Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Transarterial Chemoembolization Plus Sorafenib versus Transarterial Chemoembolization Alone for Advanced Hepatocellular Carcinoma: An Umbrella Review of Meta-Analyses and Systematic Reviews
Authors Yan J , Wen Y , Deng M, Ye B, Liu X, Zhang L
Received 6 July 2023
Accepted for publication 27 September 2023
Published 5 October 2023 Volume 2023:10 Pages 1723—1733
DOI https://doi.org/10.2147/JHC.S429352
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Mohamed Shaker
Jingxin Yan,1,2,* Yonghao Wen,1,3,* Manjun Deng,1,2 Bin Ye,4 Xinlian Liu,5 Lushun Zhang5
1Department of Hepatopancreatobiliary Surgery, Affiliated Hospital of Qinghai University, Xining, People’s Republic of China; 2Qinghai Province Key Laboratory of Hydatid Disease Research, Xining, People’s Republic of China; 3Department of Postgraduate, Qinghai University, Xining, People’s Republic of China; 4Department of General Surgery, Rongxian People’s Hospital, Zigong, People’s Republic of China; 5Department of Pathology and Pathophysiology, Chengdu Medical College, Chengdu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xinlian Liu; Lushun Zhang, Department of Pathology and Pathophysiology, Chengdu Medical College, Chengdu, People’s Republic of China, Email [email protected]; [email protected]
Background: Sorafenib is the standard treatment for most cases of advanced hepatocellular carcinoma (HCC), based on Western and Eastern clinical guidelines. Thus, an increasing number of transarterial chemoembolization (TACE) plus sorafenib combination therapies have been used in clinical practice. In addition, several systematic reviews and meta-analyses have explored the efficacy and safety of the combination of TACE and sorafenib. Therefore, we performed an umbrella review to summarize and evaluate these evidence-based studies.
Methods: PubMed, Embase, Cochrane Library, and Web of Science databases were searched up to June 1, 2023. All meta-analyses that evaluated the effect of TACE plus sorafenib on HCC were considered eligible. The quality of the included meta-analyses was evaluated by AMSTAR2 (A Measurement Tool to Assess Systematic Reviews). The quality of evidence per association provided in the meta-analyses was rated using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE). This study was registered with PROSPERO (Registration ID: CRD42023420417).
Results: We included 12 meta-analyses, including randomized clinical trials, cohort studies, and observational studies. A total of 44 associations with overall survival, survival rate, time to disease progression, overall response rate, disease control rate, and adverse events were evaluated in this umbrella review. The quality of most associations ranged from low to very low, indicating that flaws were significant in the current meta-analyses.
Conclusion: This umbrella review identified beneficial associations between TACE and sorafenib combination therapy in advanced HCC. However, owing to the low certainty of the evidence, clinicians should interpret our results with caution when applying them in clinical practice, and high-quality studies are required in the future to confirm our results.
Keywords: umbrella review, meta-analysis, systematic review, sorafenib, hepatocellular carcinoma, transarterial chemoembolization
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and the fourth leading cause of cancer-related death worldwide.1 The annual incidence of HCC is increasing and is mainly attributed to hepatitis B virus, hepatitis C virus, nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and other liver diseases, while some unknown etiologies can also be associated with HCC and cirrhosis.2–4 Because of the various etiologies and accumulation of genetic changes in HCC, it is a type of heterogeneous cancer, which makes treatment difficult.5 Surgical, systemic, locoregional, and other potential therapies have been used for the treatment of HCC, and many studies are currently underway.6,7 However, measures have been taken to improve the prognosis of patients with HCC, and the recurrence and mortality rates are still high.8 In the clinical practice, systemic therapy is recommended treatment for advanced HCC, and clinical studies found that immunotherapy, immunotherapy plus VEGF (or PDGF) inhibitor, and r multi-kinase inhibitor showed benefit when treating advance HCC.9–12 In addition to systemic therapy, transarterial chemoembolization (TACE), an approach that can embolize the hepatic artery and reduce the blood supply to HCC tissues, is a standard therapy recommended for some BCLC intermediate-stage HCC subgroups with normal liver function.13–15 In contrast, various anticancer drugs, such as 5-fluorouracil and cisplatin, can be used in TACE, which provides opportunities to control intermediate/advanced-stage HCC.16,17 Sorafenib, an oral polykinase inhibitor that can inhibit tumor angiogenesis and tumor cell proliferation, has been widely used over past years as the first-line treatment for unresectable advanced HCC.18 Sorafenib shows antitumor activity in HCC by inhibiting IL-6/STAT3 and other possible pathways.19 The efficacy and safety of sorafenib/sorafenib-related protocols have been confirmed in several clinical trials, and accordingly, studies focusing on the combination of interventional therapies and sorafenib have found that combination therapy can provide some clinical benefits compared to TACE alone.20–24
The impact of the combination of TACE and sorafenib has been broadly examined through many meta-analyses of randomized controlled trials (RCTs) and cohort studies. However, the fact that the combination of TACE and sorafenib is an effective strategy for controlling HCC remains controversial, leading to inconsistent conclusions. Therefore, conducting an umbrella review with an assessment of the overall quality of the existing evidence on this topic is exceedingly important. An umbrella review, a review of review, is a method collecting existing evidence and synthesized higher levels of evidence.25,26 Therefore, we designed this umbrella review to investigate the pooled effects of TACE and sorafenib combination found in previous meta-analyses.
