Back to Journals » Cancer Management and Research » Volume 12
Validation of Risk of Chemotherapy-Induced Neutropenia: Experience from Oncology Hospital of Nepal
Authors Sapkota B , Shrestha R, Chapagai S, Shakya DK , Bista P
Received 27 December 2019
Accepted for publication 7 May 2020
Published 20 May 2020 Volume 2020:12 Pages 3751—3758
DOI https://doi.org/10.2147/CMAR.S243916
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Beicheng Sun
Binaya Sapkota,1 Ronash Shrestha,2,* Safin Chapagai,1,* Dip Kiran Shakya,1,* Prashant Bista1,*
1Nobel College Faculty of Health Sciences, Department of Pharmaceutical Sciences, Sinamangal, Kathmandu, Nepal; 2Aadee Remedies Pvt. Ltd, Imadol, Lallitpur, Nepal
*These authors contributed equally to this work
Correspondence: Binaya Sapkota
Nobel College Faculty of Health Sciences, Department of Pharmaceutical Sciences, Sinamangal, Kathmandu, Nepal
Email [email protected]
Background: The majority of cancer patients undergoing chemotherapy show neutropenic condition which is a common side effect of myelosuppressive chemotherapy diagnosed as the reduced complete blood cell count. Such cancer patients have a higher risk of febrile neutropenia. The present study aimed to validate whether there was a risk of neutropenia in cancer patients receiving chemotherapy at Bhaktapur Cancer Hospital, Nepal.
Methods: Cross-sectional study was performed among 203 cancer patients of all age groups who attended Bhaktapur Cancer Hospital from May 2018 to January 2019 and who received a chemotherapy course. Patients receiving at least one cycle of chemotherapy as the first-line treatment were included. Statistical analysis was performed using SPSS 25. Loglinear analysis was used to analyze more than 2× 2 categories among the grades and outcome of neutropenia. Multinomial logistic regression was applied to analyze the impact of various predictor variables such as chemotherapy cycles, grades of neutropenia, and gender on the outcome of neutropenia. Variation in the absolute neutrophil count (ANC) level at various days of chemotherapy cycles was assessed with the multivariate analysis of variance (MANOVA). The p-value < 0.05 was considered significant at each condition.
Results: The main cancer type during the study period was breast cancer (41, 20.2%). Out of 163 neutropenic patients, 149 had severe neutropenia and 14 had mild neutropenia. Most patients were continued up to the 6th cycle of chemotherapy. There was significant association between the grade of neutropenia and the outcome of the condition (p-value 0.017). There were significant relations of the grade of neutropenia and smoking habit with the recovering status (p values 0.033 and 0.001, respectively). The absolute neutrophil count (ANC) level increased and decreased inconsistently (statistically non-significantly) in between treatment period of day 1 to 52.
Conclusion: Chemotherapy-induced neutropenia was a common occurrence. Majority (133, 66.5%) grade 4 neutropenic patients were recovering after the chemotherapy cycles. The physicians are warranted that they be ready for any unpredictable situation during chemotherapy treatment.
