Back to Journals » Patient Preference and Adherence » Volume 17
Patient Willingness to Use Digital Health Technologies: A Quantitative and Qualitative Survey in Patients with Cancer Cachexia
Authors Tarachandani A, Karahanoglu FI , Messere A, Tarasenko L, LaRonde-Richard AM, Kessler N, Rossulek M, Plate H, Mahoney K, Santamaria M
Received 16 January 2023
Accepted for publication 1 April 2023
Published 27 April 2023 Volume 2023:17 Pages 1143—1157
DOI https://doi.org/10.2147/PPA.S396347
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Anil Tarachandani,1 Fikret Isik Karahanoglu,1 Andrew Messere,1 Lisa Tarasenko,2 Ann-Marie LaRonde-Richard,3 Nancy Kessler,4 Michelle Rossulek,3 Hans Plate,5 Kim Mahoney,5 Mar Santamaria1
1Early Clinical Development, Pfizer Inc., Cambridge, MA, USA; 2Global Medical Affairs, Pfizer Inc., New York, NY, USA; 3Internal Medicine Research Unit, Pfizer Inc., Cambridge, MA, USA; 4Business Analytics and Insights, Pfizer Inc., New York, NY, USA; 5Pensari LLC, Baltimore, MD, USA
Correspondence: Mar Santamaria, Early Clinical Development, Pfizer Inc., 610 Main Street, Cambridge, MA, 02139, USA, Tel +1 617 852 5637, Fax +1 845 474 5357, Email [email protected]
Purpose: The objective of this study was to gain insights into the patients’ perspectives on the impact of cancer cachexia on physical activity and their willingness to wear digital health technology (DHT) devices in clinical trials.
Patients and Methods: We administered a quantitative 20-minute online survey on aspects of physical activity (on a 0– 100 scale) to 50 patients with cancer cachexia recruited through Rare Patient Voice, LLC. A subset of 10 patients took part in qualitative 45-minute web-based interviews with a demonstration of DHT devices. Survey questions related to the impact of weight loss (a key characteristic in Fearon’s cachexia definition) on physical activity, patients’ expectations regarding desired improvements and their level of meaningful activities, as well as preferences for DHT.
Results: Seventy-eight percent of patients reported that their physical activity was impacted by cachexia, and for 77% of them, such impact was consistent over time. Patients perceived most impact of weight loss on walking distance, time and speed, and on level of activity during the day. Sleep, activity level, walking quality and distance were identified as the most meaningful activities to improve. Patients would like to see a moderate improvement of activity levels and consider it meaningful to perform physical activity of moderate intensity (eg, walk at normal pace) on a regular basis. The wrist was the preferred location for wearing a DHT device, followed by arm, ankle, and waist.
Conclusion: Most patients reported physical activity limitations since the occurrence of weight loss compatible with cancer-associated cachexia. Walking distance, sleep and quality of walk were the most meaningful activities to moderately improve, and patients consider moderate physical activity as meaningful. Finally, this study population found the proposed wear of DHT devices on the wrist and around the waist acceptable for the duration of clinical studies.
Keywords: physical activity, patients’ expectations, meaningful activities, walking, DHT device
Introduction
As stated by the American Society of Clinical Oncology (ASCO) Cancer Cachexia Treatment Guidelines, cachexia is a multifactorial syndrome characterized by loss of appetite, weight, and skeletal muscle, leading to fatigue, functional impairment, increased treatment-related toxicity, poor quality of life, and reduced survival occurring in 30% of all cancer patients.1,2 The definition of cachexia changed over time with early definitions concentrating on body weight, physical performance, and patient function.2 The current diagnostic criterion for cachexia, agreed on by a panel of international experts through a Delphi consensus process in 2011, is weight loss >5% in 6 months, or weight loss >2% in individuals who are already depleted as evidenced by body mass index (BMI) <20 kg/m² or skeletal muscle mass (sarcopenia).3 To assess cachexia, the following domains should be considered: anorexia or reduced food intake, catabolic drive, muscle mass and strength, functional and psychosocial impairment.3
Amongst the 21 symptoms present in the tailored MD Anderson Symptom Inventory (MDASI) tool,4 lack of appetite, disturbed sleep, fatigue, lack of energy, and distress/feeling upset were the symptoms with the highest occurrence rates in cancer patients with cachexia. Moreover, patients with cachexia reported significantly higher severity scores than individuals without cachexia for the following categories of symptoms in the modified MDASI: pain, fatigue, disturbed sleep, lack of appetite, dry mouth, numbness, early satiety, diarrhea, anxiety, and lack of energy.4 Symptoms extend to organs and tissues beyond muscles and adipose tissue, such as heart, liver and brain.5
In addition to the symptoms described, physical inactivity has been documented in patients with cancer cachexia, eg, lung cancer patients with cachexia displayed physical activity levels drastically inferior to the activity levels of moderately active healthy individuals.6 In a longitudinal study of patients with cancer with progressive cachexia, weak but significant correlation (Spearman’s rho = −0.36, p < 0.0002) between reduction in spontaneous physical activity measured with digital health technology (DHT) devices and weight loss was reported.7 Moreover, weight loss, reduced physical functioning (Short Form Health questionnaire 36; SF-36), and bodily pain (SF-36) significantly contributed to the variability of spontaneous physical activity.7 By contrast, exercise training has been proposed as a potential therapeutic approach for cancer cachexia.8
