressive loss of body weight, skeletal muscle and adipose tissue mass, that has devastating impact. It has been known to affect up to 80% of cancer cases1 It is associated with higher mortality1,2 and poorer response rate to cancer treatment.3 It is still challenging to reverse cancer-associated cachexia when it has occurred. The probability of regaining muscle mass in patients with advanced stage of cancer and cachexia was estimated to be only 2%.4 It is therefore important to disentangle factors associated with cachexia to improve survival and response rate to treatment.
The onset of cachexia and its progression is contributed by anorexia, inflammation, and disturbance in hormonal and metabolic homeostasis.4 Vitamin D has role in skeletal muscle homeostasis, and its deficiency is correlated with decreased muscle function, increased disability, and sarcopenia, which is characterized by increased fat and reduced muscle mass.5 Meta-analyses and randomized controlled trials show advantages of vitamin D in improving age-related decline in muscle function. On the other side, the mechanism of vitamin D in the development and differentiation of muscle cells remains inconclusive.6
In cancer patients, decreased vitamin D level in the circulation was reported to be associated with impairment of glucose metabolism and insulin sensitivity. Some advantages of vitamin D supplementation observed in metastatic prostate cancer which resulted in improved muscle strength and reduced pain score,7 higher disease-free survival in breast cancer patients.8
Cancer cachexia pathogenesis is multifactorial, including general hypercatabolic state, energy deficit condition, and low-grade inflammatory response of both tumor and host origin. Systemic inflammation is an important feature and considered as the main drivers of cancer-associated cachexia.9 Proinflammatory cytokines, such as IL-6, INF-γ, and TNF-α, induced muscle protein catabolism and impair the synthesis of muscle proteins, triggering cachexia.10 Vast literatures have investigated the association of interleukin-6 (IL-6) and cachexia in cancer. However, most previous literatures used animal models or focused on a particular cancer which limited the extrapolations in human cancers in general.11–13 Moreover, vitamin D was suggested to reduce IL-6 in systemic inflammation.9,11 To the best of our knowledge, this is the first reported study from Indonesia regarding vitamin-D levels and IL-6 related to cancer-associated cachexia. The present study aims to investigate the association of IL-6 and vitamin D levels with cachexia in newly diagnosed cancer patients.
Methods
Study Population
A cross-sectional study was conducted in Dharmais National Cancer Center in Indonesia with consecutive sampling. The study population was patients aged 18 years old and above with newly diagnosed biopsy-proven from the top five of the most common type of diagnosed cancer in our hospital: nasopharyngeal cancer, lung cancer, breast cancer, cervical cancer, or non-Hodgkin lymphoma from January to December 2020. To evaluate the prevalence of cachexia on each cancer type, the number of patients is well distributed. Random sampling was used in this study. We excluded patients with more than one primary cancer. Informed consent was obtained. This study was approved by the ethical committee of Dharmais National Cancer Center, Indonesia (no. 165/KEPK/XII/2020) in accordance with the Declaration of Helsinki. Data were collected at diagnosis and prior to cancer treatment initiation.
Measurements
A 5-mL peripheral blood sample from each patient was collected by venipuncture, stored in an EDTA tube, and was centrifuged. Plasma was stored at a temperature of −80oC. IL-6 was measured using electrochemiluminescence (ECLIA) method (Cobas e601 analyzer, Roche Diagnostics GmbH). Vitamin D was measured using chemiluminescent microparticle immunoassay (CMIA) method (Abbott Architect i2000 SR).
Body weight was measured with patients wearing light clothes and without shoes, using a calibrated digital scale. Weight was recorded to the nearest 0.1 kg. Height was measured on a stadiometer and recorded to the nearest 0.1 kg. Body mass index (BMI) was calculated as weight in kilograms divided by square of height in meters. Asian BMI classification was used in this study. Cachexia was measured based on history taking from the patients and BMI measurement. It was defined as weight loss >5% over the past 6 months, or weight loss >2% over the past 6 months if current BMI <20 kg/m2.4
To estimate fat mass index, skeletal muscle mass, and visceral adipose tissue, bioelectrical impedance analysis (BIA) was used All scans were performed by trained nutritionist. DXA protocol from the manufacturer was followed. Handgrip strength in kilograms was measured using Camry digital hand dynamometer. Each hand was measured for three times. The maximum value of the dominant hand was used for the analysis.
