Back to Journals » Clinical Ophthalmology » Volume 16
Treatment of Open-Angle Glaucoma and Ocular Hypertension with the Fixed-Dose Combination of Preservative-Free Tafluprost/Timolol: Clinical Outcomes from Ophthalmology Clinics in Italy
Authors Oddone F, Scorcia V , Iester M, Sisto D, De Cilla S, Bettin P, Cagini C , Figus M , Marchini G , Rossetti L, Rossi G, Salgarello T, Scuderi GL, Staurenghi G
Received 17 March 2022
Accepted for publication 11 May 2022
Published 1 June 2022 Volume 2022:16 Pages 1707—1719
DOI https://doi.org/10.2147/OPTH.S364880
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Francesco Oddone,1 Vincenzo Scorcia,2 Michele Iester,3 Dario Sisto,4 Stefano De Cilla,5 Paolo Bettin,6 Carlo Cagini,7 Michele Figus,8 Giorgio Marchini,9 Luca Rossetti,10 Gemma Rossi,11 Tommaso Salgarello,12,13 Gian Luca Scuderi,14 Giovanni Staurenghi15 On behalf of the VISIONARY Study Group (Italy)
1Glaucoma Unit, IRCSS-Fondazione Bietti, Roma, Italy; 2Department of Ophthalmology, University Magna Græcia of Catanzaro, Catanzaro, Italy; 3Eye Clinic of Genoa, Department of Neuroscience, Rehabilitation, Ophthalmology, Genetics, Maternal and Child Health (DiNOGMI), University of Genova, Genova, Italy; 4Ophthalmology Department, University of Bari, Bari, Italy; 5Department of Health Sciences, Eye Clinic, University of Piemonte Orientale, Novara, Italy; 6Department of Ophthalmology, IRCCS San Raffaele Scientific Institute, Vita-Salute San Raffaele University, Milano, Italy; 7Department of Medicine and Surgery, Ophthalmology Section, University of Perugia, Perugia, Italy; 8Ophthalmology, Department of Surgery, Medicine, Molecular and Emergency, University of Pisa, Pisa, Italy; 9Ophthalmology Unit, Department of Neurosciences, Biomedicine and Movement, University of Verona, Verona, Italy; 10Eye Clinic, San Paolo Hospital, University of Milan, Milano, Italy; 11University Eye Clinic, Fondazione IRCCS Policlinico S. Matteo, Pavia, Italy; 12Ophthalmology Unit, Department of Ageing, Neurosciences, Head-Neck and Orthopaedics Sciences, Fondazione Policlinico Universitario A. Gemelli IRCCS, Roma, Italy; 13Institute of Ophthalmology, Università Cattolica del Sacro Cuore, Roma, Italy; 14NESMOS Department, Ophthalmology Unit, St. Andrea Hospital, Università di Roma La Sapienza, Roma, Italy; 15Eye Clinic, Department of Biomedical and Clinical Sciences Luigi Sacco, University of Milan, Milano, Italy
Correspondence: Francesco Oddone, Glaucoma Unit, IRCSS-Fondazione Bietti, Roma, Italy, Tel + 39 06 85356727, Email [email protected]
Introduction: The VISIONARY study examined the intraocular pressure (IOP)-lowering efficacy and tolerability of the preservative-free fixed-dose combination of tafluprost (0.0015%) and timolol (0.5%) (PF tafluprost/timolol FC) in a real-world setting. The country-level data reported herein comprise the largest and first observational study of PF tafluprost/timolol FC therapy in Italy.
Methods: An observational, multicenter, prospective study included adult Italian patients with open-angle glaucoma (OAG) or ocular hypertension (OHT) demonstrating insufficient response or poor tolerability with topical prostaglandin analogue (PGA) or beta-blocker monotherapy. Treatment was switched to PF tafluprost/timolol FC therapy at baseline. Primary endpoint was the absolute mean IOP change from baseline at Month 6. Exploratory and safety endpoints included change in IOP at Weeks 4 and 12, ocular signs, symptom severity and reporting of adverse events (AEs).
Results: Overall, 160 OAG/OHT patients were included. Mean ± standard deviation IOP was reduced from 19.6 ± 3.6 mmHg at baseline to 14.5 ± 2.6 mmHg at Month 6 (reduction of 5.1 ± 3.7 mmHg; 24.1%; p < 0.0001). IOP reduction was also statistically significant at Week 4 (23.1%; p < 0.0001) and Week 12 (24.7%; p < 0.0001). Based on data cutoff values for mean IOP change of ≥ 20%, ≥ 25%, ≥ 30% and ≥ 35%, respective Month 6 responder rates were 68.1%, 48.7%, 36.2% and 26.9%. Most ocular signs and symptoms were significantly reduced in severity from baseline at Month 6. Two non-serious and mild AEs were reported during the study period, among which, one AE was treatment-related (eyelash growth).
Conclusion: Italian OAG and OHT patients demonstrated a significant IOP reduction from baseline at Week 4 that was maintained over a 6-month period following a switch from topical PGA or beta-blocker monotherapy to PF tafluprost/timolol FC therapy. Severity of most ocular signs and symptoms was significantly reduced during the study period, and PF tafluprost/timolol FC was generally well tolerated.