Taken together, cachexia is a complex and heterogenous health condition. Interventions should be inclusive of all aspects of disease and aim to improve quality of life. Approaches to treat cachexia should concentrate, not only on improving the overall function but also on improving function that is meaningful to the patients. Indeed, patient-focused drug development is a systematic approach to ensure that the experiences, perspectives, needs, and priorities of patients living with a given disease and undergoing treatment for this disease are recorded and incorporated into drug development and evaluation.9 Recently, Food and Drug Administration (FDA) issued a series of guidelines on patient-focused drug development.10 Quantitative, qualitative, or mixed methods can be used to collect robust patient experience data.11,12
We performed such a quantitative and qualitative study in patients with cancer-related cachexia. The primary objective of this research was to understand from patients with cancer cachexia how weight loss impacted their everyday lives in terms of physical activities impacted and the level of impact. We also sought to understand which activities were most meaningful to patients to improve, assessed patients’ willingness to wear a DHT wearable device during treatment and inquired about what would make DHTs both convenient and comfortable with the overall goal of informing the development of novel digital endpoints for future clinical trials.
Materials and Methods
Patients
In 2020, patients with cancer cachexia (including those with non-small cell lung cancer, liver cancer, ovarian cancer, prostate cancer, colorectal cancer, or pancreatic cancer) were screened through Rare Patient Voice, LLC to identify eligible participants.
The Fearon criteria were at the basis of the patients’ eligibility, ie, weight loss of at least 5% during the past 6 months in the absence of simple starvation in individuals with BMI > 20 or of at least 2% in those with BMI <20.3 To determine their eligibility, patients were first asked if they had experienced weight loss in the past two years not due to diet, exercise changes, or other purposeful efforts to lose it. Next, patients self-reported their current, lowest, and weight 6 months prior to their lowest, along with height, to calculate BMIs at relevant time points. The inclusion criteria comprised the occurrence of weight loss fulfilling the above criteria in adults with the diagnosis of non-small lung, liver, ovarian, prostate, colorectal, pancreatic or breast cancer corresponding to some of the aspects of the definition of cachexia.3 These cancer diagnoses were chosen as they represent the most common cancer types and because it is thought that cancer cachexia in these cancer types is mediated by the GDF-15 protein, a monoclonal antibody against which is currently being tested in clinical trials.
The exclusion criteria included intentional weight loss due to diet or exercise, or patients who communicated a lack of interest in wearing a digital device for the purposes of this study. Patients unwilling/slightly willing to wear digital device were excluded as it would have been impossible to collect useful information regarding the preferences for such devices. Demographic data (age, gender) were not acquired.
Online Survey
Approached patients were asked to complete a quantitative 20-minute online survey (Supplementary Material 1). The survey was divided into a screening part and two research parts. Patients deemed eligible as detailed above were administered the actual two-part survey.
The first part titled “Cancer background” concentrated on cancer treatment, restrictions to daily activities due to weight loss and their duration and on the impact of body weight loss on selected daily activities. The activities assessed were “How fast you can walk (speed)”; “How much you walk per day (a composite of how far a person walks, how many bouts of walking in a day are done and how actively/quickly they walk)”; “How far you walk (distance)”; “How are you walking (longer or shorter steps, wobbling, balance, etc.)”; “How you are sleeping (time you spend sleeping, number of times you are waking up at night, how quickly you fall asleep or wake up)”; “How active you are during the day”; “How sedentary you are during the day”; “How much you transition from sitting/standing and how you are doing it (eg, balance, speed, timing)”. Patients were asked to assess the impact of weight loss on these activities using a 0–100 visual analogue scale (VAS), as detailed in Table 1.
 |
Table 1 VAS Scales Shown to Patients to Quantify Their Responses |
The second part titled “Wearable reaction” gauged participants’ reaction to the possibility of wearing a DHT device, their preferences concerning the anatomical location of the device, length of time they were prepared to wear the device for and the willingness to keep a diary of physical activity whilst participating in a study. Moreover, given that DHT devices can record different types of data, patients were asked to indicate what data they would consider meaningful to be recorded by the device. We next assessed the levels of improvement desired by the patients. Finally, we asked patients to nominate, which of the four levels of physical activity they would most like to pursue. The choices included: vigorous activity (eg, running), moderate activity (eg, going for a walk at a normal pace), light activity (eg, washing dishes), sedentary activity (eg, sitting and reading a book). The VAS scales and corresponding answers that appeared on screens during the survey to quantify their responses are shown in Table 1.
