Back to Journals » Infection and Drug Resistance » Volume 15
A Lung Ultrasound-Based Nomogram for the Prediction of Refractory Mycoplasma pneumoniae Pneumonia in Hospitalized Children
Authors Liu G, Wang G, Yang Z, Liu G, Ma H, Lv Y, Ma F, Zhu W
Received 8 September 2022
Accepted for publication 26 October 2022
Published 31 October 2022 Volume 2022:15 Pages 6343—6355
DOI https://doi.org/10.2147/IDR.S387890
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Gangtie Liu,1,2 Guanglei Wang,2 Zhan Yang,2 Guangfu Liu,3 Haijun Ma,3 Yong Lv,2 Feiyan Ma,2 Weiwei Zhu1
1Department of Pediatrics, Jinan Central Hospital, Shandong University, Jinan, People’s Republic of China; 2Department of Pediatrics, Taian Maternity and Child Health Care Hospital, Taian, People’s Republic of China; 3Department of Radiology, Taian Maternity and Child Health Care Hospital, Taian, People’s Republic of China
Correspondence: Weiwei Zhu, Department of Pediatrics, Jinan Central Hospital, Shandong University, No. 105 Jiefang Road, Jinan, 250013, People’s Republic of China, Tel +86-538-6620622, Email [email protected]
Purpose: Early diagnosis of refractory Mycoplasma pneumoniae pneumonia (RMPP) is challenging because of the lack of practical diagnostic imaging tools. Lung ultrasound (LUS) is an emerging tool for diagnosing childhood pneumonia. Hence, we evaluated the role of a nomogram combining LUS findings, clinical features, and laboratory indices in the early prediction of RMPP in children.
Patients and Methods: We retrospectively analyzed 225 children with Mycoplasma pneumoniae pneumonia (MPP) admitted to our hospital between Dec 2018 and Aug 2021. Logistic regression analysis incorporated LUS findings and clinical predictors into the nomogram. Ninety patients hospitalized from Sep 2021 to Dec 2021 were used for external validation of the prediction model. Receiver operating characteristics (ROC) and calibration curves were used to evaluate the performance of the nomogram in the early diagnosis of RMPP.
Results: Ultimately, Consolidation size /BSA (odds ratio (OR) 1.015, 95% confidence interval (CI) 1.536– 2.446), Pleural Effusion (OR 3.551, 95% CI 1.921– 15.600), LDH (OR 1.044, 95% CI 1.006– 1. 021) and CRP (OR 3.293, 95% CI 1.019– 1.098) were independent risk factors for the development of RMPP. The prediction model was represented visually as a nomogram. The area under the ROC curve for the predictive nomogram was 0.955 (95% CI 0.919– 0.978) in the training cohort and 0.916 (95% CI 0.838– 0.964) in the validation cohort. The calibration curve is close to the diagonal.
Conclusion: This is the first-time lung ultrasound was added to the predicted nomogram, which can more comprehensively assess the condition and more accurately predict the occurrence of RMPP early. Therefore, this nomogram can be widely used in the early diagnosis of RMPP, especially in primary care hospitals.
Keywords: predictive model, risk factors, diagnosis, Mycoplasma pneumoniae pneumonia
Introduction
Mycoplasma pneumoniae (M. pneumoniae) is common community-acquired pneumonia (CAP) pathogen in school-age children. M. pneumoniae infections occur throughout the year but are more prevalent in summer and autumn, with outbreaks occurring every 2–3 years.1 In the past ten years, among children with CAP hospitalized in northern China, the incidence of M. pneumoniae pneumonia (MPP) was as high as 37.5%.2 Conventional wisdom holds that MPP is mild, self-limiting, and not associated with severe complications.3 The incidence of refractory M. pneumoniae pneumonia (RMPP) has been increasingly reported due to the emergence of macrolide-resistant strains of M. pneumoniae.3 The diagnostic criteria for RMPP in children remain not standardized. Generally, MPP with persistent high fever and worsening lung imaging findings after treatment with azithromycin or erythromycin for seven days or more is called RMPP.4 Compared to CMPP, RMPP presents with severe clinical symptoms, longer hospital stays, and is prone to intrapulmonary complications like pleural effusion, pulmonary embolism, and pulmonary infarction, as well as extrapulmonary complications like hemolysis, mucosal skin disease, and central nervous system infections.1 Macrolide antibiotics are currently effective in treating Mycoplasma pneumoniae pneumonia,5 but patients with RMPP usually require a longer course of antibiotics and higher doses of glucocorticoids and immunoglobulins. Therefore, it is imperative to identify methods for the early diagnosis of RMPP.
