Back to Journals » Journal of Pain Research » Volume 16
Development and Validation of a Predictive Model for Chronic Postsurgical Pain After Arthroscopic Rotator Cuff Repair: A Prospective Cohort Study
Authors Dai X, Yuan M, Dang M, Liu D, Fei W
Received 15 June 2023
Accepted for publication 20 September 2023
Published 27 September 2023 Volume 2023:16 Pages 3273—3288
DOI https://doi.org/10.2147/JPR.S423110
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jinlei Li
Xiaomei Dai,1 Meijuan Yuan,1 Mengbo Dang,2 Dianwei Liu,2 Wenyong Fei3
1School of Nursing and School of Public Health, Yangzhou University, Yangzhou, People’s Republic of China; 2Dalian Medical University, Dalian, People’s Republic of China; 3Department of Orthopedics and Sports Medicine, Northern Jiangsu People’s Hospital, Affiliated to Yangzhou University, Yangzhou, People’s Republic of China
Correspondence: Wenyong Fei, Department of Orthopedics and Sports medicine, Northern Jiangsu People’s Hospital, Affiliated to Yangzhou University, Yangzhou, 225001, People’s Republic of China, Tel +86 18051061779, Email [email protected]
Purpose: Chronic pain management continues to present a significant challenge following arthroscopic shoulder surgery. Our purpose was to detect chronic postsurgical pain (CPSP) in patients who had undergone arthroscopic rotator cuff repair (ARCR) and develop a nomogram capable of predicting the associated risk.
Patients and Methods: We collected the demographic and clinical data of 240 patients undergoing ARCR in our hospital from January 2021 to May 2022. The pain level was monitored and evaluated three months after ARCR. LASSO regression was used to screen out pain-predicting factors, which were subsequently used to construct a nomogram. Internal validation was carried out using Bootstrap resampling. The data of 78 patients who underwent ARCR in our hospital from August 2022 to December 2022 were also collected for external verification of the nomogram. The predictive model was evaluated using the receiver operating characteristic curve (ROC), calibration curve, and decision curve analysis (DCA).
Results: Age, duration of preoperative shoulder pain (DPSP), C-reactive protein (CRP), number of tear tendons, and American Shoulder and Elbow Surgical Score (ASES) were screened by LASSO regression as predictive factors for CPSP. These factors were then used to construct a chronic pain risk nomogram. The area under the curve (AUC) of the predictive and validation models were 0.756 (95% CI: 0.6386– 0.8731) and 0.806 (95% CI: 0.6825– 0.9291), respectively. Furthermore, the calibration curves and decision curve analysis (DCA) for both models indicated strong performance, affirming the reliability of this predictive model.
Conclusion: The CPSP risk model that has been developed exhibits strong predictive capabilities and practical utility. It offers valuable support to clinical healthcare professionals in making informed treatment decisions, reducing the unnecessary use of analgesic drugs, and optimizing the allocation of medical resources.
Keywords: arthroscopic rotator cuff repair, chronic postoperative pain, nomogram, predictive model, risk factors
Introduction
The shoulder has become the third most common pain site in the motor system after the back and knee, which is the leading cause of musculoskeletal disabilities.1 Approximately 4.5 million patients visit healthcare facilities in the United States annually due to shoulder pain.2 For most patients, a damaged rotator cuff is the primary cause of their shoulder pain. Studies have shown that rotator cuff injuries occur in 13% to 37% of the general population, and their prevalence increases with age.3,4 Rotator cuff injuries can be treated through either conservative or surgical approaches. However, conservative treatment is often less effective, with the result that a majority of patients eventually require surgical intervention when conservative treatment proves unsuccessful.5 Currently, arthroscopic rotator cuff repair (ARCR) is the “gold standard” in the treatment of rotator cuff injury, offering advantages such as minimal incision size, reduced deltoid muscle damage, and notably improved shoulder joint function on the affected side.6,7 It is noteworthy that the surgical rate for this procedure has surged by 600% from 1996 to 2006.8
Despite advancements in surgical procedures, effective pain management remains a challenging aspect of shoulder arthroscopy, often resulting in the experience of moderate to severe pain post-surgery. The duration of pain that persists for three months or longer following a surgical procedure is referred to as chronic postsurgical pain (CPSP).9 The onset of CPSP can significantly hinder the recovery process, elevate financial burdens, increase medical resource utilization, and negatively affect the quality of family life.10,11 Historically, opioids have long been considered the gold standard for ensuring adequate pain management following orthopedic surgical procedures.12 Opioids are associated with a range of adverse effects, including nausea, vomiting, and constipation. At higher doses, these medications can lead to severe complications such as respiratory depression and hypoxia.13,14 The elevated utilization of opioid-based painkillers to manage CPSP can be dangerous, as these medications causing more deaths annually among Americans than heroin and cocaine combined.15 In recent years, multimodal pain management approaches have proven highly beneficial in addressing post-orthopedic surgery pain.16–18 Individualized postoperative pain management should begin well in advance of the surgery date. The multimodal analgesia protocol operates as a comprehensive checklist, enabling the assessment and prescription of the most appropriate analgesic category tailored to the specific patient and the expected pain intensity. Therefore, it is essential to identify high-risk patients for chronic pain preoperatively. Previous research on postoperative pain after ARCR has primarily concentrated on risk factors such as age, gender, and the type of surgical procedure.19,20 Unfortunately, there are currently no available assessment techniques to reliably identify patients at a high risk of developing CPSP.