Web-Based Interviews
A subset of 10 patients were further contacted for a qualitative 45-minute web-based interview (Supplementary Material 2) with a demonstration of the digital device, the GENEActiv (Activinsights, Kimbolton, UK; Figure 1). This subset of patients met the specific criteria for unintentional weight loss within a certain period of time of the interview. The GENEActiv device is a tri-axial accelerometer with light and temperature measurements that monitors physical activity, sleep, and everyday living behaviors. The wrist version resembles a watch. The device shown to the participants was a stand-in for very similar devices on the market from a size, weight and touch and feel perspective such as Actigraph (ActiGraph LLC, Pensacola, FL, USA) or any smartwatch.
 |
Figure 1 GENEActiv digital health technology device. |
Statistical Analysis
The descriptive summaries, including mean, standard deviation, minimum, maximum, counts, and totals/percentage of responses, of the data were computed using R version 4.2.2 and Microsoft Excel.
Results
Patient Characteristics and History of Cachexia
We approached 3795 patients with the diagnosis of cancer and asked them to take part in the study; 489 responded. After excluding patients who did not meet the inclusion criteria, 50 individuals were recruited to take part in the quantitative part of the study (Figure 2). Table 2 shows the details of diagnosis and treatment in the study population. Ten patients took part in the subsequent qualitative interviews (Figure 2, Table 2).
 |
Table 2 Patients’ Characteristics |
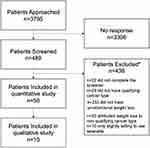 |
Figure 2 The study flow chart. Note: *Some patients met multiple exclusion criteria. |
An average weight ± SD prior to the diagnosis of cancer was 84.4 ± 23.2 kg, ranging from 46.3 to 135.2 kg. The weight loss occurred mostly at the time of surgery or other anti-cancer treatment. Average maximal weight loss, computed as the difference between the lowest weight encountered and weight prior to the diagnosis or at 6 months prior to the lowest weight, whichever was higher, equaled 19 ± 10.2 kg and ranged from 3.2 to 47.6 kg. Such weight loss corresponded to 21 ± 9% of the highest weight (range 6–48%). At the time of the survey, the average weight in the study population was 72.5 ± 20.2 kg (Table 2). In terms of BMI, an average BMI prior to the diagnosis was 30.4 ± 8.3 kg/m2. On average, at the time of maximal weight loss, patients had lost 6.6 ± 3.9 kg/m2 of BMI and have since recovered 2.3 ± 2.6 kg/m2 (Figure 3). Just over one-third of patients received weight loss treatment, mostly in the form of nutritional and vitamin supplementation (Table 2).
 |
Figure 3 The evolution of body mass index since the diagnosis of cancer in this study population. Abbreviation: BMI, body mass index. |
Impact of Weight Loss on Physical Activity
Seventy-eight percent of patients reported some level of physical activity restrictions in everyday life (Figure 4A). The restrictions ranged from the most severe inability to attend to one’s own needs in 6% of patients to the least severe inability to perform demanding physical activities in 48% of patients (Figure 4A). About one-third of the population impacted by the weight loss reported restrictions in everyday activities “at all times”; however, most commonly, patients reported that problems with physical activity were confined to “a specific period” (Figure 4B).
The activities mostly impacted by the weight loss, measured by the highest average rating, were how far (mean score ± SD; 63 ± 29) and how much a patient was able to walk per day (59 ± 30), and sleeping (59 ± 27). Half or nearly half of study population assigned a rating ≥70 to the impact experienced with these three activities (Figure 4C). Full distribution of scores depicting the impact of weight loss on activities under study is shown in Supplementary Figure 1.