Keywords: fixed-dose combination therapy, intraocular pressure, ocular hypertension, PF tafluprost/timolol FC, preservative-free topical medication, primary open-angle glaucoma
Introduction
Glaucoma is a leading global cause of blindness.1,2 An estimated 76 million people worldwide are currently diagnosed with open-angle glaucoma (OAG) or angle closure glaucoma, and this number has been projected to reach 112 million by the year 2040.1,2 Approximately 3% of the global population aged between 40 and 80 years are believed to have primary open-angle glaucoma (POAG), with prevalence ranging between 2.3% in Asia and 4.2% in Africa.1,2 Elevated intraocular pressure (IOP) is considered the most important treatable risk factor for disease development and progression.2–7 Reduction of IOP may slow down visual field loss and deterioration in vision-related quality of life.3–7
Topical IOP-lowering therapy is the most common first-line treatment for OAG.2 When IOP is insufficiently controlled using topical monotherapy, combination therapies containing a prostaglandin analogue (PGA) and beta-blocker are frequently prescribed.2,8–15 Treatment must balance IOP-lowering efficacy with tolerability since ocular surface disease (OSD) is common among people with glaucoma and can affect patient compliance.2,16–28 Interventions that improve ocular surface health and reduce exposure to toxic topical agents may enhance IOP control.18–20 Topical treatments containing preservative agents, such as benzalkonium chloride (BAK), can cause tolerability issues at the ocular surface.21–29 Preservative-free (PF) fixed-dose combination (FC) therapies reduce the number of daily medication instillations required, compared with administration of multiple monotherapies, without exposing the ocular surface to preservative agents.2,8,10–12 The PF FC formulation of tafluprost 0.0015% and timolol 0.5% (PF tafluprost/timolol FC) has demonstrated powerful IOP-lowering efficacy and a good tolerability profile in the treatment of OAG and OHT in randomized controlled trials (RCTs) and real-world observational studies.11,30–37 Although RCTs remain the gold standard for regulatory assessment and approval, restrictive inclusion criteria prevent exploration of certain facets of routine clinical practice and a high proportion of patients who may be considered appropriate for a particular treatment in the clinic would not be included in such tightly controlled studies.38–40 Real-world studies are more relevant in understanding how patients may tolerate and respond to direct medication changes in clinical situations and provide important pharmacovigilance information.35,37,39
This article reports country-level real-world data from Italy concerning a multicenter, European, observational, 6-month study (the VISIONARY study) that evaluated the IOP-lowering effectiveness, tolerability and safety of PF tafluprost/timolol FC therapy in patients with OAG and OHT, who were previously treated with either a topical PGA or beta-blocker monotherapy.35 Europe-wide data from the VISIONARY study demonstrated significant IOP reductions of up to 5.4 mmHg (approximately 24%) with PF tafluprost/timolol FC therapy from Week 4 that were maintained through Month 6 (p<0.0001).35 However, country-level data already published from the UK, Hungary, Russia and Spain revealed variations in baseline IOP and the relative change in IOP, probably because baseline pressure typically predicts treatment outcome with topical glaucoma therapy.41–45 This suggests that ophthalmologists in some countries selected patients with differing baseline IOPs for treatment intensification, which may relate to local clinical practice or treatment pathways.41–44 The current analysis represents the largest study of PF tafluprost/timolol FC treatment conducted in Italy to date, including both real-world and randomized studies. Italian ophthalmology centers enrolled more patients in the VISIONARY study than any of the other participating countries, making this dataset highly relevant when interpreting the outcomes reported from the full European study population. Analysis of data from Italy provides an important opportunity to gain insights regarding the management of OAG and OHT in Italian ophthalmologic centers.
Materials and Methods
Study Design and Visit Schedule
The VISIONARY study methodology has already been described in detail.35,41–44 Briefly, the study comprised a 6-month, observational, multicenter, prospective clinical investigation (EU PAS register number EUPAS22204). Data were prospectively collected during routine visits, between 10 April 2017 and 9 January 2019, at 14 ophthalmology centers in Italy. Patients were required to attend study visits at baseline and Month 6, whereas interim study visits (Week 4 and Week 12) were optional.35,41–44 The Institutional Review Board (IRB) or Independent Ethics Committee (IEC) at participating centers approved the protocol, and all patients provided written informed consent. The study complied with the principles of the Declaration of Helsinki.
Patient Population
Male/female adults (aged ≥18 years) with OAG or OHT currently treated with any locally available PF or preservative-containing formulation of topical PGA or beta-blocker monotherapy were allowed to enter the study. Patients were considered by the treating clinician to have shown insufficient IOP control or poor tolerance to ongoing monotherapy and were likely to benefit from a PF FC formulation. Participants were treatment-naïve for PF tafluprost/timolol FC therapy, had not undergone ophthalmic surgery (within 6 months) and were not pregnant or breast feeding. Patients with any contraindication for tafluprost or timolol treatment were not allowed to enter the study.
Investigators could indicate multiple reasons for selecting patients from the following options: insufficient IOP control or progression of glaucoma on the current monotherapy; conversion of OHT to OAG; poor local tolerance and/or insufficient adherence to the medication used; other reasons.
Study Treatment
Participants instilled one drop daily of PF tafluprost/timolol FC into their affected eye(s), as prescribed/instructed by their ophthalmologist. Study medication was either administered in the morning or evening and the preferred time of instillation was recorded at study visits.
Study Assessments and Outcome Measures
Baseline data were recorded prior to PF tafluprost/timolol FC treatment initiation (±7 days). The eye with the highest baseline IOP value was selected as the “study eye”. If IOP was equal in both eyes at baseline, the right eye was selected for analysis.
IOP was measured at each study visit using Goldmann applanation tonometry and the primary endpoint was absolute mean IOP change (mmHg) at Month 6, compared to baseline, following the initiation of PF tafluprost/timolol FC treatment. Secondary endpoints included the mean IOP change from baseline at interim visits (Weeks 4 and 12), the responder rate (defined as those patients demonstrating ≥20% IOP change from baseline at Week 12) and the percentage of responders achieving IOP reductions of at least 25%, 30% and 35% from baseline at each study visit. Sub-analysis examined the mean IOP change from baseline according to diagnostic group (containing >10 patients), baseline monotherapy (PGA or beta-blocker), the reasons for initiating PF tafluprost/timolol FC therapy, baseline presence of dry eye/ocular discomfort symptoms and the chosen time of treatment instillation (morning or evening).
Changes from baseline regarding ocular signs and subjective symptoms were assessed at Week 4, Week 12 and Month 6. Severity of conjunctival hyperemia was reported using a 4-grade scale (none, mild, moderate, severe) and best corrected visual acuity (BCVA) data were collected in decimal, logMAR or fraction (foot or meters) scales and then converted to the decimal scale.46 Other assessments included corneal fluorescein staining (CFS; Oxford Grade Scale; 0 to V grade), Schirmer’s test (mm/5 minutes, with/without anesthesia) and tear break up time (TBUT), measured in seconds(s).47 Subjective symptom severity was assessed using a 4-grade scale (none, mild, moderate and severe), and the parameters examined comprised dry eye, irritation, itching, foreign body sensation and eye pain.
Investigators used a 3-grade scale (better than prior medication, same as prior medication, worse than prior medication) to indicate their evaluation of treatment effectiveness as well as patient compliance with PF tafluprost/timolol FC therapy, compared with prior monotherapy. Patient assessment of tolerability with PF tafluprost/timolol FC treatment was reported using a 4-grade scale (very good, good, satisfactory, poor). Reported adverse events (AEs) and treatment-related AEs were documented throughout the study period.