The objective of this study was to establish and validate a predictive nomogram for CPSP following ARCR. Such a nomogram can assist healthcare professionals in formulating preventive and personalized treatment approaches during the perioperative phase. This, in turn, can lead to a reduction in the incidence of CPSP, efficient allocation of medical resources, enhanced clinical outcomes, and ultimately, an improvement in the quality of life for patients.
Materials and Methods
This study adhered to the principles outlined in the Declaration of Helsinki. Ethical clearance for this research was granted by Yangzhou University, China (YZUHL20210112), and patients either provided written or verbal consent to participate. Patients included in the study from January to September 2021 were part of a retrospective cohort study, and data was extracted from hospital records. These patients provided verbal consent. Due to the COVID-19 pandemic, discharged patients were followed up via telephone. During these follow-ups, the study’s purpose, procedures, benefits, risks, and other pertinent details were explained to the patients and their families to secure their cooperation. The Institutional Review Board has approved the use of verbal consent for these patients.
Starting from October 2021, the study shifted to a prospective cohort design. As a result, this subset of patients provided written consent statements.
Patient Source
This study encompassed a total of 318 patients who underwent ARCR at our hospital and had complete clinical data available. All surgical procedures were carried out by the same team of physicians, and every patient received a follow-up assessment three months after surgery. The data collected for this study spanned from January 2021 to May 2022 and comprised 240 patients, forming the training cohort for model development. Subsequently, between August 2022 and December 2022, an additional 78 patients were included in the validation cohort for the model. All patients attended clinic visits for reexamination at one month and three months after the surgery to evaluate their postoperative pain. In cases where patients were unable to visit the clinic, follow-up assessments were conducted via telephone.
Inclusion and Exclusion Criteria
Inclusion criteria: (1) Age ≥18 years; (2) preoperative MRI examination showing partial or full rotator cuff tear; (3) Arthroscopic rotator cuff repair performed by the same team of physicians; and (4) no communication barriers. Exclusion criteria: (1) pain in other parts except for the surgical site; (2) secondary surgery; (3) combined mental and psychological disorders, vital tissue and organ dysfunction; (4) failure to complete 3-month follow-up.
Instrument and Outcome Measures
Three scales were used to collect data. (1) The Numerical Rating Scale (NRS): This scale quantified subjective pain intensity on an 11-point continuum, ranging from 0 (indicating no pain) to 10 (representing the most severe pain). Pain ratings of NRS ≥4 were classified as indicating moderate to severe pain.21 Research has indicated that the NRS is more convenient and user-friendly when compared to other pain scales, which results in better patient compliance.22 (2) The American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form (ASES): This tool assessed shoulder function through two dimensions: pain (one item) and daily living function (ten items). Pain levels were assessed using a visual analogue scale (VAS), with 0 representing “no pain” and 10 representing “maximum pain”. Daily life functions were evaluated using a 4-point Likert scale, with “0= unable to do” and “3= not difficult”. Shoulder scores were calculated using the formula: (10 - VAS pain score) * 5 + (5/3 * the sum of the 10 daily living function scores). The maximum score achievable is 100, indicating optimal shoulder function.23 (3) The Constant-Murley Score (CMS): This assessment tool evaluated overall shoulder joint function. It consisted of two subjective measures, pain, and Activities of Daily Living (ADL), with a score range of up to 35 points, and two objective measures, range of motion (ROM) and strength, with a score range of up to 65 points. The total score on this scale is 100 points, with a higher score signifying better shoulder functioning.24
CPSP was defined as an NRS score ≥4 points 3 recorded months after ARCR.
Surgical Procedures
The surgical procedure for ARCR closely adhered to established protocols as outlined in prior studies.25 In brief, after successful anesthesia, the patient was placed in the lateral decubitus position, and routine disinfection was performed with a sterile sheet. Subsequently, access was gained to both the anterior and posterior channels of the right glenohumeral joint. Sequential examination of the glenohumeral joint was performed, and any inflamed tissue was meticulously removed using a plasma knife.