In the qualitative part of the study, 60% of the patients interviewed reported a negative impact of weight loss, 30% of them thought that weight loss had a positive impact and 10% noticed no impact. Almost all patients reported sleep disturbances and lack of energy. Sleep disturbances included inability to stay asleep, worsening of pre-cancer sleeping difficulties, need for medication, difficulties with falling asleep, feeling tired despite 8–10 h of sleep or pain preventing sleeping. Some of the patients complained of the inability to play with children or grandchildren. A few of them felt weak or out of breath. Many patients had not regularly participated in exercise prior to their weight loss, and therefore, when asked to describe their desired improvement, responded that they wanted to be able to go for walks and perform daily activities. The individuals who were more active prior to weight loss reported a desire to return to a high level of activity, but most acknowledged that they needed to accept they have cancer and may not be able to achieve vigorous activity levels. The answers showed that patients would like to be able to go for long walks, hikes, or short runs. Supplementary Table 1 includes a sample of quotes from the interviews with the patients.
Meaningful Activities and Aspired Improvements
Sleep, daytime activity level, walking distance and walking quality gauged by the question “how are you walking” were the activities, which was considered most meaningful by the respondents (Figure 5A). Full distribution of meaningful activities is shown in Supplementary Figure 2. The average meaningfulness rating score ± SD in response to “how you are sleeping” question was 84 ± 22, equivalent to “extremely meaningful” and 88% of patients assigned a rating of ≥70 (“very” and “extremely meaningful”) to this activity. For the activities “how far you walk” and “how you are walking” the average (SD) ratings were 76 ± 21 and 75 ± 23, and a rating of ≥70 was given by 68% and 64% of patients, respectively. When asked to rank the three activities in terms of how much improving them would influence the overall quality of life, the top responses that patients chose were sleep, daytime activity levels and walk quality improvements (Figure 5B). Patients would like to see a moderate level of improvement (scores 34–66; Table 1) on activities as all ratings given to the eight activities under study were in the interval of 50–64 (Figure 5C). In addition, moderate activity, such as going for a walk at a normal pace, resulted as the most meaningful activity to perform on a regular basis (Figure 5D).
Figure 5 Continued.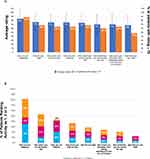
Preferences for Digital Health Technology Device
Next, patients were asked to express their preference for anatomical locations for the DHT device. The locations preferred by patients interviewed in decreasing preference order were wrist (mean rating ± SD; 90 ± 17), arm (63 ± 28), ankle (60 ± 31), and waist (42 ± 29), while least preferred were chest (28 ± 27), neck (20 ± 26), and head (12 ± 17) (Figure 6A). Our results showed that 86% of patients gave the wrist location a rating ≥70 that corresponded to “very” and “extremely preferred” answers. Patients would be willing to wear the wrist DHT device for longer than the waist device (68% versus 42% for up to 21 days on wrist and waist, respectively), albeit most respondents would wear DHTs during the entire treatment period (up to 21 days) if it could monitor changes in their physical function (Figure 6B).
Ninety-two percent of patients surveyed would approve of their doctor having access to their digital device data. During qualitative interviews, patients noted that they are often given a form to fill in at their physician’s office to assess their activity levels. Therefore, patients suggested that it would be helpful for their physicians to have access to and see data on improvements or worsening in their activity levels given appointments are often rushed.
Discussion
The development of novel digital endpoints that are meaningful to patients is a task that comprises four steps. First, a meaningful aspect of health (MAH) or of disease must be identified, ie, something that the patient wants to prevent, improve, or does not want it to get worse. Second, MAH must be subcategorized into a concept of interest (COI) that can be practically measured, and subsequently translated into a clinical outcome. Third, an outcome to be measured, eg, a clinical assessment or a task, is chosen, and finally, specific endpoints that can quantify the outcome are evaluated and validated in clinical trials.13
In this study, activities of daily living, specifically, ambulatory behavior and daily activity patterns (eg, sedentary time and sleep) were identified as MAH. As the COI, within the activities of daily living, we focused on walking behavior, sit to stand transitions, daily activity patterns and sleep. DHT devices enable to objectively monitor and quantify each outcome with multiple digital endpoints, such as gait speed, total steps, and moderate activity time.
An early study exploring the perceptions of patients with cancer cachexia and their family members regarding supportive healthcare interventions established that patients wanted their profound weight loss acknowledged.14 Moreover, patients and families also wanted to receive information about cachexia and interventions.14 Since then, the cachexia awareness amongst healthcare professionals15 and the understanding of its molecular basis16 have improved.