Keywords: chemotherapy-induced neutropenia, chemotherapy, febrile neutropenia, myelosuppressive, Nepal
Background
Globally, there are an estimated 25 million cancer cases as per the World Health Organization (WHO)’s World Cancer Report 2014.1 Neutropenia is one of the principal adverse events (AE) of antineoplastic chemotherapy and may lead to increased morbidity, mortality, and treatment costs.2,3 One of the severe forms, ie, febrile neutropenia (FN) is the incidence of fever throughout a period of severe neutropenia.4 When a neutropenic patient develops a fever, a cardinal sign of infection results in hospitalization for urgent evaluation, monitoring, and administration of intravenous antibiotics.5 Low baseline white blood cell, neutrophil counts, hemoglobin levels, and specific chemotherapy regimen are the important predictors for FN.6,7 Some regimens are more myelotoxic than others. For example, combined cyclophosphamide, methotrexate, and 5-fluorouracil are less toxic than the combination of doxorubicin (also known as adriamycin) and cyclophosphamide (combinedly known as AC). Both of these combinations are preferred in elderly patients with breast carcinoma.8 The risk of FN is greater during the first cycle of cancer treatment.7,9
Neutropenia is diagnosed by a routine complete blood count (CBC), with the accompanying differential count yielding a decrease in the absolute neutrophil count (ANC). Mild neutropenia is defined as an ANC of less than 1500 cells/mm3. A count of fewer than 1000 cells/mm3 is considered moderate; less than 500 cells/mm3 represents the severe degree of neutropenia.6 Management of neutropenia is done with chemotherapy dose modification, dose interval delays, and/or the initiation of primary prophylaxis with recombinant granulocyte-colony stimulating factors (G-CSFs) based on the individualized FN risk assessment of the patient and chemotherapy regimen.10 The National Cancer Institute Common Toxicity Criteria is the most commonly used scale for grading the severity of cytopenia associated with cancer chemotherapy; it describes neutropenia into 4 grades as shown in Table 1.11
 |
Table 1 Grades of Neutropenia |
Patients who are treated with chemotherapy are at risk for the development of neutropenic complications.6 Risk factors for neutropenia may be either patient-specific or regimen-specific; patient-specific being predominately important in cancer treatment as the chemotherapy regimens are standardized by the types of cancer. Patient-specific risk factors include not only the type of cancer but also the disease stage and age.12,13
Chemotherapy-induced neutropenia (CIN) affects the quality of life (QoL) and shows risk in people of different age groups. CIN is an important prognostic factor in metastatic colon cancer patients.14 Weycker et al5 concluded that patients hospitalized for chemotherapy-induced FN can be identified in health-care databases but they may have misclassification using the diagnosis code for neutropenia.5 Lyman et al surveyed to develop and validate a risk model for neutropenic complications in a large prospective cohort of cancer patients receiving chemotherapy.7 Crawford et al13 performed research on CIN, its complications, and impact to manage patients with cancer treated with myelosuppressive chemotherapy and identified that age, performance status, nutritional status, chemotherapy dose intensity, and low baseline blood cell counts as the different risk factors for severe FN.13
Neutropenic condition is managed by various methods of treatments including the use of antibiotics. Besides the FN, neutropenic colitis (also known as typhlitis) is also the complication of neutropenia. Patients with FN are at a higher risk of increased mortality and this neutropenic complication is known as an oncologic emergency.7 Therefore, the current study aimed to validate whether there was a risk of neutropenia in cancer patients receiving chemotherapy at one of the oldest oncology-based hospitals of Nepal, ie, Bhaktapur Cancer Hospital, Bhaktapur, Nepal.
Methods
Study Design, Study Site, and Study Population
A cross-sectional study was conducted at Bhaktapur Cancer Hospital, situated at Bhaktapur district, Nepal, from May 2018 to January 2019. It is one of the specialized tertiary cancer treatment centers in Nepal. It is equipped with state-of-the-art technology and most innovative, advanced therapies of cancer in Nepal. Patients of all age groups (eg, baby, young adults, middle-aged adults, old adults) suffering from cancer and under treatment were taken for the study. Patients’ medical records along with their laboratory parameters, chemotherapy cycles were extracted. Also, some patients came to the OPD of the hospital during the study period.
Sample Size
We included a total number of 203 cancer patients for the research purpose, taking into consideration the prevalence of 0.5, precision of 0.07, and z value of 1.96. The inclusion and exclusion criteria of the current study are as follows:
Inclusion Criteria
- Cytological or histologically diagnosed cancer patients who were not eligible for operation
- Patients receiving at least one cycle of chemotherapy as the first-line treatment
- Patients with sufficient bone marrow function and with no bone marrow metastasis
- Patients with normal hepatic and renal function
Exclusion Criteria
- Incomplete data of toxicities due to chemotherapy
- Lost follow-up
- Treatment uncooperative patients after 1 cycle
Data Collection Tools and Technique
Clinical data and information of the patients were retrieved from the medical records of the patients admitted in the hospital for chemotherapy and whose all blood tests and treatment were performed as per the institutional guidelines. Patients’ diagnosis was presented as in Appendix 1 and data collection sheet in Appendix 2.