Statistical Analysis
ICON Plc (Dublin, Ireland) conducted all statistical analyses on behalf of the VISIONARY study group. Normally distributed data were reported as mean values ± standard deviation (SD) and paired t-test was used for comparisons. Median and interquartile range (IQR) values were given for data that were not normally distributed and Wilcoxon signed rank test was used to assess the change from baseline. Comparisons between Italian and non-Italian patient demographics for age, sex and diagnosis used t-test, Chi-Square and Fisher’s Exact test, respectively. Changes from baseline concerning CFS, conjunctival hyperemia and subjective symptoms were assessed using the Bhapkar test.48 P values <0.05 indicated statistical significance.
Results
Study Population Demographics
Of the 577 patients included in the VISIONARY study full analysis set, 160 participants attended ophthalmology centers in Italy. Table 1 shows baseline study population demographics for study participants in Italy. The mean ± SD age was 68.7 ± 11.5 years (range 27–89 years) and 54.4% of the study participants were female. The most common diagnostic groups were POAG (68.1%), OHT (25.0%), pseudoexfoliation glaucoma (PEX; 2.5%) and normal tension glaucoma (NTG; 1.2%). Most participants (66.3%) were treated with PGA monotherapy prior to baseline, whereas 33.7% were using beta-blocker therapy. Among PGA monotherapy users, 21 (19.8%) were using latanoprost, 33 (31.1%) were on bimatoprost, 31 (29.2%) had been prescribed tafluprost and 21 (19.8%) were using travoprost.
 |
Table 1 Demographics of the Participants |
Change in Intraocular Pressure from Baseline
Table 2 reports the mean IOP change at each study visit for patients in Italy. Mean ± SD baseline IOP was slightly lower in the Italian population compared with the European VISIONARY population; 19.6 ± 3.6 mmHg (Italy) versus 21.5 ± 4.5 mmHg (Europe-wide population).35
 |
Table 2 Change in Intraocular Pressure from Baseline at Week 4, Week 12 and Month 6 |
The primary endpoint of absolute mean IOP change at Month 6 was statistically significant for study participants in Italy (p<0.0001; two-sided paired t-test). Mean ± SD IOP was reduced from 19.6 ± 3.6 mmHg at baseline to 14.5 ± 2.6 mmHg at Month 6, with a mean ± SD reduction of 5.1 ± 3.7 mmHg (24.1%). The mean reduction in IOP from baseline was statistically significant at each of the interim study visits (p<0.0001; two-sided paired t-test). Mean ± SD IOP reduction was 4.8 ± 3.4 mmHg (23.1%) and 5.1 ± 3.4 mmHg (24.7%) at Weeks 4 and 12, respectively. Responder rate in the Italian cohort at Month 6 was 68.1%, 48.7%, 36.2% and 26.9% based on data cutoff values for change in mean IOP from baseline of ≥20%, ≥25%, ≥30% and ≥35%, respectively (Figure 1).
Sub-analysis of Italian data according to baseline diagnosis showed that the respective mean ± SD IOP at baseline was 19.2 ± 3.7 mmHg, 20.9 ± 3.2 mmHg, 21.2 ± 4.1 mmHg and 15.0 ± 1.4 mmHg for patients with POAG, OHT, PEX and NTG. At Month 6, IOP reductions of 22.5%, 30.3%, 28.5% and 16.5% were achieved for those with POAG, OHT, PEX and NTG, respectively. The IOP reduction from baseline was statistically significant at Month 6 among those with POAG and OHT (p<0.0001; two-sided paired t-test). Statistical analysis was not conducted for the NTG and PEX subgroups because the numbers were too low.
Subgroup analysis according to prior monotherapy showed that the mean ± SD IOP was reduced from 19.5 ± 3.7 mmHg at baseline to 14.7 ± 2.3 mmHg at Month 6 among those previously treated with PGA monotherapy, representing a reduction from baseline of 4.8 ± 3.6 mmHg (22.7%; p<0.0001). Prior PGA users were treated with latanoprost (n=21), tafluprost (n=31), bimatoprost (n=33) and travoprost (n=21) with respective mean ± SD IOP at baseline being 19.9 ± 3.7 mmHg, 19.8 ± 2.7 mmHg, 19.4 ± 4.6 mmHg and 18.9 ± 3.6 mmHg. At Month 6, mean relative reductions in IOP from baseline were statistically significant in each PGA subgroup (p<0.0001; two-sided paired t-test); 27.4% (latanoprost), 24.6% (tafluprost), 21.0% (bimatoprost) and 18.0% (travoprost). Among prior beta-blocker users, the mean ± SD IOP was reduced from 19.8 ± 3.5 mmHg at baseline to 14.2 ± 3.9 mmHg at Month 6, reaching a reduction of 5.6 ± 3.9 mmHg (26.8%; p<0.0001; two-sided paired t-test).
Investigators could indicate multiple reasons for ceasing prior monotherapy and initiating PF tafluprost/timolol FC therapy. The main reasons provided were insufficient IOP control (64.4%), poor local tolerance (25.0%), progression of glaucoma (23.1%), poor compliance (3.1%) and conversion of OHT to an initial glaucomatous damage (0.6%). At Month 6, participants switching to PF tafluprost/timolol FC therapy due to insufficient IOP control and/or progression of glaucoma showed a mean relative IOP reduction of 26.8% from baseline (p<0.0001; two-sided paired t-test). Those switching due to poor tolerance with their previous monotherapy demonstrated a mean IOP change from baseline of 17.9% at Month 6 (p=0.0003; two-sided paired t-test). Patients who did not have dry eye/ocular discomfort symptoms before initiating study treatment demonstrated a mean ± SD IOP lowering from baseline of 5.6 ± 3.2 mmHg (26.9%) at Month 6 (p<0.0001; two-sided paired t-test), whereas participants with these symptoms (of any severity) at baseline showed a mean ± SD IOP reduction of 4.7 ± 4.1 mmHg (22.2%; p<0.0001; two-sided paired t-test). Most participants (85.0%) instilled PF tafluprost/timolol FC treatment in the evening. The mean ± SD relative IOP reduction from baseline in this group at Month 6 was 4.9 ± 3.6 mmHg (23.7%; p<0.0001; two-sided paired t-test). Patients who instilled study treatment in the morning achieved a reduction of 4.9 ± 4.6 mmHg (22.2%) at Month 6 (from baseline p=0.0006; two-sided paired t-test).