The current study was performed as part of a project to develop novel digital endpoints for future drug development in cancer-associated cachexia. It comprised a quantitative and a qualitative assessment of the impact of cancer cachexia-related weight loss on eight basic aspects of daily physical activity and on patients’ desired improvements to identify MAH(s) in this population. Although cancer cachexia is a multifaceted syndrome, we used “weight loss”, which is a consequence and a characteristic of cachexia, as a proxy of cancer cachexia when talking to the patients. This was driven by the fact that weight loss is easier to perceive/quantify for a patient, than fatigue or lack of energy. Of the 50 patients included in the quantitative part of the study, almost 80% reported that weight loss had an impact on their daily physical activities. An average rating of impact on the eight activities ranged between 52 and 63, and the impact of weight loss on 7/8 activities examined, fell into the 41–60, ie, “somewhat impacted” interval. Decreased physical activity and exercise level after a cancer and cachexia diagnoses was previously reported.7,17 It was further corroborated on by Bland et al18 who described a profound impact of cancer diagnosis and the experience of cachexia on physical, psychological, and social aspects of participants’ lives. Similarly to what was reported in the literature, participants reported inability to do things due to tiredness or feeling sick.18
It is hard to distinguish the specific contribution of weight loss in cancer-associated cachexia to restrictions in daily activities in such a complex period of life as that of cancer diagnosis and treatment. Almost all patients who took part in the qualitative part of the study revealed to the interviewer that their sleep quality deteriorated and they had no energy. Reduced sleep quality was reported even in patients who reported a limited impact of weight loss on their daily lives. Patients described a cyclical loop where lack of sleep caused more fatigue, which prevented them from walking for exercise. Sleep quality in cancer dependents on multiple parameters such as fatigue, pain, depression, anxiety and distress19 and so is hard to interpret. Nevertheless, a recent systematic review showed that improving sleep quality eased the symptoms of cancer-related fatigue although was unable to determine if sleep quality improvement was the sole factor behind fatigue lessening.20 Of note, patients who had been overweight before weight loss reported positive impact of weight loss, especially female patients.
Understandably, most patients would like to improve on activities that were most impacted by the weight loss and sleep improvement was considered the most important of them. Walking distance and the quality of walking emerged as the second and the third activity that patients wanted to improve. Many patients included in our web-based interviews expressed the desire to be able to walk a few miles even slowly. Several studies enquired about preferred physical activity in cancer patients. Surveys of physical activity interests and preferences of patients in palliative care and in patients with brain metastases showed that for the majority (64% in palliative care and 48% with brain metastases) walking was patients’ favorite physical activity and the activity they would be most interested in pursuing.21,22 Also, a study into exercise levels and preferences of 392 patients with various cancer diagnoses and stages identified walking as a preferred physical activity regardless of time of year.23
Other than a personal preference, “ambulating”, ie, the ability of an individual to move from one place to another and walk independently, is a key activity of daily living (ADL).24 Once a person stops walking, they require help with other ADLs, for which it is necessary to move around, such as feeding and personal hygiene. About one-third of adult patients with cancer require assistance to perform ADLs as shown by a recent meta-analysis that found that walking and transfers are amongst the most affected ADLs.25 In addition, walking has a prognostic value as gait speed is a marker of frailty that independently predicted survival in patients with several neoplasms.26–30 Last but not least, walking is also important for less essential, but still meaningful, activities such as “gardening” or “playing with children/grandchildren” that our respondents indicated as activities that they had enjoyed prior to the diagnosis and that to us represent MAHs. Taken together, we consider walking to be a COI and measure walking outcomes with digital endpoints.
Clearly, the lack of agreement on optimal endpoints in cancer cachexia trials hinders progress in devising interventions.31 Considering the importance of physical activity, eg, walking, observed in our study, paired with the molecular links between physical activity and immunological and anti-inflammatory responses,32,33 support including physical activity measurements as endpoints in cachexia trials. However, of the 65 randomized controlled trials for cancer cachexia included in a review of trial designs and endpoints, physical activity measured by questionnaires or pedometer/accelerometer was an endpoint in merely 7 (11%) trials.34
Traditionally, to understand physical activity in cancer patients, physical activity questionnaires were used.35 Although questionnaires are easy to administer, they are not objective and often do not correlate with objective accelerometer data.36 Currently, the use of wearable monitors of physical activity is on the rise.37,38 Wearables offer the possibility of a quantitative, constant, unbiased, objective and specific to the patient assessment of physical activity.37 Accelerometer data can also be stratified according to the intensity of physical activity into sedentary, low, or moderate-to-vigorous. Physical functioning and health-related quality of life correlated with the moderate-to-vigorous intensity in patients who underwent hematopoietic stem cell transplant.39
As part of our patient-focused approach, we gauged the interest of this study population in wearing a digital device for physical activity monitoring at specific anatomical locations and found that, in agreement with available literature,37 patients had a preference for a device positioned on the wrist of their nondominant arm for the intended study duration. A recent systematic review explored the use of wearables in clinical trials during cancer treatment and noted limited consensus amongst studies on which parameters to monitor during treatment. Patient adherence varied from 60 to 100%.40 Another review listed data volume, body placement, sampling frequency/range/resolution and data protection as challenges to a wide adoption of wearable devices.37 Clearly, there is room for improvement and studies like ours are needed to refine the mapping of meaningful parameters that will result in a rational standardization of the use of wearables in cancer cachexia trials.