Grades of Neutropenia
Grades of neutropenia are categorized in Table 1 based on the absolute neutrophil count.11 Outcome of neutropenia was categorized as unknown, recovering, recovered, and critical. No information about neutropenia status was categorized as unknown; neutrophil count progressing towards normal after chemotherapy was categorized as recovering; neutrophil count that became normal after chemotherapy was categorized as recovered; and the lowest possible neutrophil count was categorized as critical.
Absolute Neutrophil Count Calculation
All patients were daily checked for complete blood count (CBC)/differential count (DC) after the chemotherapy, on the basis of which the absolute neutrophil count (ANC) was calculated for each day. The ANC was calculated based on the following formula:
ANC (cells/mm3) = [(Neutrophil % + Band Cells %) × Total WBC]/10015
Since there was no information on band cells, we kept zero on this.
Ethics Approval
The study protocol was approved by the Institutional Review Committee of Nobel College (affiliated to Pokhara University) (ID: PYIRC/176/2018) and data collection approval was granted from Bhaktapur Cancer Hospital authority prior to data collection. Patients’ confidentiality was highly maintained. Patients’ informed consent was waived by the Review Committee as this was the retrospective study performed from the patients’ medical records.
Statistical Analysis
Statistical analysis was performed using IBM SPSS version 25. Loglinear analysis was used to analyze the categorical variables with more than 2×2 categories. Multinomial logistic regression was applied to analyze the impact of various predictor variables on categorical outcome variable (with more than two categories), considering the principle of the parsimony. Variation in the ANC level at various days of chemotherapy cycles was assessed with the multivariate analysis of variance (MANOVA). The p-value <0.05 was considered significant at each condition.
Results
Sociodemographic characteristics of the study population are shown in Tables 2 and 3. A total of 203 cancer patients, who met the eligibility criteria, were included in the analysis. The mean age of the patients was 45.34 (±18.432). Patients were in the age range of 5–85 years. The majority of the people diagnosed with cancer were mainly young and middle-aged adults, females and persons with blood groups A+ve and O+ve. Cancer was diagnosed more on patients with no smoking and no alcohol consumption habit (Table 2). The most commonly diagnosed cancer was breast cancer (20.2%) followed by non-Hodgkin’s lymphoma (14.3%) (Appendix 1).
 |
Table 2 Demographic Characteristics of Study Population (n = 203) |
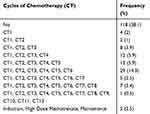 |
Table 3 Cycles of Chemotherapy |
Cycles of chemotherapy that were mostly continued were up to CT6 cycle (Table 3). There was a significant association between the grade of neutropenia and the outcome of the condition among the study participants (p-value 0.017); ie, a majority (133, 66.5%) grade 4 neutropenic patients were recovering after the chemotherapy cycles (Table 4).
 |
Table 4 Loglinear Analysis Between a Grade of Neutropenia and Outcome of Neutropenia (with a Cycle of Chemotherapy) |
There were significant relations of the grade of neutropenia and smoking habit with the recovering status (p values 0.033 and 0.001, respectively) (Table 5). The variation in the ANC level was significant at days 13, 14, 19, 28, 40, 47, and 48 days of chemotherapy (p values 0.008, 0.024, 0.010, 0.048, 0.018, 0.019, and 0.012, respectively). The ANC level increased some days and decreased some days (but in statistically non-significant ways) in between those days, beginning from day 1 to 52 (Table 6).