Ocular Signs and Symptoms
Mean ± SD and median (IQR) CFS score at baseline and at each study visit are shown in Table 3. Mean ± SD CFS score at baseline in Italy was 1.0 ± 1.1. CFS score was significantly reduced from baseline at all visits during the study period. At Month 6, mean ± SD CFS score was 0.6 ± 0.6, providing a reduction of 0.5 ± 1.1 (p=0.0004; two-sided paired t-test). The median (IQR) CFS score at baseline was 1.0 (2.00). At Week 4, Week 12 and Month 6 the median (IQR) CFS score was 0.0 (1.00). Median CFS score was reduced by 0.5 at Week 4 (p=0.0030) and by 1.0 at Week 12 (p=0.0013) and Month 6 (p=0.0003; Wilcoxon signed rank test).
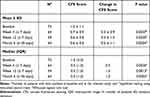 |
Table 3 Italian VISIONARY Study Population: Change in Corneal Fluorescein Staining Score (Oxford Grade Scale) During the Study Period |
Among those switching to the PF tafluprost/timolol FC due to insufficient IOP control, mean ± SD CFS grade was reduced from 2.0 ± 1.1 at baseline to 1.6 ± 0.6 at Month 6. Among those switching treatment due to poor local tolerance with PGA/beta-blocker monotherapy, mean ± SD CFS grade was reduced from 2.8 ± 1.1 at baseline to 1.8 ± 0.7 at Month 6. Patients who switched therapy due to insufficient IOP control demonstrated significantly greater reductions in mean CFS score at Month 6, compared to those switching due to poor local tolerance (p=0.0376; two-sided paired t-test).
Schirmer's test and TBUT showed slight improvement without reaching a statistically significant change. Median (IQR) Schirmer’s test result was increased from 10.0 (11.0) mm/5 minutes at baseline to 11.0 (12.0) mm/5 minutes at Month 6 (p=0.628; two-sided Wilcoxon signed rank test). Median (IQR) TBUT increased from 6.0 (5.0) s at baseline to 8.0 (4.0) s at Month 6 (p=0.156; two-sided Wilcoxon signed rank test). The median (IQR) BCVA decimal score was unchanged at Month 6 from baseline (1.0 [0.2]).
Severity of conjunctival hyperemia was significantly reduced from baseline, at all study visits through Month 6 in the Italian dataset (p<0.0001; Bhapkar test). Conjunctival hyperemia was mild or absent at baseline for 60.0%, 82.6%, 42.3% and 47.7% of prior PGA users on latanoprost, tafluprost, bimatoprost and travoprost, respectively (Figure 2). Moderate or severe hyperemia was reported for the remaining patients in these subgroups at baseline. Reductions in hyperemia were observed across each PGA subgroup, although statistical significance could not be assessed due to low patient numbers. Conjunctival hyperemia was either mild or absent in 91.8% of patients on beta-blocker therapy at baseline. At Month 6, the severity of conjunctival hyperemia was either stabilized (43.2%) or improved (35.1%) among prior beta-blocker users.
Despite subjective symptoms being typically mild or absent at baseline, statistically significant reductions from baseline were reported at Month 6 regarding severity of dry eye symptoms (p<0.001; Bhapkar test), irritation (p=0.0002; Bhapkar test) and foreign body sensation (p=0.0008; Bhapkar test). The change in severity of itching (p=0.1975; Bhapkar test) and eye pain (p=0.2184; Bhapkar test) symptoms was not significant at Month 6, compared with baseline.
Physician and Patient Assessments
When comparing the clinical effectiveness against baseline monotherapy, most investigators (78.8%) reported improved IOP control with PF tafluprost/timolol FC treatment at Month 6, whereas 19.2% of them considered the study treatment to be equally effective. Investigators perceived patient compliance with PF tafluprost/timolol FC treatment to be the same as (46.4%) or improved (52.3%), compared with baseline medication, at Month 6. Patients typically reported tolerability with PF tafluprost/timolol FC treatment to be good or very good at Week 4 (87.4%), Week 12 (94.0%) and Month 6 (91.7%).
Discontinuations from Study Treatment and Safety Outcomes
Overall, 19 patients (11.9%) discontinued their PF tafluprost/timolol FC treatment during the study period. Investigators were allowed to report multiple reasons for discontinuation from the study treatment. Reasons for ceasing treatment were poor local tolerance (4 patients), poor compliance (1 patient) and other reasons (3 patients). Data were missing for 11 patients. No discontinuation was attributed to insufficient IOP control.
In total, 2 AEs were reported by 2 different patients during the 6-month study period (Table 4). All AEs were non-serious and mild in severity, and 1 case of eyelash growth was considered to be related to study treatment.