There are several strengths to this study. First, from the point of view of inclusion criteria, all major cancer diagnoses linked to weight loss were included. Second, the study is part of a patient-focused drug development, a paradigm that will stably replace the traditionally used physician-centered approaches. The involvement of patients early on in a clinical development project engages the patient in decision-making, resulting in greater motivation and better clinical outcomes.41 Finally, methodology-wise, we designed a study composed of two phases – a quantitative and a qualitative phase – to explore the scale and details of patient’s views and, moreover, we explored both impact of cachexia on patients’ lives and future expectations of the patients. Structured interviews were utilized for data consistency, albeit open questions were also included to make sure that all non-standard patient experience was captured.
Limitations of the study include lack of demographic data, and small sample size, especially in cancers represented by less than 10 patients. However, the focus here has been on cancer cachexia independently from the underlying cancer. Although cancer cachexia occurs in up to 30% of cancer patients, it is not easy to find patients who are well enough to participate in surveys like this one. Moreover, in patient-reported surveys, data accuracy relies on the individual’s perception, opinion, or recall. These may often be subjective or inaccurate and cannot be verified. Without doubt, such factors are inevitable in patient-focused approaches as are high values of standard deviations due to variability of patients’ responses. Another weak point of this survey was that of not having been able to ship samples of the wearables to the patients due to COVID-19 restrictions, which could have generated interesting data. Patients were shown the device during the video interviews.
Conclusion
In conclusion, most patients reported physical activity limitations since the occurrence of cancer-related cachexia. The participants stated the order of preference of activity improvements with walking distance, sleep and walking quality ranking the highest and indicated “moderate” both as a degree of improvement desired and as a meaningful physical activity level to pursue on a regular basis. Finally, the participants found the proposed wear of the digital device on the wrist and around the waist acceptable for duration of the treatment. This study provides patient centric evidence for the importance and meaningfulness of measuring activities of daily living with DHTs. Future investigation of meaningful aspects of health in cancer cachexia within a larger clinical trial will help establish integration of digital endpoints in future interventional trials.
Abbreviations
ADL, activity of daily living; BMI, body mass index; COI, concept of interest; DHT, digital health technology; MAH, meaningful aspect of health; MDASI, MD Anderson Symptom Inventory; SD, standard deviation; VAS, visual analogue scale.
Data Sharing Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Ethics Approval and Informed Consent
This study was deemed exempt from ethical approval in accordance with 45 CFR, part 46, subpart A, exempt research involving benign behavioral interventions in conjunction with the collection of information from an adult subject through verbal or written responses. All participants provided consent for taking part in the study.
Acknowledgments
Pensari, LLC performed the research. Medical writing support was provided by Alicja M. Gruszka, MD PhD on behalf of TransPerfect, and was funded by Pfizer.
Author Contributions
Study concept and design: A.M, M.S, A.T., L.T., A.-M. LR-R., N.K, M.R. Data collection and study execution: H.P., K.M. Analysis: H.P., K.M., F.I.K. Critical revision of all versions of the manuscript for important intellectual content: All. Accepted the final version of the manuscript: All. Agreed on the journal to which the article was submitted: All. Agreed to take responsibility and be accountable for the contents: All.
Funding
This study and preparation of the manuscript were funded by Pfizer Inc.
Disclosure
F. Isik Karahanoglu, Andrew Messere, Lisa Tarasenko, Ann-Marie LaRonde-Richard, Nancy Kessler, Michelle Rossulek, and Mar Santamaria are current employees of Pfizer. Anil Tarachandani was employed by Pfizer at the time of the study. Hans Plate and Kim Mahoney are current employees of Pensari LLC and were contracted by Pfizer to provide data for the development of this manuscript. The authors report no other conflicts of interest in this work.