 |
Table 5 Multinomial Logistic Regression of the Outcome of Neutropenia with Various Predictor Variablesa |
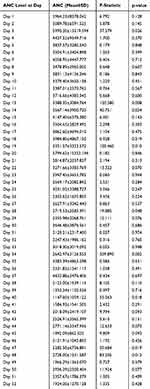 |
Table 6 Multivariate Analysis of Variance of the ANC Level at Various Days of Chemotherapy Cycles |
Discussion
In the present study, a fairly large number of CIN was associated with older age (46, 22.7% among 36–45 years). Increasing age is related to the more risk of CIN due to immunosenescence phenomena (ie, natural, gradual deterioration of the immune system with aging).14,16 Elderly patients may have reduced bone marrow reserves or declined renal and hepatic function, and are more prone to treatment-related complications.17,18 Thus, with age, the benefits of cancer chemotherapy may be reduced and the risks may be increased.
Epidemiological data have identified chronic alcohol consumption as a significant risk factor for upper alimentary tract cancer, including cancer of oropharynx, larynx, esophagus, and liver because acetaldehyde is predominantly responsible for alcohol-associated carcinogenesis.19–21 Combined exposure to alcohol and tobacco consumption showed a greater than multiplicative synergistic effect.22 Alcohol ingestion is associated with an increased risk of breast cancer among postmenopausal women.23 However, the present research showed indirect proportion between alcohol consumption and cancer.
In the present study, the majority of the cancer patients were in the blood groups A+ve, O+ve, and AB+ve. For several decades, ABO type antigens were suspected to have a role in the development of cancer. Aird et al24 explored the association of peptic ulcer and gastric carcinoma with ABO blood types.25 Huang et al also concluded the association of ABO blood types in the development of gastrointestinal and urinary tract cancers.24
In this study, we observed the neutropenic and non-neutropenic conditions of the patients receiving chemotherapy and also calculated the grade of neutropenia during chemotherapy. Out of 203 patients, 163 were suffered from neutropenia during chemotherapy, 14 of them suffered from grade 1 whereas 149 from grade 4 neutropenia (ie, severe neutropenia). Fortner et al found that neutropenia-related hospitalizations prevented the patients from normal activities provided useful information for future modeling of additional factors such as disease status and chemotherapy schedule.26 Hashiguchi et al (2015) reported maximum number of chemotherapy-induced neutropenia from chemotherapy cycles 1–5.27 Nearly similar was the case here in our research as well where we had maximum number of cycles that were up to 6th cycle. Li et al (2016) also concluded that there was very limited information and insight on the quantitative relationships between severity and duration of CIN.28
Educational programs directed toward this population may reduce the risk of preventable infections and decrease morbidity and mortality.29 Similarly, the involvement of clinical pharmacists can improve patient care.30 Since the grade of neutropenia and outcome of the condition were significantly associated with each other (p-value 0.017), ie, majority (133, 66.5%) grade 4 neutropenic patients were recovering after the chemotherapy cycles, prediction of neutropenic attack could have been made. This helps the physicians better prepare for the aggravating condition if any. The present study showed infrequent increment and decrement of the ANC level in between the whole treatment period from day 1 to 52.
Limitations
The present study was single-centric, hence, necessitating future multi-centric studies of a similar nature. Also, the cross-sectional nature of the study might have limited the exact cause–effect relationship among the risk factor and the outcome of interest, ie, neutropenia. Therefore, a cohort study of a similar type might be warranted with the findings of the present study.
Conclusion
The present study documented the CIN but the phenomenon is not in the unidirectionally ordered manner, ie, increasing and decreasing in between. This might warrant the physicians that they be ready for any unpredictable situation during chemotherapy treatment.
Abbreviations
AE, adverse events; ANC, absolute neutrophil count; CBC, complete blood count; CIN, chemotherapy-induced neutropenia; FN, febrile neutropenia; G-CSFs, granulocyte-colony stimulating factors; QoL, quality of life; WHO, World Health Organization.