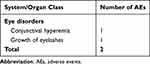 |
Table 4 Adverse Events Reported in Italy During the Study Period |
Discussion
The current analysis of data from Italian patients, predominantly with POAG and OHT, participating in the Europe-wide VISIONARY study demonstrated that a rapid, sustained and significant IOP reduction from baseline was achieved when switching from PGA or beta-blocker monotherapy to the PF tafluprost/timolol FC. Statistically and clinically significant IOP reduction was shown from Week 4 with PF tafluprost/timolol FC therapy and continued over the 6-month study period. Treatment effectiveness, tolerability and compliance were rated as high with PF tafluprost/timolol FC therapy, with only 2 non-serious and mild AEs reported, while the severity of most ocular signs and symptoms was significantly reduced at Month 6. The study was conducted in a real-world setting that reflected usual clinical practice regarding glaucoma and OHT treatment changes in Italy. No wash-out period was implemented during the therapeutic switch, providing an indication of the outcomes that ophthalmologists may observe in their own centers when stepping up to PF tafluprost/timolol FC therapy from prior monotherapy.35,37 The Italian data analysis was broadly aligned with that of the full Europe-wide VISIONARY study population, highlighting the reproducibility of treatment results with PF tafluprost/timolol FC therapy.35,41–44 The current analysis represents the largest real-world/randomized study of PF tafluprost/timolol FC therapy conducted to date in Italy and the Italian cohort represents the largest country-level subgroup included in the VISIONARY study.35,41–44 These data strengthen the existing evidence base regarding the role of PF tafluprost/timolol FC therapy in clinical practice.11,31–37
Italian participants achieved a mean IOP reduction from baseline of 5.1 ± 3.7 mmHg at Month 6 (24.1%; p<0.0001), which was similar to the primary outcome shown in the full VISIONARY study population (5.7 ± 4.1 mmHg [24.9%; p<0.0001]), despite the Italian cohort having a slightly lower baseline IOP (19.6 ± 3.6 mmHg) compared with the Europe-wide dataset (21.5 ± 4.5 mmHg) and pre-treatment pressure being predictive of IOP reduction.35,45 Published baseline IOP levels from the Hungarian (20.8 mmHg), UK (22.0 mmHg), Russian (23.8 mmHg) and Spanish (21.9 mmHg) VISIONARY datasets suggest that ophthalmologists in Italy tended to select PGA/beta-blocker monotherapy users with lower pressures for treatment intensification to FC therapy compared to other countries.41–44 In addition, the data from Italy indicate that even patients with IOP values below 20 mmHg may experience significant clinical benefit following a switch to PF tafluprost/timolol FC.45 Indeed, the relative change in IOP at Month 6 observed in the Italian dataset was slightly greater than the reductions published for Hungary and was similar to those for the UK and Spain where the starting IOP was marginally higher.41,42,44 At Month 6, the majority of the Italian ophthalmologists considered PF tafluprost/timolol FC to be associated with better IOP control (78.8%), compared with previous monotherapy, and this is likely to have been supported by the reassuringly high levels of treatment compliance observed by investigators during this real-world study. Change in IOP was significant, regardless of whether patients instilled their medication in the morning or evening. The fact that the majority administered their medication at night-time might be related to countrywide clinical practice and recommendations on timing in Italy.
Response rates showed that approximately 68% of the participants achieved an IOP reduction ≥20% at Month 6. In addition, around 49%, 36% and 27% achieved an IOP reduction exceeding 25%, 30% and 35%, respectively. These outcomes were similar to the published Europe-wide responder rates, reflecting the clinically meaningful IOP reductions that may be achieved when changing to PF tafluprost/timolol FC treatment from prior PGA or beta-blocker monotherapy.35,41–44 Participants with POAG and OHT achieved significant IOP reductions from baseline (p<0.0001) and those with NTG and PEX demonstrated numerical improvements. Italian patients with OHT and PEX achieved the highest mean IOP reductions from baseline (30.3% and 28.5%, respectively), whereas the same subgroups in the Europe-wide analysis demonstrated mean reductions of 26.1% and 17.6%.35 The IOP reduction achieved in Italy for the OHT subgroup was impressive, particularly as the starting IOP was lower in this group (20.9 ± 3.18 mmHg) than the corresponding European subgroup (22.3 ± 4.1 mmHg).35,45
Patients treated with beta-blocker monotherapy at baseline achieved greater IOP reductions (26.8%) at Month 6, compared with prior PGA users (22.7%). These data are similar to the findings for the Europe-wide VISIONARY population regarding IOP reductions in prior beta-blocker (28.5%) and PGA (23.6%) users.35 Italian patients previously using latanoprost, tafluprost and bimatoprost demonstrated mean IOP reduction of 21.0–27.4% at Month 6, which was comparable with the European VISIONARY dataset (20.5–25.9%).35 Italian patients previously using travoprost showed a smaller IOP reduction at Month 6 (18.0%) compared with other PGA user groups and the travoprost subgroup in the European VISIONARY population (21.3%).35 However, baseline IOP was slightly higher in the other PGA user groups and the Europe-wide travoprost subgroup, and this may have influenced the difference in outcomes observed here.35,45 Subjects enrolled in Italy due to insufficient IOP control and/or progression of OAG demonstrated greater IOP reductions (26.8%) compared to those who were switched due to poor tolerance with prior medication (17.9%), which was expected – based upon the published results for the Europe-wide study population.35
A significant IOP reduction was achieved irrespective of whether patients had dry eye/ocular discomfort symptoms at baseline (p<0.0001), although the presence of symptoms was associated with a slightly smaller mean reduction in IOP at Month 6 (22.2%), versus those without dry eye (26.9%). PF tafluprost/timolol FC therapy was associated with improved tolerability outcomes and indicators of ocular health, regardless of prior monotherapy. Significant improvements were observed concerning conjunctival hyperemia across the whole Italian cohort (p<0.0001) and regardless of the prior PGA therapy used. These data reflect outcomes in the Europe-wide study population.35 Although median TBUT and Schirmer's test improved, the changes from baseline were not significant. BCVA remained unchanged at Month 6. Similar future studies would benefit from the collection and reporting of additional data relating to visual field parameters at baseline and follow-up study visits to establish the impact of PF tafluprost/timolol FC treatment on the rate of disease progression and associated sight loss.
Despite ocular symptoms being reported as generally mild or absent at baseline, significant improvements in severity were demonstrated concerning dry eye (p<0.001), irritation (p=0.0002) and foreign body sensation (p=0.0008) at Month 6, while eye pain and itching were unchanged. Given that people with glaucoma are susceptible to OSD, and even PF PGA formulations may be associated with tolerability issues (potentially caused by eye drops excipients), any improvements in signs or symptoms of ocular surface health and/or tolerability that accompany a switch to topical PF FC therapy from prior monotherapy may be welcomed by patients and their treating clinician.16–29,49,50
Patient-reported tolerability was typically good/very good (91.7%) at Month 6 and only 2 non-serious AEs were reported; both were mild in severity and 1 (eyelash growth) was considered to be treatment-related. Early termination was recorded in 19 patients (11.9%). Multiple reasons could be given for discontinuation from study treatment and the known reasons for study discontinuation were poor ocular tolerance (4 patients), poor compliance (1 patients) and other reasons (3 patients). It should be noted that no discontinuations were attributed to insufficient IOP control. Due to the observational nature of the investigation the reason of study discontinuation was not clarified for 11 participants. This loss of information, resulting from participants choosing not to attend all visits, represents a limitation of the study that is also reflective of the practical challenges with patient follow-up faced by ophthalmologists in routine clinical practice. As data were collected during routine ophthalmology appointments in the VISIONARY study, it was not possible to measure diurnal IOP variation to understand the level of IOP control (and fluctuation) achieved over a 24-hour period with PF tafluprost/timolol FC therapy. Future studies would also benefit from a design that allowed deeper analysis of IOP change alongside indicators of ocular surface improvement and the inclusion of a control group so that outcomes with PF tafluprost/timolol FC therapy may be compared against those for patients treated with other PGA/timolol FC treatments.