The arthroscope was then transitioned to the subacromial space, with the establishment of lateral and posterior lateral channels. Here, the damaged tendon was meticulously explored, and the insertion of the affected tendon was securely fixed using an anchor and mattress suture technique.
Closure of the incision was accomplished through sutures, and cotton pads were applied over the surgical area as a protective dressing. The shoulder and arm of the operated limb were immobilized to prevent inadvertent movement. Postoperatively, patients received cefazolin for anti-inflammatory purposes and parecoxib for analgesic treatment.
Variable Collection
We set up a data collection team comprising a physician, a nurse, and a therapist. The factors and variables that could impact postoperative pain were identified by reviewing published literature and drawing on clinical experience. At the time of admission, various preoperative factors were recorded, such as age, sex, body mass index (BMI), smoking history, alcohol history, trauma history, pain site, duration of preoperative shoulder pain (DPSP), complications (hypertension, diabetes, cerebral infarction), frozen shoulder, osteoporosis, blood routine and blood biochemical indicators (leukocyte, erythrocyte sedimentation rate, c-reactive protein, creatinine, bilirubin), Preoperative NRS, ASES, and Constant-Murly scores. Intraoperative factors were collected after completion of the surgery, including the number, location, and area of tendon tears (small tear<1cm, medium tear 1–3cm, large tear 3–5cm, massive tear >5cm), full-thickness tear, surgery times, Acromioplasty, number of anchors, bone morphogenetic protein (BMP) and chitin injection.
Statistical Analysis
All data were statistically analyzed using SPSS software (version 27.0) and R-studio software (version 4.2.2). For continuous variables following a normal distribution, descriptive statistics were presented as Mean ± SD, and group comparisons were conducted using the t-test. For non-normally distributed continuous variables, median values with Interquartile Range (IQR) were utilized, and between-group comparisons were performed using the rank-sum test. Categorical variables were expressed as counts (n) and percentages (%) and were analyzed using the chi-square test. Statistical significance was determined at a threshold of p<0.05. The least absolute shrinkage and selection operator (LASSO) method was used in R-studio software to screen the predictive factors of chronic pain after ARCR. A nomogram was created with a “rms” package, and 200 Bootstrap samples were completed with an insert package for internal model validation. The receiver operating characteristic (ROC) curve, calibration curve, and decision curve analysis (DCA) were generated to evaluate the predictive performance of the prediction model.
Results
Variable Distribution Information for Training and Validation Cohort
A total of 318 patients were included in our study based on our inclusion and exclusion criteria. Table 1 presents a comparison of the baseline characteristics of the patients in the training and validation cohorts, consisting of 240 and 78 patients, respectively. Among the variables examined, excepting pain site, DPSP, history of trauma, frozen shoulder status, performance of Acromioplasty, and preoperative NRS scores, there were no significant differences between the two groups. However, it’s noteworthy that the rate of postoperative chronic pain differed slightly, with 12.1% among patients in the training cohort and 17.9% among those in the validation cohort.
 |
Table 1 Comparison of Variable Distribution Information for Training and Validation Cohort |
Univariate Analysis of Training Cohort
According to the definition of CPSP, the training cohort was divided into a pain group (n=29) and a control group (n=211). As shown in Table 2, several tear tendons (p=0.021), ASES (p=0.003), intraoperative chitin injection (p=0.041), and postoperative 1-month NRS (p<0.001) showed significant differences between the two groups.
 |
Table 2 Univariate Analysis of Training Cohort |
LASSO Regression Screening Variables
Considering the excessive number of variables and there is collinearity between them, we adopted the LASSO regression method to further screen variables. Figure 1 illustrates the path of individual variables in LASSO regression across different penalty factors. LASSO regression can eliminate the coefficients of irrelevant features by setting them to zero, and through the utilization of 10-fold cross-validation, we identified the variables with non-zero coefficients, as depicted in Figure 2. According to the results of regression analysis, we screened five possible independent risk factors, including age, DPSP, CRP, ASES, and the number of tear tendons.