Data Sharing Statement
The data used in the current study available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The study protocol was approved by the Institutional Review Board of Nobel College (ID: PYIRC/176/2018). Patients’ informed consent was waived by the Review Committee as this was the retrospective study performed from the patients’ medical records.
Acknowledgment
The authors would like to acknowledge all cancer patients who showed their willingness to participate in the present research. The authors are also thankful to clinical and administrative team of Bhaktapur Cancer Hospital for their kind support throughout the study. We are thankful to the software engineer Samit Ghimire for his contribution in information technology-related issues.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Stewart BW, Wild CP. World Cancer Report 2014. World Health Organization and International Agency for Research on Cancer; 2014:1–630.
2. Lyman GH, Lyman CH, Agboola O. Risk models for predicting chemotherapy-induced neutropenia. Oncologist. 2005;10:427–437. doi:10.1634/theoncologist.10-6-427
3. Schwenkglenks M, Jackisch C, Constenla M, et al. Neutropenic event risk and impaired chemotherapy delivery in six European audits of breast cancer treatment. Support Care Cancer. 2006;14:901–909. doi:10.1007/s00520-006-0034-9
4. Patel K, West H. Febrile neutropenia. JAMA Oncol. 2017;3(12):1751. doi:10.1001/jamaoncol.2017.1114
5. Weycker D, Sofrygin O, Seefeld K, Deeter RG, Legg J, Edelsberg J. Technical evaluation of methods for identifying chemotherapy-induced febrile neutropenia in healthcare claims databases. BMC Health Serv Res. 2013;13:60. doi:10.1186/1472-6963-13-60
6. Lyman GH. Impact of chemotherapy dose intensity on cancer patient outcomes. J Natl Compr Canc Netw. 2009;7(1):99–108. doi:10.6004/jnccn.2009.0009
7. Lyman GH, Kuderer NM, Crawford J, et al. Predicting individual risk of neutropenic complications in patients receiving cancer chemotherapy. Cancer. 2011;117(9):1917–1927. doi:10.1002/cncr.25691
8. Lyman GH, Kuderer NM, Balducci L. Cost-benefit analysis of granulocyte colony-stimulating factor in the management of elderly cancer patients. Curr Opin Hematol. 2002;9:207–214. doi:10.1097/00062752-200205000-00006
9. Crawford J, Dale DC, Kuderer NM, et al. Risk and timing of neutropenic events in adult cancer patients receiving chemotherapy: the results of a prospective nationwide study of oncology practice. J Natl Compr Cancer Netw. 2008;6(2):109–118. doi:10.6004/jnccn.2008.0012
10. Lustberg MB. Management of neutropenia in cancer patients. Clin Adv Hematol Oncol. 2012;10(12):825–826.
11. Muturi-Kioi V, Lewis D, Launay O, et al. Neutropenia as an Adverse Event following Vaccination: Results from Randomized Clinical Trials in Healthy Adults and Systematic Review. PLoS One. 2016;11(8):e0157385.
12. Shayne M, Crawford J, Dale DC, Culakova E, Lyman GH. Predictors of reduced dose intensity in patients with early-stage breast cancer receiving adjuvant chemotherapy. Breast Cancer Res Treat. 2006;100(3):255–262. doi:10.1007/s10549-006-9254-4
13. Crawford J, Dale DC, Lyman GH. Chemotherapy‐induced neutropenia: risks, consequences, and new directions for its management. Cancer. 2004;100(2):228–237. doi:10.1002/cncr.11882
14. Rambach L, Bertaut A, Vincent J, Lorgis V, Ladoire S, Ghiringhelli F. Prognostic value of chemotherapy-induced hematological toxicity in metastatic colorectal cancer patients. World J Gastroenterol. 2014;20(6):1565–1573. doi:10.3748/wjg.v20.i6.1565
15. ClinicalAdvisor.com. Absolute neutrophil count calculator. Haymarket Media, Inc. Available from: https://www.clinicaladvisor.com/home/my-practice/medical-calculators/absolute-neutrophil-count-calculator/.