Conclusions
In a real-world setting, Italian patients with OAG/OHT demonstrated statistically and clinically significant reductions in IOP following a switch to PF tafluprost/timolol FC treatment from PGA or beta-blocker monotherapy. Despite some minor differences in baseline characteristics, the clinical outcomes reported in Italy were similar to those of the European VISIONARY dataset. A significant IOP reduction was observed from Week 4 and maintained over a 6-month period. PF tafluprost/timolol FC was generally well tolerated, with most ocular signs and symptoms being significantly reduced in severity during the study period.
The VISIONARY Study Group (Italy)
Francesco Oddone (IRCSS-Fondazione Bietti, Roma), Lucia Tanga (IRCSS-Fondazione Bietti, Roma), Vincenzo Scorcia (University Magna Græcia of Catanzaro, Catanzaro), Giuseppe Giannaccare (University Magna Græcia of Catanzaro, Catanzaro), Michele Iester (University of Genova, Policlinico San Martino Hospital, Genova), Carlo Enrico Traverso (Eye Clinic of Genoa, Department of Neuroscience, Rehabilitation, Ophthalmology, Genetics, Maternal and Child Health (DiNOGMI), University of Genova, Policlinico San Martino Hospital, Genova), Dario Sisto (University of Bari, Bari), Rossella Favale (University of Bari, Bari), Stefano De Cilla (University of Piemonte Orientale, Novara), Antonella Clemente (University of Piemonte Orientale, Novara), Paolo Bettin (IRCCS San Raffaele Scientific Institute, Vita-Salute San Raffaele University), Francesca Gorgoni (IRCCS San Raffaele Scientific Institute, San Raffaele Hospital, Milano), Carlo Cagini (University of Pisa, Pisa), Elena Venceslai (University of Perugia, Perugia), Michele Figus (University of Pisa, Pisa), Chiara Posarelli (University of Pisa, Pisa), Giorgio Marchini (Azienda Ospedaliera Universitaria Integrata, University Hospital, Verona), Roberta Morbio (University of Verona, Verona), Luca Rossetti (University of Milan, Milano), Laura Ottobelli (University of Milan, Milano), Gemma Rossi (Fondazione IRCCS Policlinico S. Matteo, Pavia), Tommaso Salgarello (Fondazione Policlinico Universitario A. Gemelli IRCCS, Roma; Università Cattolica del Sacro Cuore, Roma), Andrea Giudiceandrea (Fondazione Policlinico Universitario A. Gemelli IRCCS, Roma; Università Cattolica del Sacro Cuore, Roma), Gian Luca Scuderi (Università di Roma La Sapienza, Roma), Andrea Perdicchi (Università di Roma La Sapienza, Roma), Giovanni Staurenghi (University of Milan, Milano), Angelica Dipinto (University of Milan, Milano).
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Statement of Ethics
The study was conducted in accordance with the Declaration of Helsinki of 1964, as revised in 2013, and was registered under the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP®) European Union electronic Register of Post-Authorisation Studies (EU PAS register number EUPAS22204). The study protocol was approved by the Institutional Review Board (IRB) or Independent Ethics Committee (IEC) at each center/institution and written informed consent was obtained from subjects prior to enrolment.
Acknowledgments
Claudia Fassari, Melania Simone, Stefano Marsico, Akemi Soejima, Gabriela Saborio and Claire Lea provided input concerning the design and implementation of the study as well as the analyses and reporting of data on behalf of Santen. Medical writing services were provided on behalf of the authors by Rebecca Down at Copperfox Communications Limited. The authors thank the participants for their valuable contribution to the VISIONARY study.
Author Contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study was funded by Santen SA, Switzerland. Sponsorship funding for medical writing services and Article Publishing Charges was provided by Santen Italy. The contribution of the authors affiliated with the IRCCS Fondazione Bietti was supported by the Italian Ministry of Health and by Fondazione Roma.
Disclosure
Francesco Oddone has received consultancy fees from Santen, Allergan, Sooft, Thea, Omikron Italia, Centervue and Novartis. Vincenzo Scorcia has no relevant financial disclosures. Michele Iester has received consultancy fees from SIFI and Thea, speaker grant from Santen, Omikron Italia and Thea. Dario Sisto has no relevant financial disclosures. Stefano De Cilla has no relevant financial disclosures. Paolo Bettin has not received any fee from any company in the last two years. Carlo Cagini has no relevant financial disclosures. Michele Figus has no relevant financial disclosures. Giorgio Marchini has no relevant financial disclosures. Luca Rossetti received fees from Aerie, Alcon, Allergan, Centervue, Novartis, NTC, Omikron. Gemma Rossi has no relevant financial disclosures. Tommaso Salgarello received no specific funding for this work. Gian Luca Scuderi has received consultancy fees from Santen, Allergan, Sooft, Omikron Italy. Giovanni Staurenghi has received consultant/advisor fees from Heidelberg Engineering, Centervue, Apellis, Allergan, Astellas, Bayer, Boehringer, Genentech, Iveric, Novartis, Roche and Chengdu Kanghong Biotechnology Co, Kyoto Drug Discovery & Development Co; grant support from Heidelberg Engineering, Optos, Optovue, Quantel Medical, Centervue, Carl Zeiss Meditec, Nidek and Topcon; lecture fees from Heidelberg Engineering, Nidek, Bayer, Novartis and Roche; patents/royalty from Ocular Instruments. The authors report no other conflicts of interest in this work.
References
1. Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi:10.1016/j.ophtha.2014.05.013
2. European Glaucoma Society. Terminology and guidelines for glaucoma 5th edition; 2020. Available from: https://www.eugs.org/eng/egs_guidelines_download.asp.
3. Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol. 1998;126:498–505. doi:10.1016/S0002-9394(98)00272-4
4. The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS investigators. Am J Ophthalmol. 2000;130:429–440. doi:10.1016/S0002-9394(00)00538-9
5. Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the early manifest glaucoma trial. Arch Ophthal. 2002;120:1268–1279. doi:10.1001/archopht.120.10.1268
6. Rulli E, Quaranta L, Riva I, et al. Visual field loss and vision-related quality of life in the Italian Primary Open Angle Glaucoma Study. Sci Rep. 2018;8(1):619. doi:10.1038/s41598-017-19113-z
7. Janz NK, Wren PA, Lichter PR, et al. The Collaborative Initial Glaucoma Treatment Study: interim quality of life findings after initial medical or surgical treatment of glaucoma. Ophthalmology. 2001;108:1954–1965. doi:10.1016/S0161-6420(01)00874-0
8. Holló G, Katsanos A, Boboridis KG, Irkec M, Konstas AG. Preservative-free prostaglandin analogs and prostaglandin/timolol fixed combinations in the treatment of glaucoma: efficacy, safety and potential advantages. Drugs. 2018;78(1):39–64. doi:10.1007/s40265-017-0843-9
9. Tabet R, Stewart WC, Feldman R, Konstas AG. A review of additivity to prostaglandin analogs: fixed and unfixed combinations. Surv Ophthalmol. 2008;53(1):S85–92. doi:10.1016/j.survophthal.2008.08.011
10. Holló G, Topouzis F, Fechtner RD. Fixed-combination intraocular pressure lowering therapy for glaucoma and ocular hypertension: advantages in clinical practice. Expert Opin Pharmacother. 2014;15(12):1737–1747. doi:10.1517/14656566.2014.936850
11. Konstas AG, Katsanos A, Athanasopoulos GP, et al. Preservative-free tafluprost/timolol fixed combination: comparative 24-h efficacy administered morning or evening in open-angle glaucoma patients. Expert Opin Pharmacother. 2018;19:1981–1988. doi:10.1080/14656566.2018.1534958
12. Figus M, Agnifili L, Lanzini M, et al. Topical preservative-free ophthalmic treatments: an unmet clinical need. Expert Opin Drug Deliv. 2020;18:655–672.
13. Konstas AGP, Haidich AB, Rossetti L, et al. Prostaglandin-timolol fixed combinations efficacy: myth or reality? Editorial Eur J Ophthalmol. 2012;22:1–4. doi:10.5301/ejo.5000077
14. Aptel F, Cucherat M, Denis P. Efficacy and tolerability of prostaglandin-timolol fixed combinations: a meta-analysis of randomized clinical trials. Eur J Ophthalmol. 2012;22:5–18. doi:10.5301/ejo.5000009
15. Quaranta L, Biagioli E, Riva I, et al. Prostaglandin analogs and timolol-fixed versus unfixed combinations or monotherapy for open-angle glaucoma: a systematic review and meta-analysis. J Ocul Pharmacol Ther. 2013;29:382–389. doi:10.1089/jop.2012.0186
16. Zhang X, Vadoothker S, Munir WM, Saeedi O. Ocular surface disease and glaucoma medications: a clinical approach. Eye Contact Lens. 2019;45(1):11–18. doi:10.1097/ICL.0000000000000544
17. Ferreras A, Figus M, Fogagnolo P, Iester M, Frezzotti P. Managing side effects on ocular surface caused by glaucoma eye drops. Curr Med Chem. 2019;26(22):4223–4224. doi:10.2174/092986732622190920092210
18. Dubrulle P, Labbé A, Brasnu E, et al. Influence of treating ocular surface disease on intraocular pressure in glaucoma patients intolerant to their topical treatments: a report of 10 cases. J Glaucoma. 2018;27(12):1105–1111. doi:10.1097/IJG.0000000000001041
19. Di Staso S, Agnifili L, Cecannecchia S, DI Gregorio A, Ciancaglini M. In vivo analysis of prostaglandins-induced ocular surface and periocular adnexa modifications in patients with glaucoma. In Vivo. 2018;32(2):211–220. doi:10.21873/invivo.11227
20. Batra R, Tailor R, Mohamed S. Ocular surface disease exacerbated glaucoma: optimizing the ocular surface improves intraocular pressure control. J Glaucoma. 2014;23(1):56–60. doi:10.1097/IJG.0b013e318264cd68
21. Jaenen N, Baudouin C, Pouliquen P, Manni G, Figueiredo A, Zeyen T. Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur J Ophthalmol. 2007;17(3):341–349. doi:10.1177/112067210701700311
22. Baudouin C. Detrimental effect of preservatives in eyedrops: implications for the treatment of glaucoma. Acta Ophthalmol. 2008;86(7):716–726. doi:10.1111/j.1755-3768.2008.01250.x
23. Pisella PJ, Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol. 2002;86(4):418–423. doi:10.1136/bjo.86.4.418
24. Asiedu K, Abu SL. The impact of topical intraocular pressure lowering medications on the ocular surface of glaucoma patients: a review. J Curr Ophthalmol. 2018;31(1):8–15. doi:10.1016/j.joco.2018.07.003
25. Aguayo Bonniard A, Yeung JY, Chan CC, Birt CM. Ocular surface toxicity from glaucoma topical medications and associated preservatives such as benzalkonium chloride (BAK). Expert Opin Drug Metab Toxicol. 2016;12(11):1279–1289. doi:10.1080/17425255.2016.1209481
26. Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17:350–355. doi:10.1097/IJG.0b013e31815c5f4f
27. Erb C, Gast U, Schremmer D. German register for glaucoma patients with dry eye. I. Basic outcome with respect to dry eye. Graefes Arch Clin Exp Ophthalmol. 2008;246:1593–1601. doi:10.1007/s00417-008-0881-9
28. Garcia-Feijoo J, Sampaolesi JR. A multicentre evaluation of ocular surface disease prevalence in patients with glaucoma. Clin Ophthalmol. 2012;6:441–446. doi:10.2147/OPTH.S29158
29. Boimer C, Birt CM. Preservative exposure and surgical outcomes in glaucoma patients: the PESO study. J Glaucoma. 2013;22:730–735. doi:10.1097/IJG.0b013e31825af67d
30. Pfeiffer N, Traverso CE, Lorenz K, et al. A 6-month study comparing efficacy, safety, and tolerability of the preservative-free fixed combination of tafluprost 0.0015% and timolol 0.5% versus each of its individual preservative-free components. Adv Ther. 2014;31:1228–1246. doi:10.1007/s12325-014-0163-3