Statistical Analysis
Descriptive analyses are presented in count and proportion for categorical variables. Continuous variables are presented in mean (standard deviation [SD]) or median (Q1 – Q3), depending on the data distribution. To compare characteristics and body composition profile between patient with and without cachexia, chi-square test or Mann–Whitney U-test was performed. Visualization using Boxplot and Mann–Whitney test was applied to show the difference in IL-6 and vitamin D levels between patients with and without cachexia. Correlation between IL-6 and vitamin D as well as correlation of body composition with IL-6 or vitamin D were assessed using Spearman correlation. All statistical analyses were performed using Stata version 16 (StataCorp, Texas, USA). A p-value <0.05 was considered statistically significant.
Results
Patients’ Characteristics
A total of 150 patients was included in the study, in which 64% (n = 96) patients were females and had median age of 52 years old. The prevalence of cachexia was 57.3% (n = 86). Underweight was found in 8% (n = 12) patients, obesity in 10% (n = 15) patients. Nasopharynx cancer is the type of cancer with most cachexia patients, followed by lung, and in the third place there were breast, cervix cancer and non-Hodgkin lymphoma, with same number of patients. No difference in sex, age, type of cancer, or comorbidities among patients with cachexia vs without cachexia (all P > 0.05) (Table 1).
 |
Table 1 Patients’ Characteristics |
Association of Body Composition and Handgrip Strength with Cachexia
Patients with cachexia had lower BMI, waist circumference (WC), fat mass index (FMI), and visceral adipose tissue compared to patients without cachexia (P < 0.05). There was no difference in skeletal muscle mass and handgrip strength between patients with cachexia and without cachexia (Table 2).
 |
Table 2 Association of Body Composition and Handgrip Strength with Cachexia |
Interleukin-6 and Vitamin D Levels in Patients with Cachexia and without Cachexia
IL-6 level was found to be higher in patients with cachexia than in patients without cachexia (P = 0.025), whereas no difference in vitamin D level was observed between patients with and without cachexia (P = 0.787). Vitamin D and IL-6 levels between patients with and without cachexia are shown in Figure 1A and B.
 |
Figure 1 (A) Difference in IL-6 levels in patients with cachexia vs without cachexia; (B) difference in vitamin D levels in patients with cachexia vs without cachexia. |
Correlation of IL-6 and Vitamin D Levels with Body Weight, BMI, Fat Mass Index, Muscle Mass, Visceral Fat, and Handgrip Strength
There was no correlation of IL-6 with body weight, BMI, fat mass index, muscle mass, visceral fat, or handgrip strength. On the other hand, vitamin D level was negatively correlated with fat mass index, whereas positively correlated with muscle mass, visceral fat, and handgrip strength (all P < 0.05) (Table 3).
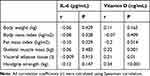 |
Table 3 Correlation of IL-6 and Vitamin D Levels with Body Weight, BMI, Fat Mass Index, Muscle Mass, Visceral Fat, and Handgrip Strength |
Correlation Between IL-6 and Vitamin D Levels
No significant correlation between IL-6 and vitamin D levels was found (r spearman = 0.01; P = 0.892) (Table 3). When stratified by the presence of cachexia, no correlation between IL-6 and vitamin D levels was observed among patients with cachexia (r = 0.03; P = 0.811) or among patients without cachexia (r = −0.006, P = 0.960).
Discussion
Our study demonstrated that IL-6 was higher in cancer patients with cachexia than those without cachexia. This confirms previous findings from human and mice studies.12–14 Some mechanisms on the effect of IL-6 on cancer cachexia have been suggested. IL-6 may trigger tissue catabolism, and therefore attenuate the development of weight loss and muscle wasting.15 Moreover, IL-6 may accelerate autophagy of muscle cells in cancer environment.16 The present study showed an association of cachexia with IL-6, but not with vitamin D levels in cancer patients. Besides, vitamin D was found to be correlated with handgrip strength and several body composition components, such as fat mass index, skeletal muscle mass, and visceral adipose tissue. No correlation between IL-6 and vitamin D was found.
While vitamin D deficiency has been shown to cause skeletal muscle abnormalities and contribute to muscle wasting,17 we found null association between vitamin D levels and cachexia in cancer patients. Similarly, vitamin D supplementation did not improve muscle wasting in hepatoma-induced rats.1 However, the animal study showed an overexpression of vitamin D receptor in rats with cancer cachexia regardless of the vitamin D levels.1
As previously reported, cachexia was associated with lower BMI, smaller waist circumference, lower fat mass index, and lower visceral adipose tissue in the present study. Interestingly, no difference in skeletal muscle mass and handgrip strength was observed between patients with and without cachexia. The latter finding was contrast to an international consensus indicating that muscle wasting (sarcopenia), instead of fat loss, was predominant in cancer-associated cachexia.4 The studies used in the consensus mainly involved patients with advanced stages of cancer or had received chemotherapy as the study population.4 Taken together, these findings imply that depletion of adipose tissue, compared to loss of skeletal muscle, might be more prominent in cachexia among treatment-naïve cancer; meanwhile, muscle wasting might contribute more to cachexia among cancer patients undergoing treatment or in advanced stages.