Early diagnosis of RMPP is challenging due to the lack of practical imaging diagnostic tools. The mainstream early predictive models combine clinical symptoms, physical examination findings, and laboratory indices. Among predictive models, including clinical symptoms, researchers have found that age,6 the duration of fever,7 and pleural effusion8 are common predictors of RMPP. The risk of RMPP increases with increasing age at onset and prolonged duration of fever. Among predictive models, including laboratory indicators, recent studies have found that the levels of LDH and CRP9 in the blood are the best predictors of RMPP. Some studies have also found inflammatory indicators in the blood, such as DD,10 SF, IL-18,11 ALB,12 the neutrophil-to-lymphocyte ratio (NLR) and the mean platelet volume-to-lymphocyte ratio (MPVLR),13 are suitable for predicting RMPP. Increased IL-17A in bronchoalveolar lavage fluid is an independent risk factor for RMPP.14
In actual clinical practice, lung images of RMPP often show lobar or segmental changes, and imaging assessment primarily relies on chest X-ray (CXR).15 Therefore, some researchers have added CXR findings to the design of RMPP prediction models. Bi et al7 found that large-area lung consolidation was a risk factor for developing RMPP; Cheng et al12 found that CXR showed that consolidation in more than two-thirds of the lung was predictive of bronchiolitis obliterans (BO) in children with RMPP. The findings of these studies indicate that pulmonary imaging assessment is vital for the early diagnosis and prognosis determination of RMPP. However, the poor sensitivity of CXR in diagnosing pneumonia,16 coupled with the drawbacks of radiation exposure and the lack of specific quantitative indicators, make it challenging to enter CXR results into predictive models of RMPP. Although chest CT is superior to CXR in diagnosis, its radiation harm is more severe than that of CXR, limiting its application in children. Therefore, exploring a safe, accurate, efficient, and quantifiable diagnostic imaging tool for children is urgent. Numerous studies have confirmed that lung ultrasound (LUS), a safe, portable, and effective new diagnostic imaging tool,17 can be applied to diagnose CAP in children with high sensitivity and specificity.
LUS is widely used to diagnose and follow up CAP in adults and children. LUS is an ideal alternative to CXR, and its diagnostic value in CAP in children is comparable to or even better than that of CXR.17,18 Consolidation is the most common manifestation of CAP in LUS in children. Researchers have demonstrated that LUS is more sensitive than CXR in detecting consolidation,19 and LUS has higher accuracy than chest CT.20 There are few studies evaluating pneumonia conditions, especially in identifying RMPP.
This study aimed to explore the role of a nomogram combining LUS parameters, clinical features, and laboratory indices in the early prediction of RMPP in hospitalized children.
Materials and Methods
Study Design and Patients
In this retrospective observational study, children aged 1–14 years with MPP combined with pulmonary consolidation who were hospitalized in Taian Maternity and Child Health Care Hospital were analyzed. It is a 700-bed tertiary care children’s hospital. The Ethics Committee of Taian Maternity and Child Health Care Hospital approved the study (LW202203). Data collection began only after written informed consent was obtained from the children’s families. This study complies with the Declaration of Helsinki.
In the training cohort, the retrospective study included 247 children admitted to Taian Maternity and Child Health Care Hospital in Shandong Province, China, between Dec 2018 and Aug 2021. In the validation cohort, 90 children were hospitalized at Taian Maternal and Child Health Care Hospital from September 2021 to December 2021. All children were admitted to the hospital with signs and symptoms of pneumonia, including fever, cough, lung rales, and solid lung changes on chest radiograph or chest CT. The diagnosis of MPP was based on one of the following laboratory findings: a positive serological test result (MP-IgM positive or antibody titer>1:160) or a positive nasopharyngeal swab for MP-RNA. The exclusion criteria included: 1. Children with underlying medical conditions, including congenital pulmonary dysplasia, heart disease, pulmonary tuberculosis, and autoimmune disease; 2. LUS was not completed within five days after admission; 3. Children with similar medical conditions in the last three months; 4. Nasopharyngeal secretions from children were positive for the respiratory syncytial virus, influenza virus, adenovirus, parainfluenza virus, and chlamydia trachomatis; 5. children have laboratory indicators that may indicate the presence of a bacterial infection (positive sputum culture, positive blood culture, etc.); 6. children whose condition worsened and required transfer to the pediatric intensive care unit (PICU); and 7. the patient’s treatment was incomplete, or the child’s family refused to participate in the study.
Data Collection
The data collected from the enrolled children included: demographic information (age, sex, and weight of the children), clinical manifestations (the duration of fever before admission and the presence of hyperpyrexia, chest /abdominal pain, rash, wheezing, and sputum sounds), and laboratory indicators (white blood cell count, neutrophil ratio, C-reactive protein, alanine aminotransferase, lactate dehydrogenase, and prealbumin). Lung Ultrasound parameters, including the position and size of subpleural consolidation (as measured on the transverse axis, longitudinal axis, and sagittal axis), B-line around the consolidation, air-bronchogram sign, liquid-bronchogram sign, Doppler of consolidation area, and pleural effusion, were examined.