References
1. Law ML. Cancer cachexia: pathophysiology and association with cancer-related pain. Mini review. Front Pain Res. 2022;2022:3. doi:10.3389/fpain.2022.971295
2. Roeland EJ, Bohlke K, Baracos VE, et al. Management of cancer cachexia: ASCO guideline. J Clin Oncol. 2020;38(21):2438–2453. doi:10.1200/JCO.20.00611
3. Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–495. doi:10.1016/S1470-2045(10)70218-7
4. Zhou T, Yang K, Thapa S, Liu H, Wang B, Yu S. Differences in symptom burden among cancer patients with different stages of cachexia. J Pain Symptom Manage. 2017;53(5):919–926. doi:10.1016/j.jpainsymman.2016.12.325
5. Cao Z, Scott AM, Hoogenraad NJ, Osellame LD. Mediators and clinical treatment for cancer cachexia: a systematic review. JCSM Rapid Commun. 2021;4(2):166–186. doi:10.1002/rco2.30
6. Moses AW, Slater C, Preston T, Barber MD, Fearon KC. Reduced total energy expenditure and physical activity in cachectic patients with pancreatic cancer can be modulated by an energy and protein dense oral supplement enriched with n-3 fatty acids. Br J Cancer. 2004;90(5):996–1002. doi:10.1038/sj.bjc.6601620
7. Fouladiun M, Korner U, Gunnebo L, Sixt-Ammilon P, Bosaeus I, Lundholm K. Daily physical-rest activities in relation to nutritional state, metabolism, and quality of life in cancer patients with progressive cachexia. Clin Cancer Res. 2007;13(21):6379–6385. doi:10.1158/1078-0432.CCR-07-1147
8. Tsitkanou S, Murach KA, Washington TA, Greene NP. Exercise counteracts the deleterious effects of cancer cachexia. Cancers. 2022;14(10):2512. doi:10.3390/cancers14102512
9. Lacombe D, O’Morain C, Casadei B, et al. Moving forward from drug-centred to patient-centred research: a white paper initiated by EORTC and developed together with the BioMed alliance members. Eur Respir J. 2019;53(2):1801870. doi:10.1183/13993003.01870-2018
10. Food and Drug Administration. FDA patient-focused drug development guidance series for enhancing the incorporation of the patient’s voice in medical product development and regulatory decision making. Available from: https://www.fda.gov/drugs/development-approval-process-drugs/fda-patient-focused-drug-development-guidance-series-enhancing-incorporation-patients-voice-medical.
11. Food and Drug Administration. Guidance document: patient-focused drug development: collecting comprehensive and representative input. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-focused-drug-development-collecting-comprehensive-and-representative-input.
12. Food and Drug Administration. Guidance document: patient-focused drug development: methods to identify what is important to patients. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-focused-drug-development-methods-identify-what-important-patients.
13. Manta C, Patrick-Lake B, Goldsack JC. Digital measures that matter to patients: a framework to guide the selection and development of digital measures of health. Digit Biomark. 2020;4(3):69–77. doi:10.1159/000509725
14. Reid J, McKenna HP, Fitzsimons D, McCance TV. An exploration of the experience of cancer cachexia: what patients and their families want from healthcare professionals. Eur J Cancer Care. 2010;19(5):682–689. doi:10.1111/j.1365-2354.2009.01124.x
15. Reid J, Jatoi A, Enriquez-Hesles E, Porter S. Managing cancer cachexia: multi-disciplinary healthcare perspectives. Original research. Palliat Med Hosp Care. 2019;5(1):14–22. doi:10.17140/PMHCOJ-5-132
16. Yeom E, Yu K. Understanding the molecular basis of anorexia and tissue wasting in cancer cachexia. Exp Mol Med. 2022;54(4):426–432. doi:10.1038/s12276-022-00752-w
17. Wasley D, Gale N, Roberts S, et al. Patients with established cancer cachexia lack the motivation and self-efficacy to undertake regular structured exercise. Psychooncology. 2018;27(2):458–464. doi:10.1002/pon.4512
18. Bland KA, Krishnasamy M, Parr EB, et al. ”I want to get myself as fit as I can and not die just yet” - perceptions of exercise in people with advanced cancer and cachexia: a qualitative study. BMC Palliat Care. 2022;21(1):75. doi:10.1186/s12904-022-00948-x
19. Souza R, Dos Santos MR, Das Chagas Valota IA, Sousa CS, Costa Calache ALS. Factors associated with sleep quality during chemotherapy: an integrative review. Nurs Open. 2020;7(5):1274–1284. doi:10.1002/nop2.516
20. Dean R. Can improving quality of sleep reduce the symptoms of cancer-related fatigue in adults?: a systematic review. Eur J Cancer Care. 2022;31(4):e13597. doi:10.1111/ecc.13597
21. Lowe SS, Danielson B, Beaumont C, Watanabe SM, Courneya KS. Physical activity interests and preferences of cancer patients with brain metastases: a cross-sectional survey. BMC Palliat Care. 2016;15:7. doi:10.1186/s12904-016-0083-x
22. Lowe SS, Watanabe SM, Baracos VE, Courneya KS. Physical activity interests and preferences in palliative cancer patients. Support Care Cancer. 2010;18(11):1469–1475. doi:10.1007/s00520-009-0770-8
23. Avancini A, Pala V, Trestini I, et al. Exercise levels and preferences in cancer patients: a cross-sectional study. Int J Environ Res Public Health. 2020;17(15):5351. doi:10.3390/ijerph17155351
24. Edemekong PF, Bomgaars DL, Sukumaran S, Levy SB. Activities of daily living. In: StatPearls. StatPearls Publishing; 2022.