16. Lyman GH, Abella E, Pettengell R. Risk factors for febrile neutropenia among patients with cancer receiving chemotherapy: a systematic review. Crit Rev Oncol/Hematol. 2014;90(3):190–199. doi:10.1016/j.critrevonc.2013.12.006
17. Balducci L, Hardy CL, Lyman GH. Hemopoiesis and aging. Cancer Treat Res. 2005;124:109–134.
18. Shrestha S, Shrestha S, Khanal S. Polypharmacy in elderly cancer patients: challenges and the way clinical pharmacists can contribute in resource-limited settings. Aging Med. 2019;2(1):42–49. doi:10.1002/agm2.12051
19. Pöschl G, Seitz HK. Alcohol and Cancer. Alcohol Alcohol. 2004;39(3):155–165. doi:10.1093/alcalc/agh057
20. Bagnardi V, Blangiardo M, Vecchia CL, Corrao G. Alcohol consumption and the risk of cancer: a meta-analysis. Alcohol Res. 2001;25(4):263.
21. Rehm J, Shield KD. Alcohol and mortality: global alcohol-attributable deaths from cancer, liver cirrhosis, and injury in 2010. Alcohol Res. 2014;35(2):174–183.
22. Goldstein BY, Chang S, Hashibe M, Vecchia CL, Zhang Z. Alcohol consumption and cancer of the oral cavity and pharynx from 1988 to 2009: an update. Eur J Cancer Prev. 2010;19:431–465. doi:10.1097/CEJ.0b013e32833d936d
23. Dorgan JF, Baer DJ, Albert PS, et al. Serum hormones and the alcohol–breast cancer association in postmenopausal women. J Natl Cancer Inst. 2001;93(9):710–715. doi:10.1093/jnci/93.9.710
24. Aird I, Bentall HH, Mehigan JA, Roberts JAF. The blood groups in relation to peptic ulceration and carcinoma of colon, rectum, breast, and bronchus. Br Med J. 1954;2(4883):315–321. doi:10.1136/bmj.2.4883.315
25. Huang JY, Wang R, Gao Y, Yuan J. ABO blood type and the risk of cancer–findings from the Shanghai Cohort Study. PLoS One. 2017;12(9):e0184295. doi:10.1371/journal.pone.0184295
26. Fortner BV, Tauer K, Zhu L, et al. Medical visits for chemotherapy and chemotherapy-induced neutropenia: a survey of the impact on patient time and activities. BMC Cancer. 2004;4:22. doi:10.1186/1471-2407-4-22
27. Hashiguchi Y, Kasai M, Fukuda T, Ichimura T, Yasui T, Sumi T. Chemotherapy-induced neutropenia and febrile neutropenia in patients with gynecologic malignancy. Anticancer Drugs. 2015;26:1054–1060. doi:10.1097/CAD.0000000000000279
28. Li Y, Klippel Z, Shih X, Reiner M, Wang H, Page JH. Relationship between severity and duration of chemotherapy-induced neutropenia and risk of infection among patients with nonmyeloid malignancies. Support Care Cancer. 2016;24:4377–4383. doi:10.1007/s00520-016-3277-0
29. Dunbar A, Tai E, Nielsen DB, Shropshire S, Richardson LC. Preventing infections during cancer treatment: development of an interactive patient education website. Clin J Oncol Nurs. 2014;18(4):426–431. doi:10.1188/14.CJON.426-431
30. Shrestha S, Shrestha S, Palaian S. Can clinical pharmacists bridge a gap between medical oncologists and patients in resource-limited oncology settings? An experience in Nepal. J Oncol Pharm Pract. 2019;25(3):765–768. doi:10.1177/1078155218784734
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