LUS Procedure
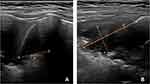
Three attending ultrasonographers completed the LUS examinations within five days of the admission of the children. All three sonographers had more than ten years of experience in pediatric ultrasound and had been performing LUS examinations in children for more than one year. LUS was performed using a Philips (EPIQ5, EPIQ7) ultrasound machine with convex array probe C5-1 and linear array probe eL18-4.21 The children were placed in a sitting or supine position. The children were first assessed for lung consolidation in both lungs using the convex array probe (1–5 MHz); the lungs were divided bilaterally into twelve regions, six on the right (right anterior superior, right anterior inferior, right superior axillary, right inferior axillary, right posterior superior, and right posterior inferior regions) and six on the left (left anterior superior, left anterior inferior, left superior axillary, left inferior axillary, right posterior superior, and right posterior inferior regions). The sonographer scanned each lung region of the children longitudinally (with the probe perpendicular to the rib cage) and transversely (with the probe parallel to the rib space) and saved the images. The maximum dimension on each of three-axis on LUS was recorded (Figure 1). Then, the air-bronchogram sign, liquid-bronchogram sign, blood flow, and other parameters in the area of lung consolidation were assessed using a linear array probe (4–18MHz).
Definitions
The B-line is a comet tail-like artifact arising from the pleural line, perpendicular to the pleura in a radial pattern, extending to the edge of the screen. It generally moves with the lung slide.22 Scattered B lines: the number of B lines in one rib gap≤3; Dense B-lines: The B-lines fuse with each other throughout the rib space and are difficult to distinguish and count, while the acoustic rib shadow remains visible; Fused B-lines: the entire intercostal space shows too dense B-lines, failing to deliver the acoustic rib shadow;21 The air-bronchogram sign is defined as punctate or linear hyperechoic artifacts within the lung consolidation.23 The liquid-bronchogram sign is defined as a dendritic hypoechoic distribution along the airways within the lung consolidation.24 The vascular pattern on Color Doppler appears as a dendritic blood flow signal observed in solid lung tissue. The maximum diameter of lung consolidation defines the size of the consolidation. The ratio of the size of the consolidation to the body surface area (consolidation size /BSA) was used to correct the size of solid lung lesions at different ages. Diagnostic procedures for RMPP: Three pediatricians reviewed all clinical and imaging data (chest radiographs or chest CT) of the enrolled children but not the LUS findings. They voted on the diagnosis of RMPP based on the diagnostic criteria and their clinical experiences. The diagnostic criteria for RMPP were persistent high fever and worsening lung imaging findings despite treatment with azithromycin or erythromycin for more than seven days.4 Those who met the above criteria were included in the RMPP group, and those who did not were included in the CMPP group.
Design and Validation of the Predictive Nomogram
This study used binary logistic regression analysis to evaluate the factors predicting the occurrence of RMPP. The independent variables that were significant in the univariate analysis were included in the logistic regression analysis to screen for independent risk factors predicting the event of RMPP and build a prediction model. The prediction model was converted into a visual nomogram in R software. The accuracy of the nomogram model was evaluated using receiver operating characteristic (ROC) curves, and calibration curves validated the nomogram model.
Statistical Analysis
SPSS Statistics (version 21.0) and R software (version 4.0.3) were used for statistical analysis. The mean ± standard deviation (mean ± SD) or median (interquartile range (IQR)) was used to express differences in continuous variables between the two groups. When the data were normally distributed, an independent-sample t-test was used. The Mann–Whitney test was used when the data were normally distributed. Categorical variables are expressed as a rate (%) and were compared between two groups using the Pearson χ2 or Fisher exact test. P < 0.05 was considered statistically significant.
Results
General Features
In the training cohort, as shown in the study flow chart (Figure 2), we finally enrolled 225 children, 126 boys, and 99 girls, with a median age of 5 (3.4–6.8) years. Twenty-two children were excluded from the study, including 9 children who did not complete the LUS examination within 5 days of admission, 6 children with incomplete clinical data, 5 children with co-infection with bacteria, 1 child with underlying medical conditions, and 1 child with similar diseases in the last three months.
 |
Figure 2 Study flow chart. (A) Training cohort; (B) Validation cohort. Abbreviations: CMPP, common M. pneumoniae pneumonia; RMPP, Refractory M. pneumoniae pneumonia. |
In the validation cohort, 90 hospitalized children were enrolled, 40 boys and 50 girls, with a median age of 5.3 (3.5–6.6) years, and their data were used for external validation of the prediction model (Table 1).