 |
Figure 1 Variation trajectory of variables in LASSO regression under different penalty factors. |
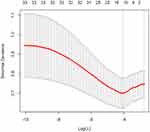 |
Figure 2 LASSO regression 10-fold cross validation results. |
Development and Validation of Nomogram
We created a predictive risk nomogram for CPSP using the five identified predictive factors, as shown in Figure 3. The scoring criteria were formulated according to the size of the regression coefficients of all predictive indicators. By computing the total score for the multiple predictive indicators of each patient, we can determine the probability of CPSP for that patient. Meanwhile, the ROC curves of the training cohort and the external validation cohort of the prediction model were obtained, with the AUC of 0.756 (95% CI: 0.6386–0.8731) and 0.806 (95% CI: 0.6825–0.9291), respectively, indicating that the model had the good distinguishing ability (Figure 4a and d). Furthermore, the calibration curves for both cohorts demonstrated favorable calibration (Figure 4b and e). The results from Decision Curve Analysis (DCA) suggested that this nomogram holds significant practical utility in a clinical setting for predicting the likelihood of chronic pain following arthroscopic rotator cuff repair (Figure 4c and f). By applying this nomogram to a 45-year-old woman who had been experiencing shoulder pain for 16 months before surgery, had elevated C-reactive protein levels, suffered from tears in both the supraspinatus and infraspinatus tendons, and presented with an ASES score of 45 upon admission, we can calculate her total score as follows: Total Score = Age (55) + DPSP (10) + CRP (20) + Number of Tear Tendons (58) + ASES Score (50) = 193. Based on this total score, the patient has an estimated probability of approximately 70% of developing CPSP. This assessment allows for the identification of high-risk individuals on the day of surgery, enabling the implementation of individualized perioperative pain management strategies tailored to the specific needs of each patient.
 |
Figure 3 Predictive nomogram of CPSP constructed by LASSO regression-screened predictors. |
Comparison of Roc and Net Reclassification Index
To evaluate the accuracy of our newly developed CPSP prediction nomogram, we compared it with the ASES scoring system. In terms of predicting chronic pain at 3 months post-surgery, the ASES scoring system had an area under the ROC curve of 0.606 (95% CI: 0.535–0.677). However, our newly created nomogram outperformed the ASES system, showing a higher net reclassification index of 13.28%, as illustrated in Figure 5.
 |
Figure 5 Compared to the ASES, the net weight classification diagram of the CPSP prediction nomogram. |
Discussion
Research has indicated that the overall rate of post-surgical pain ranges from 10% to 50%, with approximately 2% to 10% of patients eventually experiencing severe chronic pain.26 Daisy et al27 conducted a one-year follow-up study on 908 patients who underwent outpatient surgery and reported a CPSP incidence of 15.3%. Our study found that the incidence of CPSP was 12.1% in the training cohort, 17% in the validation cohort, and 13.5% overall, which is consistent with previous research. Hence, the issue of chronic pain following ARCR demands careful consideration. Given the absence of a consensus on the risk factors linked to inadequate pain control after ARCR, we embarked on a prospective study aimed at investigating the predictive factors contributing to CPSP both before and during surgery. Subsequently, we developed and validated risk prediction nomograms, which enabled us to identify high-risk patients susceptible to CPSP. This approach allows for timely interventions to address the issue of chronic pain in these individuals.
In our study, we employed the LASSO regression method as a strategy to mitigate the impact of overfitting and collinearity, thereby enhancing the accuracy of variable prediction.28 One significant factor we investigated was age, which emerged as a crucial determinant in the development of chronic pain. This finding is consistent with several previous studies,29–31 which have also noted that younger patients tend to experience higher levels of postoperative pain intensity when compared to older patients. The relationship between age and pain perception can be attributed to various factors associated with the aging process. As individuals age, the function and electrophysiology of the peripheral nervous system can be affected. This includes reduced nerve regeneration and diminished neurotransmitter content. Furthermore, there is a decrease in nerve density in facial skin and the density of epidermal nerve fibers. Epidermal nerve endings play a role in converting external stimuli into electrical signals, which can influence pain perception and sensitivity. These age-related changes in neural function may contribute to the observed differences in postoperative pain experiences between younger and older patients. A reduction in the density of epidermal nerves leads to decreased sensitivity to mechanical stimuli, touch, and other sensations, which consequently causes a decline in pain sensitivity with age.32–34 Although age is a non-interventive risk factor, gaining insight into high-risk groups allows for the development of personalized analgesia plans and the provision of appropriate health education in advance. Likewise, the duration of preoperative shoulder pain is a crucial predictor of CPSP. As the duration of preoperative pain increases, adverse clinical outcomes follow. Numerous previous studies focusing on the shoulder and neck have identified pain duration as a prognostic factor for both pain intensity and functional outcomes.35,36 This may be related to prior memories of pain. Bill et al37 conducted a study on the connection between memory and chronic pain, and the results showed that chronic pain has an emotional connection with previous pain stimuli. The persistent operation of pain plasticity mechanisms, akin to memory retention or the inability to erase pain-related memories, can contribute to the transition from acute pain to chronic pain.