Methods
Umbrella Review Methods and Registration
Our umbrella review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement,27 and the protocol was registered with PROSPERO (Registration ID: CRD42023420417).
Search Strategy
A comprehensive search of Embase, Cochrane Library, Web of Science, and PubMed for English-language systematic reviews and meta-analyses of TACE combined with sorafenib versus TACE alone published before June 1, 2023. The search strategy can be found in Table 1.
 |
Table 1 Index and Keyword Terms Used in the Databases |
Selection Criteria
The eligibility criteria were systematic reviews and meta-analyses of observational studies or RCTs measuring the clinical outcomes of TACE plus sorafenib versus TACE alone for advanced HCC. The exclusion criteria were as follows: (1) narrative reviews, primary studies, protocols, theses, and conference papers; (2) studies with statistical errors or other serious flaws; (3) articles that did not assess the outcomes of interest (including but not limited to overall survival, overall response rate, disease control rate, disease progression rate, alpha-fetoprotein, time of disease progression, progression-free rate, and vascular endothelial growth factor); and (4) non-English meta-analysis.
Data Extraction
Two independent reviewers (YHW and JXY) performed the research and data extraction, and disagreements were resolved by a third author (MJD). The following information was extracted from all eligible studies: first author, publication year, number of included studies, study population, and sample size. All the outcomes associated with the included studies were extracted from the original meta-analysis.
Quality Assessment
Two independent reviewers (YHW and LSZ) used AMSTAR2 (A Measurement Tool to Assess Systematic Reviews) to assess the methodological quality of eligible articles. The AMSTAR2 questionnaire uses 16 measures to classify systematic reviews as high, moderate, low, or critically low quality.28
Data Synthesis and Analysis
Extraction and analysis were performed using R (R Development Core Team) software version 3.6.1. To assess the certainty of the evidence, the authors used a GRADEproGDT-independent assessment based on the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) Handbook, which considers the following characteristics: study design (observational, randomized clinical trials), inconsistencies between studies (I2 statistics), imprecision of study results, indirection, publication bias, size effects, and the presence of dose–response gradients.29–33
Results
Search Strategy Outcome
A PRISMA flowchart of the literature search process is shown in Figure 1. A preliminary search yielded 1076 articles, of which 568 were duplicates. After removing duplicates using automated tools, we rearranged the abstracts of the remaining studies; 496 articles did not meet the inclusion criteria. Finally, 12 studies34–45 met the eligibility criteria and were included in the umbrella review, and Table 2 summarizes the characteristics of the included studies.
 |
Table 2 Description of the Selected Studies: Objectives, Numbers of Studies and Most Important results |
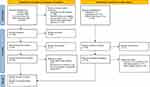 |
Figure 1 PRISMA 2020 flow diagram for new systematic reviews which included searches of databases, registers, and other sources. |
A description of the selected studies: objectives, number of studies, and most important results is included in Table 2. Li,34 Zeng,38 and Xie36 only included RCTs, whereas the others included both observational studies and RCTs.
Outcome of Quality Assessment
Table 3 shows AMSTAR2 item evaluations for included meta-analyses. Overall, of the 11 papers, eight were of low quality and four were of very low quality. In particular, all included meta-analyses used appropriate methods for statistical method (Q11, 11/12) and a significant publication bias assessment (Q15, 11/12), while none of the review authors provided a list of exclusion studies or justified the exclusion.
 |
Table 3 The Results of the Methodological Quality Assessment of the Meta-Analysis |
The HR, OR, RR, and SMD of the most important outcomes and adverse events are presented in Supplementary Tables S1 and S2.
Main Outcomes
Overall Survival
Ten meta-analyses34,35,37–42,44,45 reported data on OS, and seven (7/10, 70%) found a significant improvement in OS in the TACE plus sorafenib group compared to TACE alone, with low-level certainty of evidence according to the GRADE (Supplementary Table S1).