 |
Table 1 Baseline Characteristics of All Patients in the Training and Validation Cohorts |
Eventually, 161 children were enrolled in the CMPP group, and 64 were enrolled in the RMPP group. There were no significant differences in sex, age, or weight between the two groups (P>0.05). The duration of fever, the proportion of children with hyperpyrexia, the white blood cell count, the neutrophil ratio, CRP, ALT, prealbumin, and LDH were higher in the RMPP group than in the CMPP group, and the differences were statistically significant (P<0.05). There were no significant differences in other clinical symptoms, such as the duration of fever before admission and the proportion of children with wheezing and sputum sounds, between the two groups (P>0.05). The above-detailed results are shown in Table 2.
 |
Table 2 Baseline Information of the CMPP and RMPP Patients |
LUS Findings of the CMPP and RMPP Patients
LUS examinations were completed within five days of the admission of children in both the RMPP and CMPP groups. The comparison of the ultrasound manifestations and color Doppler ultrasound findings between the two groups is shown in Table 3. This study focused on analyzing the main LUS parameters of MPP, including B-lines, air-bronchogram signs, liquid-bronchogram signs, lung consolidations, and pleural effusion. We measured the lateral, longitudinal, and sagittal dimensions of the lung consolidation area and recorded the maximum values. Consolidations were mainly unilateral in the RMPP and CMPP groups (92.2% vs.84.5%, χ2=2.359, P>0.05). The mean maximum diameter of the lung consolidation (or atelectasis) area in the RMPP group was 6.3 cm, significantly greater than the 3.2 cm in the CMPP group (χ2=−10.336, P<0.001). Consolidation size /BSA represents the size of the consolidation after correction of the body surface area, with a median of 8.3 cm in the RMPP group, which was also significantly greater than the 4.0 cm in the CMPP group (χ2=−9.277, P<0.001). Dense B-lines were found around the lung consolidations in the RMPP group, but no scattered B-lines or fused B-lines were found, with no statistically significant differences between the two groups (P>0.05). In the RMPP group, the air bronchogram signs in lung consolidation areas were less than those in the CMPP group (73.4% vs 95.7%), but the liquid bronchogram signs were significantly more than those in the RMPP group (15.6% vs 1.9%, χ2=23.717, P<0.001). The pleural effusion incidence was significantly higher in the RMPP group than in the CMPP group (64.1% vs 29.8%, χ2=22.467, P<0.001). Color Doppler ultrasound was mainly used to evaluate the blood flow within the consolidation. We found that the two groups of children had similar blood flow distribution in the consolidation area, with no statistically significant difference (97.5% vs 96.9%, P>0.05).
 |
Table 3 LUS Findings of the CMPP and RMPP Patients |
Developing the Nomogram in the Training Cohort
Pleural effusion (odds ratio (OR) 3.551, 95% confidence interval (CI) 1.921–15.600), LDH (OR 1.044, 95% CI 1.006–1.021), CRP (OR 3.293, 95% CI 1.019–1.098), and Consolidation size /BSA (OR 1.015, 95% CI 1.536–2.446), when entered the prediction model, were independent risk factors for the occurrence of RMPP (Table 4). Predictive models are represented as nomogram visualizations (Figure 3).
 |
Table 4 Stepwise Logistic Regression Analysis for the Independent Variable Predicting the RMPP |
 |
Figure 3 Nomogram for predicting RMPP. Abbreviations: RMPP, Refractory M. pneumoniae pneumonia; Consolidation Size/BSA, Size of Consolidation corrected by BSA; BSA, Body surface area. |
Validation of the Nomogram
In the training cohort, the AUC of the predictive model was 0.955 (95% CI 0.919–0.978). (Figure 4). The AUC of the predictive model in the validation cohort was 0.916 (95% CI 0.838–0.964). The calibration curve of the nomogram shows a high degree of agreement between the predicted and observed probabilities in the training cohort and validation cohort (Figure 5).