The supraspinatus muscle is the most frequently affected site in cases of rotator cuff tears. As the size of the supraspinatus tear increases, it has the potential to involve the infraspinatus or subscapularis muscles as well. In our study, we observed that supraspinatus tears accounted for 68.9% of all cases, and in the pain group, the median number of damaged tendons was 1 (1, 2), with a significant p-value of 0.021. Existing literature on the association between damaged tendons and pain presents mixed findings. While some researchers argue that the severity of the affected tendon and tears does not correlate with pain, other studies have yielded different conclusions.38 While some researchers have found that intact healthy tendons generally lack innervation, injured tendons tend to undergo extended nerve growth, especially during the tendon repair process. This extensive nerve growth into the tendon has been associated with the abnormal expression of pro-inflammatory and nociceptive tissue responses.39,40 This may lead to an increase in the number of tendons involved and a greater tendency for the patient to develop pain. It is well known that inflammation is an important factor in rotator cuff tears.41
C-reactive protein (CRP) is a widely used marker of systemic inflammation. Studies have shown a strong correlation between elevated levels of CRP and increased pain sensitivity, indicating that CRP levels can serve as a predictor of CPSP.42 Hodges et al conducted a cross-sectional observational study investigating the connection between musculoskeletal pain and fluctuations in CRP levels. They analyzed and compared 17,624 patients with chronic pain, 11,962 patients with acute pain, and 29,604 patients without pain. The results showed that individuals with chronic pain exhibited significantly higher CRP levels compared to those with acute pain and individuals without pain.43 Interestingly, a recent study showed that limited vitamin D synthesis is associated with higher CRP concentrations.44 Supplementing sufficient vitamin D can increase anti-inflammatory activity and reduce the production of proinflammatory cytokines. Skrobot et al45 investigated the impact of vitamin D supplementation on inflammation markers and pain severity in patients who had undergone posterior lumbar intervertebral fusion (PLIF) surgery over a five-week period. The findings demonstrated that vitamin D supplementation could effectively lower systemic inflammatory markers and alleviate the intensity of pain experienced by patients. Based on the insights from these studies, preoperative vitamin D supplementation has the potential to mitigate postoperative pain, particularly in patients with elevated CRP levels.
ASES is one of the self-reported outcome assessment tools for patients after ARCR. Its superiority over other specific patient self-reported outcome measurement tools in detecting changes in clinical status after ARCR and shoulder arthroplasty has been demonstrated in previous research studies.46,47 In our study, patients with lower ASES scores are more prone to chronic pain, and their post-surgery shoulder function recovery is considerably less compared to patients with higher ASES scores before undergoing surgery. Previous studies have shown that preoperative pain related status measured using the patient self-reported outcome assessment tool can predict the pain intensity of patients after surgery.48,49 In this context, the ASES has demonstrated its predictive capability for forecasting the onset of chronic pain three months after surgery, with an associated area under ROC curve of 0.606. However, our newly developed nomogram, boasting a net weight classification index of 13.28%, exhibits superior predictive prowess for chronic postsurgical pain (CPSP) when compared to the ASES.
Intraoperative BMP injection was identified as a significant factor through LASSO regression screening. However, it was not included in the final model due to the limited number of injections administered. BMP, renowned for its robust osteoinductive properties, plays a pivotal role in osteogenesis and chondrogenesis processes.50 The clinical use of rhBMP-2 was approved by the US Food and Drug Administration (FDA) in 2002 for surgical procedures.51 Notably, some researchers have employed rhBMP-2 in conjunction with hydroxyapatite for posterolateral lumbar fusion surgery. As per their findings, both study groups exhibited notable enhancements in pain scores, functional scores, and quality of life scores when compared to the baseline group, with no significant adverse effects related to the treatment observed.52 Greiner et al53 implanted rhBMP-12/resorbable collagen sponge at the tendon bone interface and reported no adverse clinical outcomes during their 26-week follow-up. Moreover, they found that rhBMP-12, when used at a concentration of 0.015 mg/mL, poses no safety concerns in the context of rotator cuff repair. In our study, we administered rhBMP-2 directly into the tendon-bone interface, without utilizing biomaterials as a carrier, at a concentration of approximately 0.010 mg/mL. We diligently monitored patients for nearly 2 years. Notably, in our research, patients who received intraoperative rhBMP-2 injections did not report postoperative chronic pain, possibly owing to the positive influence of rhBMP-2 on the healing of the tendon-bone interface. Nevertheless, it’s crucial to acknowledge that the number of injections administered was limited, and further extensive data are needed to firmly establish the link between these injections and pain reduction. Furthermore, a deeper investigation into the precise mechanism of action is warranted.
We screened age, DPSP, CRP, the number of torn tendons, and ASES as potential risk factors for chronic pain following ARCR. Subsequently, we developed a chronic pain risk nomogram that exhibited robust performance. The AUC for the training cohort was 0.756, and for the validation cohort, it was 0.806. Unlike complex equation formulas, the nomogram offers a more user-friendly and visually accessible tool for clinical application. DCA further confirms the clinical utility and value of this nomogram.