Survival Rate
Three meta-analyses37,41,45 reported data on 0.5-year, 1-year, and 2-year survival rates, and all these meta-analyses found significant improvements in the TACE plus sorafenib group compared to TACE alone, with low to very low level certainty of evidence according to the GRADE (Supplementary Table S1).
Time of Disease Progression
TTP was mentioned in nine meta-analyses,34,35,37–42,44 and seven (7/9, 77.8%) found a significant improvement in TTP in the TACE plus sorafenib group compared to TACE alone, with a moderate level certainty of evidence according to the GRADE (Supplementary Table S1).
Overall Response Rate
Ten34,36–39,41–45 included meta-analyses reporting data on ORR, and seven (7/10, 70%) included meta-analyses found a significant improvement in ORR in the TACE plus sorafenib group compared to TACE alone, with a moderate level of certainty of evidence according to the GRADE (Supplementary Table S1).
Disease Control Rate
Seven meta-analyses34,36,38,40–42,45 reported data on DCR, and four (4/7, 57.14%) found a significant improvement in ORR in the TACE plus sorafenib group compared to TACE alone, with moderate certainty of evidence according to the GRADE (Supplementary Table S1).
Adverse Events
All Grade Adverse Event
Five meta-analyses34,38,42,44,45 reported data on fatigue, and four (4/5, 80%) found that the incidence of fatigue was higher in the TACE plus sorafenib group than in the TACE alone group, with a moderate level of certainty of evidence according to the GRADE (Supplementary Table S2).
Two studies34,44 included meta-analyses reporting data on nausea, and one (1/2, 50%) meta-analysis found that the incidence of nausea was higher in the TACE plus sorafenib group than in the TACE alone group, with a low level certainty of evidence according to the GRADE (Supplementary Table S2).
Three34,42,44 included meta-analyses reporting data on alopecia, and two (2/3, 50%) included meta-analyses which found that the incidence of alopecia was higher in the TACE plus sorafenib group than in the TACE alone group, with a low-level certainty of evidence according to the GRADE (Supplementary Table S2).
Five meta-analyses34,38,42,44,45 reported data on hand–foot skin reactions, and all included meta-analyses found that the incidence of hand–foot skin reactions was higher in the TACE plus sorafenib group than in the TACE alone group, with low level certainty of evidence according to the GRADE (Supplementary Table S2).
Five meta-analyses34,38,42,44,45 reported data on rashes, and all included meta-analyses showed that the incidence of rashes was higher in the TACE plus sorafenib group than in the TACE alone group, with a low level of certainty of evidence according to the GRADE (Supplementary Table S2).
Two meta-analyses42,44 reported data on hematological events, and none of the included meta-analyses found that the incidence of hematological events was higher in the TACE plus sorafenib treatment group than in the TACE alone group, with very low certainty of evidence according to the GRADE (Supplementary Table S2).
Five meta-analyses34,38,42,44,45 reported data on diarrhea, and all included meta-analyses found that the incidence of diarrhea was higher in the TACE plus sorafenib treatment group than in the TACE alone group, with low level certainty of evidence according to the GRADE (Supplementary Table S2).
Five meta-analyses34,38,42,44,45 reported that the incidence of hypertension was higher in the TACE plus sorafenib treatment group than in the TACE alone group, with low certainty of evidence according to the GRADE (Supplementary Table S2).
One34 included meta-analysis reported data on fever, abdominal pain, anorexia, ALT elevation, AST elevation, leukopenia, elevated bilirubin levels, and oral mucosal inflammation. This meta-analysis found that the incidence of fever, abdominal pain, anorexia, ALT elevation, AST elevation, and leukopenia was not significant in the TACE plus sorafenib treatment group compared to TACE alone, with high-level certainty of evidence according to the GRADE (Supplementary Table S2). In addition, in the TACE plus sorafenib treatment group, the incidence of elevated bilirubin levels and oral mucosal inflammation was higher than in the TACE alone group, with a high level of certainty of evidence according to the GRADE (Supplementary Table S2).
Grade 1/2 Adverse Event
One36 included meta-analysis reported data on fatigue, nausea, hand–foot skin reactions, diarrhea, and alopecia. This meta-analysis found that the incidence of fatigue, nausea, and alopecia was not significant in the TACE plus sorafenib group compared to the TACE alone group, with a high level of certainty of evidence according to the GRADE (Supplementary Table S2). In addition, in the TACE plus sorafenib treatment group, the incidence of hand–foot skin reactions and diarrhea were higher than that in the TACE alone group, with moderate certainty of evidence according to the GRADE (Supplementary Table S2).