25. Neo J, Fettes L, Gao W, Higginson IJ, Maddocks M. Disability in activities of daily living among adults with cancer: a systematic review and meta-analysis. Cancer Treat Rev. 2017;61:94–106. doi:10.1016/j.ctrv.2017.10.006
26. Dociak-Salazar E, Barrueto-Deza JL, Urrunaga-Pastor D, Runzer-Colmenares FM, Parodi JF. Gait speed as a predictor of mortality in older men with cancer: a longitudinal study in Peru. Heliyon. 2022;8(2):e08862. doi:10.1016/j.heliyon.2022.e08862
27. Dulaney CR, McDonald AM, Wallace AS, Fiveash J. Gait speed and survival in patients with brain metastases. J Pain Symptom Manage. 2017;54(1):105–109. doi:10.1016/j.jpainsymman.2017.03.013
28. Hantel A, DuMontier C, Odejide OO, et al. Gait speed, survival, and recommended treatment intensity in older adults with blood cancer requiring treatment. Cancer. 2021;127(6):875–883. doi:10.1002/cncr.33344
29. Liu MA, DuMontier C, Murillo A, et al. Gait speed, grip strength, and clinical outcomes in older patients with hematologic malignancies. Blood. 2019;134(4):374–382. doi:10.1182/blood.2019000758
30. Pamoukdjian F, Levy V, Sebbane G, et al. Slow gait speed is an independent predictor of early death in older cancer outpatients: results from a prospective cohort study. J Nutr Health Aging. 2017;21(2):202–206. doi:10.1007/s12603-016-0734-x
31. Laird BJA, Balstad TR, Solheim TS. Endpoints in clinical trials in cancer cachexia: where to start? Curr Opin Support Palliat Care. 2018;12(4):445–452. doi:10.1097/SPC.0000000000000387
32. Cortiula F, Hendriks LEL, van de Worp W, et al. Physical exercise at the crossroad between muscle wasting and the immune system: implications for lung cancer cachexia. J Cachexia Sarcopenia Muscle. 2022;13(1):55–67. doi:10.1002/jcsm.12900
33. Halle JL, Counts BR, Carson JA. Exercise as a therapy for cancer-induced muscle wasting. Sports Med Health Sci. 2020;2(4):186–194. doi:10.1016/j.smhs.2020.11.004
34. Naito T. Evaluation of the true endpoint of clinical trials for cancer cachexia. Asia Pac J Oncol Nurs. 2019;6(3):227–233. doi:10.4103/apjon.apjon_68_18
35. Schrack JA, Gresham G, Wanigatunga AA. Understanding physical activity in cancer patients and survivors: new methodology, new challenges, and new opportunities. Cold Spring Harb Mol Case Stud. 2017;3(4):a001933. doi:10.1101/mcs.a001933
36. Douma JAJ, de Beaufort MB, Kampshoff CS, et al. Physical activity in patients with cancer: self-report versus accelerometer assessments. Support Care Cancer. 2020;28(8):3701–3709. doi:10.1007/s00520-019-05203-3
37. Dadhania S, Williams M. Wearable accelerometers in cancer patients. In: Lim C-P, Chen Y-W, Vaidya A, Mahorkar C, Jain LC, editors. Handbook of Artificial Intelligence in Healthcare: Vol 2: Practicalities and Prospects. Springer International Publishing; 2022:109–147.
38. Gresham G, Schrack J, Gresham LM, et al. Wearable activity monitors in oncology trials: current use of an emerging technology. Contemp Clin Trials. 2018;64:13–21. doi:10.1016/j.cct.2017.11.002
39. Morishita S, Kaida K, Yamauchi S, et al. Relationship of physical activity with physical function and health-related quality of life in patients having undergone allogeneic haematopoietic stem-cell transplantation. Eur J Cancer Care. 2017;26(4):e12669. doi:10.1111/ecc.12669
40. Beauchamp UL, Pappot H, Hollander-Mieritz C. The use of wearables in clinical trials during cancer treatment: systematic review. JMIR Mhealth Uhealth. 2020;8(11):e22006. doi:10.2196/22006
41. Allarakhia M. Exploring open innovation with a patient focus in drug discovery: an evolving paradigm of patient engagement. Expert Opin Drug Discov. 2015;10(6):571–578. doi:10.1517/17460441.2015.1037271
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