This study also has several limitations. Firstly, it is a single-center study with a relatively limited sample size. Secondly, we did not incorporate certain relevant factors such as psychological aspects, sleep patterns, and lifestyle choices into the variables under consideration. Finally, the follow-up period for monitoring pain-related symptoms was limited to three months, and it would be beneficial to conduct more extended follow-ups to assess the pain status of patients over a longer duration post-surgery. The predictive nomogram serves as a valuable tool for identifying patients at high risk of CPSP early in the process. In our future research, we intend to focus on strategies for preventing CPSP, particularly by addressing preoperative pain as a significant risk factor. This may involve effective preoperative pain management or early administration of analgesics to patients at high risk, with the goal of potentially reducing the incidence of CPSP.
Conclusion
The CPSP nomogram developed in this study for ARCR demonstrates strong predictive capabilities and clinical utility. It enables healthcare professionals to effectively identify high-risk patients, implement early interventions, offer targeted health education and psychological support, and tailor perioperative and post-discharge pain management. This approach not only reduces the unnecessary use of analgesic medications but also conserves valuable medical resources.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the Yangzhou University, China (YZUHL20210112), and written or verbal consent was obtained from the patients for their anonymized information to be published in this article. The patients collected from January to September 2021 were included for a retrospective cohort study using data extracted from the outcomes of patient visits to the hospital, and these patients provided verbal consent statements. Due to the epidemic situation of COVID-19, the discharged patients were followed up by telephone, and the purpose, steps, benefits, risks and other details of the study were informed to the patients so that patients and their families can cooperate. Verbal consent from these patients has been approved by the Institutional Review Board.
Patients after October 2021 were included for a prospective cohort study; therefore, this subset of patients provided written consent statements.
Acknowledgments
We would like to thank the researchers and study participants for their contributions.
Author Contributions
All authors made a significant contribution to the work reported, in the conception, study design, execution, acquisition of data, analysis, and interpretation; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Orthopaedic and sports Rehabilitation Clinical Medical Research Center (2021-NCRC-CXJJ-PY-07).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Narvy SJ, Didinger TC, Lehoang D, et al. Direct cost analysis of outpatient arthroscopic rotator cuff repair in medicare and non-medicare populations. Orthop J Sports Med. 2016;4:2325967116668829.
2. Weber S, Chahal J. Management of rotator cuff injuries. J Am Acad Orthop Surg. 2020;28:e193–e201. doi:10.5435/JAAOS-D-19-00463
3. Reilly P, Macleod I, Macfarlane R, et al. Dead men and radiologists don’t lie: a review of cadaveric and radiological studies of rotator cuff tear prevalence. Ann R Coll Surg Engl. 2006;88:116–121. doi:10.1308/003588406X94968
4. Yamamoto A, Takagishi K, Osawa T, et al. Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg. 2010;19:116–120. doi:10.1016/j.jse.2009.04.006
5. Schemitsch C, Chahal J, Vicente M, et al. Surgical repair versus conservative treatment and subacromial decompression for the treatment of rotator cuff tears: a meta-analysis of randomized trials. Bone Joint J. 2019;101:1100–1106. doi:10.1302/0301-620X.101B9.BJJ-2018-1591.R1
6. Day MA, Westermann RW, Bedard NA, et al. Trends associated with open versus arthroscopic rotator cuff repair. Hss j. 2019;15:133–136. doi:10.1007/s11420-018-9628-2
7. Adam JR, Nanjayan SKT, Monga P. Management of rotator cuff tears - Key historical landmarks. J Clin Orthop Trauma. 2021;18:6–12. doi:10.1016/j.jcot.2021.03.019
8. Colvin AC, Egorova N, Harrison AK, et al. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94:227–233. doi:10.2106/JBJS.J.00739
9. Schug SA, Lavand’homme P, Barke A, et al. The IASP classification of chronic pain for ICD-11: chronic postsurgical or posttraumatic pain. Pain. 2019;160:45–52. doi:10.1097/j.pain.0000000000001413
10. Taylor RS, Ullrich K, Regan S, et al. The impact of early postoperative pain on health-related quality of life. Pain Pract. 2013;13:515–523. doi:10.1111/papr.12026
11. Parsons B, Schaefer C, Mann R, et al. Economic and humanistic burden of post-trauma and post-surgical neuropathic pain among adults in the United States. J Pain Res. 2013;6:459–469. doi:10.2147/JPR.S44939
12. Schoenfeld AJ, Jiang W, Chaudhary MA, et al. Sustained prescription opioid use among previously opioid-naive patients insured through TRICARE (2006–2014). JAMA Surg. 2017;152(12):1175–1176. doi:10.1001/jamasurg.2017.2628
13. Coyle DT, Pratt CY, Ocran-Appiah J, et al. Opioid analgesic dose and the risk of misuse, overdose, and death: a narrative review. Pharmacoepidemiol Drug Saf. 2018;27:464–472. doi:10.1002/pds.4366
14. Webster LR. Risk factors for opioid-use disorder and overdose. Anesth Analg. 2017;125:1741–1748. doi:10.1213/ANE.0000000000002496
15. Centers for Disease Control and Prevention. Vital signs: overdoses of prescription opioid pain relievers---United States, 1999--2008. MMWR Morb Mortal Wkly Rep. 2011;60:1487–1492.