 |
Figure 4 ROC curves. (A) Training cohort; (B) Validation cohort. Abbreviation: AUC, the area under the ROC curves. |
 |
Figure 5 Calibration curve for predictive models. (A) Training cohort; (B) Validation cohort. |
Discussion
Macrolides are the first-line antibiotics for treating MPP. As macrolide-unresponsive RMPP continues to emerge, only drugs such as minocycline or fluoroquinolones are available for treatment; however, the safety of these two classes of drugs in children remains to be studied.25 Zhai et al26 found that the course of antibiotics for RMPP was significantly longer than that for children with CMPP, requiring two or even longer infusion courses. Because RMPP has an overwhelming immune response and is prone to extrapulmonary complications such as mycoplasma encephalopathy, liver injury, myocardial injury, glucocorticoid, immunoglobulin, liver-protecting, and heart-protecting drugs are also used in its treatment. Fiberoptic bronchoscopy is also a method of treatment for RMPP combined with pulmonary atelectasis.27 Glucocorticoids were applied in our collection of children in the RMPP group; nearly 1/3 of them underwent alveolar lavage treatment via fiberoptic bronchoscopy. The above treatment options indicate that RMPP is much more complex and expensive than CMPP. Early diagnosis of RMPP can help clinicians to provide more aggressive treatment and thus reduce the incidence of complications and sequelae. In this study, we developed an early prediction model of RMPP in children, which included LUS parameters, clinical manifestations, and laboratory indicators. To achieve this goal, we screened the predictive variables for RMPP through logistic regression analysis to build a nomogram. Finally, consolidation size /BSA, pleural effusion, LDH, and CRP have entered the predictive model. The ROC and calibration curves analysis confirmed that the prediction nomogram has good discriminatory and calibration power.
Pulmonary imaging in RMPP usually shows extensive consolidation or atelectasis in one or more lobes and pleural effusion.15 In practical clinical work, imaging evaluation primarily relies on CXR or chest CT.28 CXR is fast, inexpensive, and widely used in outpatient, emergency, and primary ward hospitals. However, CXR lacks accuracy and reliability in diagnosing pneumonia and exposes children to ionizing radiation; furthermore, the interobserver agreement is low.16,29 CT has been used as the gold standard for pneumonia imaging in studies of adult patients, showing a high degree of accuracy.30 Although chest CT is superior to CXR, its radiation harm is more severe than CXR, limiting its application in children. Because of the cognitive state of children, sedation or even anesthesia is often needed for chest CT examination, which also increases the difficulty of implementing chest CT.
Furthermore, CT equipment’s high cost and training costs limit its popularization and application in primary hospitals.31 Therefore, exploring a safe, accurate, efficient, and economical imaging diagnostic tool for children is urgent. Many studies have confirmed18 that LUS, as a safe, portable, and effective new imaging diagnostic tool, can be applied to diagnose pneumonia in children. In children with suspected CAP, LUS is more effective than physical examination alone or physical examination combined with laboratory indices and CXR in diagnosing the disease.32 Compared with CXR, LUS has better sensitivity and similar specificity in diagnosing pneumonia in children and can detect abnormal lung lesions that are not easily seen by CXR, such as small lung consolidations and pleural effusions.33,34 The above studies mainly focus on the role of LUS in diagnosing suspected pneumonia in children, but few studies evaluate pneumonia conditions, especially identifying RMPP. Our study filled this gap and found that LUS can effectively identify RMPP.
Through LUS, we found that 92.2% of lung consolidation (or atelectasis) caused by RMPP was unilateral. The median size of consolidation in RMPP was 6.3 cm, significantly greater than the 3.2 cm in CMPP (χ2=−10.336, P<0.001). Assessment of the consolidation size is influenced by age because children are a particular group of patients whose lung volume increases with age. While there is no accepted method to correct for age, we tried to correct the consolidation size in children of different ages using the ratio of the actual measured size to the body surface area (BSA), and the corrected values were expressed as consolidation size /BSA. The median consolidation size /BSA value in the RMPP group was 8.3, significantly higher than that in the CMPP group. Our study confirmed that consolidation size corrected for BSA remained an independent risk factor for RMPP and entered the prediction model. Several studies confirmed7,12,26 that unilateral pulmonary solidity or atelectasis was the most critical risk factor for developing RMPP, which was consistent with our findings. Musolino et al35 showed that 63.6% of patients with complicated pneumonia had a lung consolidation size ≥5 cm in LUS. Pulmonary consolidation under LUS is tissue-like hypoechoic areas under the pleura, indicating that air is replaced by fluid in the alveoli of the lesion area, and large consolidation can appear directly as liver-like tissue. Electronic bronchoscopy in children with RMPP shows that the necrotic bronchial mucosa loses its ability to expel sputum and many secretions or mucus embolize the distal bronchus, which is the main reason for the failure of pulmonary consolidation or atelectasis to improve; therefore, lobar pulmonary solidity or atelectasis is the primary imaging features of RMPP. In our current study, the assessment of consolidation sizes by LUS confirmed the above point.