31. Holló G, Hommer A, Antón López A, et al. Efficacy, safety, and tolerability of preservative-free fixed combination of tafluprost 0.0015%/timolol 0.5% versus concomitant use of the ingredients. J Ocul Pharmacol Ther. 2014;30:468–475. doi:10.1089/jop.2013.0229
32. Holló G, Katsanos A. Safety and tolerability of the tafluprost/timolol fixed combination for the treatment of glaucoma. Expert Opin Drug Saf. 2015;14:609–617. doi:10.1517/14740338.2015.1010507
33. Kaarniranta K, Ikaheimo K, Mannermaa E, et al. Pharmacokinetics, efficacy, and safety of the preservative-free fixed combination of tafluprost 0.0015% and timolol 0.5% in healthy volunteers: a Phase I comparison vs. the corresponding preservative-free monotherapies. Clin Pharmacokinet. 2016;55:485–494. doi:10.1007/s40262-015-0331-x
34. Hoy SM. Tafluprost/timolol: a review in open-angle glaucoma or ocular hypertension. Drugs. 2015;75:1807–1813. doi:10.1007/s40265-015-0476-9
35. Oddone F, Tanga L, Kóthy P, Holló G; VISIONARY Study Group. Treatment of open-angle glaucoma and ocular hypertension with preservative-free tafluprost/timolol fixed-dose combination therapy: the VISIONARY Study. Adv Ther. 2020;37(4):1436–1451. doi:10.1007/s12325-020-01239-8
36. Pillunat LE, Erb C, Ropo A, Kimmich F, Pfeiffer N. Preservative-free fixed combination of tafluprost 0.0015% and timolol 0.5% in patients with open-angle glaucoma and ocular hypertension: results of an open-label observational study. Clin Ophthalmol. 2017;11:1051–1064. doi:10.2147/OPTH.S128453
37. Bourne RRA, Kaarniranta K, Lorenz K, et al. Changes in ocular signs and symptoms in patients switching from bimatoprost–timolol to tafluprost–timolol eye drops: an open-label Phase IV study. BMJ Open. 2019;9:e024129. doi:10.1136/bmjopen-2018-024129
38. Spitzer E, Cannon CP, Serruys PW. Should real-world evidence be incorporated into regulatory approvals? Expert Opin Drug Saf. 2018;9:1–5.
39. Eichler HG, Bloechl-Daum B, Broich K, et al. Data rich, information poor: can we use electronic health records to create a learning healthcare system for pharmaceuticals? Clin Pharmacol Ther. 2019;105(4):912–922. doi:10.1002/cpt.1226
40. Food and Drug Administration. Use of real-world evidence to support regulatory decision-making for medical devices: guidance for industry and food and drug administration staff; 2018. Available from: www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM513027.pdf.
41. Holló G, Kóthy P; A magyarországi VISIONARY-vizsgálók. The Hungarian VISIONARY Study: Hungarian results in the European multicenter preservative-free tafluprost/timolol fixed combination investigation. Ophthalmologia Hungarica. 2020;157(3):196–201. Hungarian.
42. Ansari E, Pavicic-Astalos J, Ayan F, et al. Treatment of open-angle glaucoma and ocular hypertension with preservative-free tafluprost/timolol fixed-dose combination therapy: UK and Ireland results from the VISIONARY Study. Adv Ther. 2021;38(6):2990–3002. doi:10.1007/s12325-021-01725-7
43. Karlova EV, Petrov SY, Germanova VN. Preservative-free fixed combination in the treatment of open-angle glaucoma and ocular hypertension: the VISIONARY Study (EUPAS22204). Vestn Oftalmol. 2020;136(4):76–84. Russian. doi:10.17116/oftalma202013604176
44. Garcia-Medina JJ, Benitez-del-Castillo J, Rodríguez-Agirretxe I, Lopez-Lopez F, Moreno-Valladares A; The VISIONARY study group (Spain). Treatment of open-angle glaucoma and ocular hypertension with preservative-free tafluprost/timolol fixed-dose combination therapy: results from the VISIONARY study population in Spain. J Ocul Pharmacol Ther. 2022;38:252–260. doi:10.1089/jop.2021.0099
45. Holló G, Vuorinen J, Tuominen J, Huttunen T, Ropo A, Pfeiffer N. Fixed-dose combination of tafluprost and timolol in the treatment of open-angle glaucoma and ocular hypertension: comparison with other fixed-combination products. Adv Ther. 2014;31(9):932–944. doi:10.1007/s12325-014-0151-7
46. NIDEK Co. LTD. Conversion table for representation of visual acuity. Available from: https://www.nidek-intl.com/visual_acuity.html.
47. Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22(7):640–650. doi:10.1097/00003226-200310000-00008
48. Bhapkar V. A note on the equivalence of two test criteria for hypotheses in categorical data. J Am Stat Assoc. 1966;61(313):228–235. doi:10.1080/01621459.1966.10502021
49. Pacwa A, Machowicz J, Wojtyniak A, et al. SCD1-fatty acid desaturase inhibitor MF-438 alleviates latent inflammation induced by preservative-free prostaglandin analog eye drops. J Inflamm Res. 2022;15:793–806. doi:10.2147/JIR.S347784
50. Trzeciecka A, Paterno JJ, Toropainen E, et al. Long-term topical application of preservative-free prostaglandin analogues evokes macrophage infiltration in the ocular adnexa. Eur J Pharmacol. 2016;788:12–20. doi:10.1016/j.ejphar.2016.06.014
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Retrospective Analysis of Switching Bimatoprost 0.01% to Bimatoprost 0.03% in Patients with Various Types of Glaucoma and Ocular Hypertension
Xu KM, Cho R, Chan TYB
Clinical Ophthalmology 2022, 16:2385-2390
Published Date: 29 July 2022
Steroid Response Following Dropless Cataract Surgery Using Subconjunctival Triamcinolone
Wu AM, Pitts KM, Pineda R, Chen SH, Wang M, Johnson G, Shen LQ, Margeta MA
Clinical Ophthalmology 2023, 17:2803-2814
Published Date: 22 September 2023
Risk Factors and Management of Intraocular Pressure Elevation After Vitrectomy Combined with Silicone Oil Tamponade
Ge L, Su N, Fan W, Yuan S
International Journal of General Medicine 2024, 17:447-456
Published Date: 3 February 2024
Micropulse Transscleral Cyclophotocoagulation in Non-Incisional Eyes with Ocular Hypertension and Primary Open-Angle Glaucoma
Murtaza F, Kaba Q, Somani S, Tam ES, Yuen D
Clinical Ophthalmology 2024, 18:1295-1312
Published Date: 11 May 2024