16. Sabesan VJ, Shahriar R, Petersen-Fitts GR, et al. A prospective randomized controlled trial to identify the optimal postoperative pain management in shoulder arthroplasty: liposomal bupivacaine versus continuous interscalene catheter. J Shoulder Elbow Surg. 2017;26:1810–1817. doi:10.1016/j.jse.2017.06.044
17. Patel MA, Gadsden JC, Nedeljkovic SS, et al. Brachial plexus block with liposomal bupivacaine for shoulder surgery improves analgesia and reduces opioid consumption: results from a multicenter, randomized, double-blind, controlled trial. Pain Med. 2020;21:387–400. doi:10.1093/pm/pnz103
18. Kim YS, Park Y, Koh HJ, Hebelka H, Lagerstrand KM. Is there a difference between perineural dexamethasone with single-shot interscalene block (SSIB) and interscalene indwelling catheter analgesia (IICA) for early pain after arthroscopic rotator cuff repair? A pilot study. J Clin Med. 2022;12:11. doi:10.3390/jcm12010011
19. Maher A, Leigh W, Young S, et al. Do age, demographics, and tear characteristics affect outcomes after rotator cuff repair? Results of Over 2000 rotator cuff repairs at 5-year follow-up. Orthop J Sports Med. 2022;10:23259671221119222. doi:10.1177/23259671221119222
20. Daniels SD, Stewart CM, Garvey KD, et al. Sex-based differences in patient-reported outcomes after arthroscopic rotator cuff repair. Orthop J Sports Med. 2019;7:2325967119881959. doi:10.1177/2325967119881959
21. Downie WW, Leatham PA, Rhind VM, et al. Studies with pain rating scales. Ann Rheum Dis. 1978;37:378–381. doi:10.1136/ard.37.4.378
22. Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005;14:798–804. doi:10.1111/j.1365-2702.2005.01121.x
23. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3:347–352. doi:10.1016/S1058-2746(09)80019-0
24. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;1987:160–164.
25. Wu XL, Baldwick C, Briggs L, et al. Arthroscopic undersurface rotator cuff repair. Tech Shoulder Elb Surg. 2009;10:112–118. doi:10.1097/BTE.0b013e3181ac1a92
26. Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–1625. doi:10.1016/S0140-6736(06)68700-X
27. Hoofwijk DM, Fiddelers AA, Peters ML, et al. Prevalence and predictive factors of chronic postsurgical pain and poor global recovery 1 year after outpatient surgery. Clin J Pain. 2015;31:1017–1025. doi:10.1097/AJP.0000000000000207
28. Zhou Y, Shi W, Zhao D, et al. Identification of immune-associated genes in diagnosing aortic valve calcification with metabolic syndrome by integrated bioinformatics analysis and machine learning. Front Immunol. 2022;13:937886. doi:10.3389/fimmu.2022.937886
29. Barrington JW, Lovald ST, Ong KL, et al. Postoperative pain after primary total knee arthroplasty: comparison of local injection analgesic cocktails and the role of demographic and surgical factors. J Arthroplasty. 2016;31:288–292. doi:10.1016/j.arth.2016.05.002
30. van Dijk JFM, Zaslansky R, van Boekel RLM, et al. Postoperative pain and age: a retrospective cohort association study. Anesthesiology. 2021;135:1104–1119. doi:10.1097/ALN.0000000000004000
31. Bellville JW, Forrest WH, Miller E, et al. Influence of age on pain relief from analgesics. A study of postoperative patients. JAMA. 1971;217:1835–1841. doi:10.1001/jama.1971.03190130039008
32. Verdú E, Ceballos D, Vilches JJ, et al. Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst. 2000;5:191–208. doi:10.1111/j.1529-8027.2000.00026.x
33. Kaliappan S, Simone DA, Banik RK. Nonlinear Inverted-U shaped relationship between aging and epidermal innervation in the rat plantar hind paw: a laser scanning confocal microscopy study. J Pain. 2018;19:1015–1023. doi:10.1016/j.jpain.2018.04.002
34. Simone DA, Nolano M, Johnson T, et al. Intradermal injection of capsaicin in humans produces degeneration and subsequent reinnervation of epidermal nerve fibers: correlation with sensory function. J Neurosci. 1998;18:8947–8959. doi:10.1523/JNEUROSCI.18-21-08947.1998