In the RMPP group, nearly two-thirds of the children had pleural effusion, and in the CMPP group, less than one-third had pleural effusion. Logistic regression analysis identified pleural effusion as an independent risk factor for developing RMPP and entered the prediction model (OR 3.551, 95% CI 1.921–15.600, P=0.01). As a traditional tool for evaluating pleural effusion, LUS can identify a small amount of pleural effusion with high sensitivity.20 Although their study enrolled only 18 patients, Kurian et al found that chest ultrasound and chest CT were similar in detecting pleural effusion due to pneumonia. These findings are consistent with the majority of previously published studies. Musolino et al found pleural effusions in 63.1% of children with complicated pneumonia.35 Lee et al8 confirmed that pleural effusion is a risk factor for nonresponse to treatment or the progression of MPP in children (OR 5.127, 95% CI 1.404–18.727). YY Ling et al13 found that pleural effusion was an independent risk factor for RMPP in children over six years of age (OR 3.023; 95% CI 1.424–6.420, P=0.004). The current study only deals with the qualitative assessment of pleural effusion and lacks a quantitative evaluation, which may affect its contribution to the early diagnosis of RMPP.
Both groups had air bronchograms in the consolidation area, consistent with Urbankowska E et al,36 who found air bronchograms in 76.5% of lung consolidations. The air bronchogram sign in the CMPP group was significantly greater than that in the CMPP group (95.7% vs 73.4%), while the liquid bronchogram sign was less than that in the CMPP group (1.9% vs 15.6%, χ2=23.717, P<0.001). Musolino et al35 found that an increase in the air bronchogram sign in the consolidation area is a sign of the regression of lung consolidation. In contrast, the presence of bronchial fluid in the consolidation area is a sign of the progression of lung consolidation. Therefore, the results indicate that the fact of an air bronchogram is indicative primarily of milder disease. Color ultrasound Doppler was used to assess blood flow in areas of consolidation (or atelectasis). We found a good blood flow distribution in both groups in consolidation areas, indicating that consolidation due to MPP mainly affects ventilation and has less impact on blood flow imaging, except in conditions such as necrotizing pneumonia, which was not present in our enrolled patients.
The B-line is also a critical LUS indicator; our study showed that all children had B-lines. Scattered B-lines and confluent B-lines appeared only in the non-consolidated areas of CMPP; dense B-lines mostly appeared around areas of lung consolidation. Pathological B-lines are generally referred to as interstitial syndromes. The simultaneous appearance of three or more separate B-lines or the fusion of B-lines has been associated with a thickening of the interlobular septal membrane in adult CT.37 This association suggests that interstitial edema is a common feature of CMPP and RMPP.
In our study, Consolidation size /BSA, pleural effusion, CRP, and LDH were significant indicators of RMPP development, and we developed a nomogram prediction tool through R software. The predictive nomograms of RMPP currently in use rely on clinical manifestations, laboratory indicators, and inflammatory factors. This reliance is related to the pathogenesis of RMPP, as studies have confirmed that immune damage plays an essential role in the pathogenesis of RMPP and direct pathogenic damage. Cheng et al38 used LASSO regression to screen for risk factors for RMPP and found that LDH, albumin, the neutrophil ratio, and high fever were four independent risk factors; the AUC of the nomogram was greater than 0.8 in both the training and validation groups. Bi et al7 took six indicators, including age, fever days, CRP, ATL, LDH, and CXR results, and developed a predictive nomogram for RMPP with a sensitivity of 78.3% and a specificity of 86.2%. The AUC of the predictive scale was 0.871. LUS parameters were added to the RMPP prediction model, which had never been used before. The external validation confirmed the accuracy and stability of the model, and good calibration of the model was confirmed by bootstrapping and calibration curves, indicating that the model has a high diagnostic power, which suggests that the model provides a reasonable degree of discrimination.
We also found some limitations in this study during the research process. This study was a single-center prospective study of only hospitalized children. We did not include children from the emergency department or the intensive care unit; therefore, selection bias exists. The sample size of the RMPP group in this study was small, and an evaluation of the nomogram in a large sample of children is still needed. Regarding the diagnostic criteria for RMPP, we used three experts to review the study because there is still a lack of a gold-standard method for diagnosing RMPP. Therefore, we consider the risk of bias in this study to be uncertain. In future work, we should conduct further multicenter, large-sample, prospective studies to confirm further the value of LUS parameters in the early diagnosis of RMPP.
Conclusion
In conclusion, LUS can accurately assess the distribution, size, ventilation, and blood flow of consolidation areas due to MPP. A nomogram based on LUS parameters combined with clinical presentation and laboratory indicators can accurately predict the development of RMPP in children hospitalized with MPP. The nomogram can be widely used in primary care to alert clinicians to personalize the care and treatment of children with MPP who are likely to develop RMPP.
Data Sharing Statement
The original data in this study can be obtained from the corresponding authors.
Ethics Statement
The Ethics Committee of Taian Maternity and Child Health Care Hospital approved the study (LW202203). Data collection began only after written informed consent was obtained from the children’s families.
Acknowledgments
This work is supported by the Shandong Maternal and Child Health Science and Technology Innovation Program Project (contact name: Gang-Tie Liu).
Disclosure
The authors have reported no potential conflicts of interest.