Although we found an association between IL-6 and cancer-associated cachexia and associations of body composition components with cachexia, no correlation between IL-6 and body composition components (ie, body weight, BMI, fat mass index, skeletal muscle mass, and visceral adipose tissue) was observed. This finding suggests that body compositions measured using BIA did not serve as a mediator in the association between IL-6 and cachexia. Catabolism induced by proinflammatory cytokines including IL-6 was rather observed in molecular level as shown by previous studies.18,19 Majority of patients with obesity in this current study had no cachexia. However, loss of muscle mass and function could also be occurred in obesity patients, defined as sarcopenic obesity, in which in this study we did not evaluate further.20
Our study indicated evidence of a positive correlation of vitamin D levels with skeletal muscle mass and handgrip strength in a cancer population. In line with our finding, a study in head and neck cancer patients also found a positive correlation between vitamin D level with skeletal muscle mass both before and after treatment.21 Moreover, a corresponding correlation was also seen among elderly with sarcopenia22 and patients undergoing regular hemodialysis.23 A proposed mechanism underlying this correlation is that vitamin D binding to the receptors stimulates phosphate uptakes energy-rich phosphate compound is formed and contribute to muscle cell contractility.24 Vitamin D also promotes the activation of mitogen-activated protein kinase signaling pathways which may result in myogenesis and cell proliferation.13 Nevertheless, whether vitamin D supplementation in cancer patients may improve their muscle mass and muscle strength warrants further investigation in the future.
The present study did not detect any correlation between vitamin D and IL-6 levels. On the other hand, some studies showed that vitamin D administration downregulated several proinflammatory cytokines including IL-6.9,11 The study population in those previous studies was patients with systemic infection. It is possible that the interaction between vitamin D and IL-6 in cancer is somewhat distinct from the interaction between them in the case of systemic infection.
This study is the first study that we know of to evaluate the role of vitamin D and IL-6 with cachexia and body compositions in naïve-treatment cancer patients in Indonesia. Longitudinal study design should be performed in the future to investigate the causal association between IL-6 and cancer-associated cachexia. Limitation in this study includes small patient numbers, and variety in cancer types. Another limitation is that although BIA provides precise measures of body composition, BIA could not assess visceral fat and skeletal muscle mass at the tissue organ level, which may contribute to the null correlation of body composition with IL-6 and vitamin D in the present study. Recall bias might have occurred since we relied on patients’ history to dietary intake. In addition, data of smoking, kidney or liver diseases, hypo- or hyperparathyroidism, or other conditions that might affect vitamin D or IL-6 levels were not available.
Conclusions
Cancer-associated cachexia in this study population is associated with a higher level of IL-6, lower BMI, lower fat mass index, and lower visceral adipose tissue. We also found that muscle mass, muscle strength, and visceral adipose tissue are positively correlated with vitamin D levels in patients with cancer.
Disclosure
There is no conflict of interests within authors on this research.
References
1. Camperi A, Pin F, Costamagna D, et al. Vitamin D and VDR in cancer cachexia and muscle regeneration. Oncotarget. 2017;8(13):21778–21793. doi:10.18632/oncotarget.15583
2. Bourdel-Marchasson I, Diallo A, Bellera C, et al. One-year mortality in older patients with cancer: development and external validation of an MNA-based prognostic score. PLoS One. 2016;11(2):1–16. doi:10.1371/journal.pone.0148523
3. Rounis K, Makrakis D, Tsigkas A-P, et al. Cancer cachexia syndrome and clinical outcome in patients with metastatic non-small cell lung cancer treated with PD-1/PD-L1 inhibitors: results from a prospective, observational study. Transl Lung Cancer Res. 2021;10(8):3538–3549.
4. Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–495. doi:10.1016/S1470-2045(10)70218-7
5. Kim S-H, Kwon HS, Hwang H-J. White blood cell counts, insulin resistance, and vitamin D levels and sarcopenia in Korean elderly men. Scand J Clin Lab Invest. 2017;77:228–233.