35. Hoving JL, de Vet HCW, Twisk JWR, et al. Prognostic factors for neck pain in general practice. Pain. 2004;110:639–645. doi:10.1016/j.pain.2004.05.002
36. Bot SD, van der Waal JM, Terwee CB, et al. Predictors of outcome in neck and shoulder symptoms: a cohort study in general practice. Spine. 2005;30:E459–70. doi:10.1097/01.brs.0000174279.44855.02
37. McCarberg B, Peppin J. Pain pathways and nervous system plasticity: learning and memory in pain. Pain Med. 2019;20(12):2421–2437. doi:10.1093/pm/pnz017
38. Dunn WR, Kuhn JE, Sanders R, et al. Symptoms of pain do not correlate with rotator cuff tear severity: a cross-sectional study of 393 patients with a symptomatic atraumatic full-thickness rotator cuff tear. J Bone Joint Surg Am. 2014;96:793–800. doi:10.2106/JBJS.L.01304
39. Ackermann PW, Alim MA, Pejler G, et al. Tendon pain - what are the mechanisms behind it? Scand J Pain. 2023;23:14–24. doi:10.1515/sjpain-2022-0018
40. Shih CA, Wu KC, Shao CJ, et al. Synovial fluid biomarkers: association with chronic rotator cuff tear severity and pain. J Shoulder Elbow Surg. 2018;27:545–552. doi:10.1016/j.jse.2017.09.020
41. Longo UG, Berton A, Papapietro N, et al. Epidemiology, genetics and biological factors of rotator cuff tears. Med Sport Sci. 2012;57:1–9.
42. Afari N, Mostoufi S, Noonan C, et al. C-reactive protein and pain sensitivity: findings from female twins. Ann Behav Med. 2011;42:277–283. doi:10.1007/s12160-011-9297-6
43. Hodges S, Guler S, Sacca V, et al. Associations among acute and chronic musculoskeletal pain, sleep duration, and C-reactive protein (CRP): a cross-sectional study of the UK biobank dataset. Sleep Med. 2023;101:393–400. doi:10.1016/j.sleep.2022.11.013
44. Laird E, McNulty H, Ward M, et al. Vitamin D deficiency is associated with inflammation in older Irish adults. J Clin Endocrinol Metab. 2014;99:1807–1815. doi:10.1210/jc.2013-3507
45. Krasowska K, Skrobot W, Liedtke E, et al. The Preoperative supplementation with vitamin D attenuated pain intensity and reduced the level of pro-inflammatory markers in patients after posterior lumbar interbody fusion. Frontiers in pharmacology. 2019;10:527. doi:10.3389/fphar.2019.00527
46. Baumgarten KM, Chang PS. The American shoulder and elbow surgeons score highly correlates with the simple shoulder test. J Shoulder Elbow Surg. 2021;30:707–711. doi:10.1016/j.jse.2020.07.015
47. Baumgarten KM, Barthman BJ, Chang PS. The American shoulder and elbow score is highly correlated with the western Ontario rotator cuff index and has less responder and administrator burden. Arthrosc Sports Med Rehabil. 2021;3:e1637–e1643. doi:10.1016/j.asmr.2021.07.019
48. Berliner JL, Brodke DJ, Chan V, et al. Can preoperative patient-reported outcome measures be used to predict meaningful improvement in function after TKA? Clin Orthop Relat Res. 2017;475:149–157. doi:10.1007/s11999-016-4770-y
49. Berliner JL, Brodke DJ, Chan V, et al. John Charnley award: preoperative patient-reported outcome measures predict clinically meaningful improvement in function after THA. Clin Orthop Relat Res. 2016;474:321–329. doi:10.1007/s11999-015-4350-6
50. Salazar VS, Gamer LW, Rosen V. BMP signalling in skeletal development, disease and repair. Nat Rev Endocrinol. 2016;12:203–221. doi:10.1038/nrendo.2016.12
51. Burkus JK, Transfeldt EE, Kitchel SH, et al. Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2. Spine. 2002;27:2396–2408. doi:10.1097/00007632-200211010-00015
52. Cho M, You KH, Yeom JS, et al. Mid-term efficacy and safety of Escherichia coli-derived rhBMP-2/hydroxyapatite carrier in lumbar posterolateral fusion: a randomized, multicenter study. Eur Spine J. 2023;32:353–360. doi:10.1007/s00586-022-07440-3
53. Greiner S, Ide J, Van Noort A, et al. Local rhBMP-12 on an absorbable collagen sponge as an adjuvant therapy for rotator cuff repair - a phase 1, randomized, standard of care control, multicenter study: safety and Feasibility. Am J Sports Med. 2015;43:1994–2004. doi:10.1177/0363546515584756
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