References
1. Waites KB, Xiao L, Liu Y, Balish MF, Atkinson TP. Mycoplasma pneumoniae from the respiratory tract and beyond. Clin Microbiol Rev. 2017;30(3):747–809. doi:10.1128/CMR.00114-16
2. Gao L-W, Yin J, Hu Y-H, et al. The epidemiology of paediatric Mycoplasma pneumoniae pneumonia in North China: 2006 to 2016. Epidemiol Infect. 2019;147:e192. doi:10.1017/S0950268819000839
3. Tsai T-A, Tsai C-K, Kuo K-C, Yu H-R. Rational stepwise approach for Mycoplasma pneumoniae pneumonia in children. J Microbiol Immunol Infect. 2021;54(4):557–565. doi:10.1016/j.jmii.2020.10.002
4. Tamura A, Matsubara K, Tanaka T, Nigami H, Yura K, Fukaya T. Methylprednisolone pulse therapy for refractory Mycoplasma pneumoniae pneumonia in children. J Infect. 2008;57(3):223–228. doi:10.1016/j.jinf.2008.06.012
5. Shah SS, Test M, Sheffler-Collins S, Weiss AK, Hall M. Macrolide therapy and outcomes in a multicenter cohort of children hospitalized with Mycoplasma pneumoniae pneumonia. J Hosp Med. 2012;7(4):311–317. doi:10.1002/jhm.1904
6. Lu A, Wang C, Zhang X, Wang L, Qian L. Lactate Dehydrogenase as a Biomarker for Prediction of Refractory Mycoplasma pneumoniae Pneumonia in Children. Respir Care. 2015;60(10):1469–1475. doi:10.4187/respcare.03920
7. Bi Y, Zhu Y, Ma X, et al. Development of a scale for early prediction of refractory Mycoplasma pneumoniae pneumonia in hospitalized children. Sci Rep. 2021;11(1):6595. doi:10.1038/s41598-021-86086-5
8. Lee E, Lee YY. Predictive factors of the responses to treatment of pneumonia. J Clin Med. 2021;10(6):1154.
9. Zhang Y, Zhou Y, Li S, Yang D, Wu X, Chen Z. The clinical characteristics and predictors of refractory Mycoplasma pneumoniae Pneumonia in children. PLoS One. 2016;11(5):e0156465. doi:10.1371/journal.pone.0156465
10. Huang X, Li D, Liu F, Zhao D, Zhu Y, Tang H. Clinical significance of D-dimer levels in refractory Mycoplasma pneumoniae pneumonia. BMC Infect Dis. 2021;21(1):14. doi:10.1186/s12879-020-05700-5
11. Choi Y-J, Jeon J-H, Oh J-W. Critical combination of initial markers for predicting refractory Mycoplasma pneumoniae pneumonia in children: a case control study. Respir Res. 2019;20(1):193. doi:10.1186/s12931-019-1152-5
12. Cheng Q, Zhang H, Shang Y, et al. Clinical features and risk factors analysis of bronchitis obliterans due to refractory Mycoplasma pneumoniae pneumonia in children: a nomogram prediction model. BMC Infect Dis. 2021;21(1):1085. doi:10.1186/s12879-021-06783-4
13. Ling Y, Ning J, Xu Y. Explore the predictive value of peripheral blood cell parameters in refractory pneumonia in children over 6 years old. Front Pediatr. 2021;9:659677. doi:10.3389/fped.2021.659677
14. Yang M, Meng F, Wang K, et al. Interleukin 17A as a good predictor of the severity of Mycoplasma pneumoniae pneumonia in children. Sci Rep. 2017;7(1):12934. doi:10.1038/s41598-017-13292-5
15. Yan Y, Wei Y, Jiang W, Hao C. The clinical characteristics of corticosteroid-resistant refractory Mycoplasma Pneumoniae pneumonia in children. Sci Rep. 2016;6:39929. doi:10.1038/srep39929
16. Ben Shimol S, Dagan R, Givon-Lavi N, et al. Evaluation of the World Health Organization criteria for chest radiographs for pneumonia diagnosis in children. Eur J Pediatr. 2012;171(2):369–374. doi:10.1007/s00431-011-1543-1
17. Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38(4):577–591. doi:10.1007/s00134-012-2513-4
18. Pereda MA, Chavez MA, Hooper-Miele CC, et al. Lung ultrasound for the diagnosis of pneumonia in children: a meta-analysis. Pediatrics. 2015;135(4):714–722. doi:10.1542/peds.2014-2833
19. Shah VP, Tunik MG, Tsung JW. Prospective evaluation of point-of-care ultrasonography for the diagnosis of pneumonia in children and young adults. JAMA Pediatr. 2013;167(2):119–125. doi:10.1001/2013.jamapediatrics.107