6. Garcia M, Seelaender M, Sotiropoulos A, Coletti D, Lancha AH. Vitamin D, muscle recovery, sarcopenia, cachexia, and muscle atrophy. Nutrition. 2019;60:66–69. PMID: 30529188. doi:10.1016/j.nut.2018.09.031
7. Van Veldhuizen PJ, Taylor SA, Williamson S and Drees BM. Treatment of vitamin D deficiency in patients with metastatic prostate cancer may improve bone pain and muscle strength. Journal of Urology. 2000;163(1):187–190. doi:10.1016/S0022-5347(05)68001-9
8. Zeichner SB, Koru-Sengul T, Shah N, et al. Improved Clinical Outcomes Associated With Vitamin D Supplementation During Adjuvant Chemotherapy in Patients With HER2+ Nonmetastatic Breast Cancer. Clinical Breast Cancer. 2015;15(1):e1–e11. doi:10.1016/j.clbc.2014.08.001
9. Miroliaee AE, Salamzadeh J, Shokouhi S, Sahraei Z. The study of vitamin D administration effect on CRP and Interleukin-6 as prognostic biomarkers of ventilator associated pneumonia. J Crit Care. 2018;44:300–305.
10. Paval DR, Patton R, McDonald J, et al. A systematic review examining the relationship between cytokines and cachexia in incurable cancer. J Cachexia Sarcopenia Muscle. 2022;13(2):824–838. doi:10.1002/jcsm.12912
11. Visser MPJ, Dofferhoff ASM, van den Ouweland JMW, et al. Effects of Vitamin D and K on Interleukin-6 in COVID-19 [Internet]. Front Nutr. 2022;8. doi:10.3389/fnut.2021.761191
12. Han J, Lu C, Meng Q, Halim A, Yean TJ, Wu G. Plasma concentration of interleukin-6 was upregulated in cancer cachexia patients and was positively correlated with plasma free fatty acid in female patients. Nutr Metab. 2019;16(1):80. doi:10.1186/s12986-019-0409-9
13. Hou Y-C, Wang C-J, Chao Y-J, et al. Elevated serum interleukin-8 level correlates with cancer-related cachexia and sarcopenia: an indicator for pancreatic cancer outcomes. J Clin Med. 2018;7(12):15.
14. Baltgalvis KA, Berger FG, Pena MMO, Davis JM, Muga SJ, Carson JA. Interleukin-6 and cachexia in Apc (Min/+) mice. Am J Physiol Integr Comp Physiol. 2008;294(2):R393–R401.
15. Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89(2):381–410.
16. Pettersen K, Andersen S, Degen S, et al. Cancer cachexia associates with a systemic autophagy-inducing activity mimicked by cancer cell-derived IL-6 trans-signaling. Sci Rep. 2017;7(1):2046. doi:10.1038/s41598-017-02088-2
17. Ceglia L. Vitamin D and its role in skeletal muscle. Curr Opin Clin Nutr Metab Care. 2009;12(6):628–633. doi:10.1097/MCO.0b013e328331c707
18. White JP. IL-6, cancer and cachexia: metabolic dysfunction creates the perfect storm. Transl Cancer Res. 2017;6(Suppl 2):S280–S285.
19. Han J, Meng Q, Shen L, Wu G. Interleukin-6 induces fat loss in cancer cachexia by promoting white adipose tissue lipolysis and browning. Lipids Health Dis. 2018;17(1):14. doi:10.1186/s12944-018-0657-0
20. Donini LM, Busetto L, Bischoff SC, et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Obes Facts. 2022;15(3):321–335.
21. Nejatinamini S, Debenham BJ, Clugston RD, et al. Poor vitamin status is associated with skeletal muscle loss and mucositis in head and neck cancer patients. Nutrients. 2018;10:1236.
22. Verlaan S, Maier AB, Bauer JM, et al. Sufficient levels of 25-hydroxyvitamin D and protein intake required to increase muscle mass in sarcopenic older adults – the PROVIDE study. Clin Nutr. 2018;37(2):551–557. doi:10.1016/j.clnu.2017.01.005
23. Gordon PL, Sakkas GK, Doyle JW, Shubert T, Johansen KL. Relationship between vitamin D and muscle size and strength in patients on hemodialysis. J Ren Nutr off J Counc Ren Nutr Natl Kidney Found. 2007;17(6):397–407.
24. Marcinkowska E. A run for a membrane vitamin D receptor. Biol Signals Recept. 2001;10(6):341–349.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