20. Llamas-álvarez AM, Tenza-Lozano EM, Latour-Pérez J. Accuracy of lung ultrasonography in the diagnosis of pneumonia in adults: systematic review and meta-analysis. Chest. 2017;151(2):374–382. doi:10.1016/j.chest.2016.10.039
21. Mojoli F, Bouhemad B, Mongodi S, Lichtenstein D. Lung ultrasound for critically ill patients. Am J Respir Crit Care Med. 2019;199(6):701–714. doi:10.1164/rccm.201802-0236CI
22. Lichtenstein DA, Mezière GA, Lagoueyte J-F, Biderman P, Goldstein I, Gepner A. A-lines and B-lines: lung ultrasound as a bedside tool for predicting pulmonary artery occlusion pressure in the critically ill. Chest. 2009;136(4):1014–1020. doi:10.1378/chest.09-0001
23. Lichtenstein D, Mezière G, Seitz J. The dynamic air bronchogram. A lung ultrasound sign of alveolar consolidation ruling out atelectasis. Chest. 2009;135(6):1421–1425. doi:10.1378/chest.08-2281
24. Jaworska J, Komorowska-Piotrowska A, Pomiećko A, et al. Consensus on the application of lung ultrasound in pneumonia and bronchiolitis in children. Diagnostics. 2020;10(11). doi:10.3390/diagnostics10110935
25. Kawai Y, Miyashita N, Kubo M, et al. Therapeutic efficacy of macrolides, minocycline, and tosufloxacin against macrolide-resistant Mycoplasma pneumoniae pneumonia in pediatric patients. Antimicrob Agents Chemother. 2013;57(5):2252–2258. doi:10.1128/AAC.00048-13
26. Zhai Y-Y, Wu S-Z, Yang Y, et al. An analysis of 20 clinical cases of refractory mycoplasma pneumonia in children. Ann Palliat Med. 2020;9(5):2592–2599. doi:10.21037/apm-19-497
27. Lin M, Shi L, Huang A, Liang D, Ge L, Jin Y. Efficacy of levofloxacin on macrolide-unresponsive and corticosteroid-resistant refractory Mycoplasma pneumoniae pneumonia in children. Ann Palliat Med. 2019;8(5):632–639. doi:10.21037/apm.2019.10.05
28. Jang MS, Kim BG, Kim J. Prediction model for prolonged fever in patients with Mycoplasma pneumoniae pneumonia: a retrospective study of 716 pediatric patients. BMC Pulm Med. 2021;21(1):168. doi:10.1186/s12890-021-01534-2
29. Levinsky Y, Mimouni FB, Fisher D, Ehrlichman M. Chest radiography of acute paediatric lower respiratory infections: experience versus interobserver variation. Acta Paediatrica. 2013;102(7):e310–e314. doi:10.1111/apa.12249
30. Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277–2284. doi:10.1056/NEJMra072149
31. Zar HJ, Andronikou S, Nicol MP. Advances in the diagnosis of pneumonia in children. BMJ. 2017;358:j2739. doi:10.1136/bmj.j2739
32. Zhou J, Song J, Gong S, et al. Lung ultrasound combined with procalcitonin for a diagnosis of ventilator-associated pneumonia. Respir Care. 2019;64(5):519–527. doi:10.4187/respcare.06377
33. Balk DS, Lee C, Schafer J, et al. Lung ultrasound compared to chest X-ray for diagnosis of pediatric pneumonia: a meta-analysis. Pediatr Pulmonol. 2018;53(8):1130–1139. doi:10.1002/ppul.24020
34. Iorio G, Capasso M, Prisco S, et al. Lung ultrasound findings undetectable by chest radiography in children with community-acquired pneumonia. Ultrasound Med Biol. 2018;44(8):1687–1693. doi:10.1016/j.ultrasmedbio.2018.04.007
35. Musolino AM, Tomà P, Supino MC, et al. Lung ultrasound features of children with complicated and noncomplicated community acquired pneumonia: a prospective study. Pediatr Pulmonol. 2019;54(9):1479–1486. doi:10.1002/ppul.24426
36. Urbankowska E, Krenke K, Drobczyński Ł, et al. Lung ultrasound in the diagnosis and monitoring of community acquired pneumonia in children. Respir Med. 2015;109(9):1207–1212. doi:10.1016/j.rmed.2015.06.011
37. Lichtenstein D, Mézière G, Biderman P, Gepner A, Barré O. The comet-tail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care Med. 1997;156(5):1640–1646. doi:10.1164/ajrccm.156.5.96-07096
38. Cheng S, Lin J, Zheng X, et al. Development and validation of a simple-to-use nomogram for predicting refractory Mycoplasma pneumoniae pneumonia in children. Pediatr Pulmonol. 2020;55(4):968–974. doi:10.1002/ppul.24684
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.