Grade 3/4 Adverse Event
Three meta-analyses36,39,44 reported data on fatigue, and two of the included meta-analyses found that the incidence of fatigue was higher in the TACE plus sorafenib treatment group than in the TACE alone group, with a low-level certainty of evidence according to the GRADE (Supplementary Table S2).
Two36,44 included meta-analyses reported data on nausea, and none of the included meta-analyses found that the incidence of nausea was higher in the TACE plus sorafenib treatment group than in the TACE alone group, with a low level of certainty of evidence according to the GRADE (Supplementary Table S2).
Two meta-analyses36,44 reported data on hematological events, and none of the included meta-analyses found that the incidence of hematological events was higher in the TACE plus sorafenib treatment group than in the TACE alone group, with a moderate level of certainty of evidence according to the GRADE (Supplementary Table S2).
Two meta-analyses36,44 reported data on anorexia, and none of the included meta-analyses found that the incidence of anorexia was higher in the TACE plus sorafenib treatment group than in the TACE alone group, with very low certainty of evidence according to the GRADE (Supplementary Table S2).
Four meta-analyses36,38,39,44 reported data on hand–foot skin reactions, and three of the included meta-analyses found that the incidence of hand–foot skin reactions was higher in the TACE plus sorafenib treatment group than in the TACE alone group, with moderate level certainty of evidence according to the GRADE (Supplementary Table S2).
Four meta-analyses36,38,39,44 reported data on rash, and all included meta-analyses found that the incidence of rash was higher in the TACE plus sorafenib treatment group than in the TACE alone group, with a moderate level of certainty of evidence according to the GRADE (Supplementary Table S2).
Four meta-analyses36,38,39,44 reported data on diarrhea, and all of the included meta-analyses found that the incidence of diarrhea was higher in the TACE plus sorafenib treatment group than in the TACE alone group, with moderate level certainty of evidence according to the GRADE (Supplementary Table S2).
Three meta-analyses36,39,44 reported data on hypertension, and two of the included meta-analyses found that the incidence of hypertension was higher in the TACE plus sorafenib treatment group than in the TACE alone group, with a moderate level of certainty of evidence according to the GRADE (Supplementary Table S2).
Discussion
To date, 12 systematic reviews and meta-analyses have explored the clinical outcomes of TACE combined with sorafenib in advanced HCC. However, it is too early to draw robust conclusions that TACE combined with sorafenib is an effective and safe method for patients with advanced HCC, as the methodological quality of the included meta-analyses is consistent. For example, none of the included meta-analyses stated Q7 of AMSTAR2: Did the review authors provide a list of excluded studies and justify the exclusions? In addition, the certainty of most evidence is low to very low, indicating that flaws in current evidence should be noted. Although this umbrella review was conducted, further high-quality RCTs and evidence-based studies are required to explore the safety and efficacy of TACE in combination with sorafenib for advanced HCC.
Although the certainty of evidence is low to very low, in this umbrella review, TACE in combination with sorafenib showed positive results in prolonging survival and increasing tumor responses compared to TACE alone. A possible explanation for these results is that TACE and sorafenib play different roles in the treatment of HCC. Sorafenib, a multi-kinase inhibitor that can inhibit angiogenesis by selectively targeting VEGF and PDGF receptors, has been widely used for the treatment of HCC over the years, and has shown survival benefits for advanced HCC.11,46,47 Sorafenib provided molecular insights into the metabolic changes in the miR-494/G6pc axis, together with HIF-1A activation, regulating glycogen and lipid storage.48 Based on a Phase III randomized double-blind placebo-controlled trial conducted in the Asia-Pacific region, sorafenib prolonged the median OS from 4.2 to 6.5 months (HR=0.68; 95% CI=0.50–0.93).49 TACE is recommended by the European Society for Medical Oncology and the American Society for Medical Oncology.50–52 In addition, TACE directly injects anticancer drugs into the cancer cell supply arteries with a high local drug concentration in the tumor areas. In such settings, a combination of TACE and sorafenib has been used in the treatment of advanced HCC for years, and many clinical and evidence-based studies have been published. However, the effect of combination therapy is being questioned as some studies have shown negative results. For example, a large-sample multicenter RCT recently completed in South Korea indicated that TACE combined with sorafenib did not show survival benefits compared with TACE alone.53 In addition, clinical evidence has shown that the combination of TACE and sorafenib has many more advantages than TACE alone. To solve this problem, more than ten systematic reviews and meta-analyses have been conducted to investigate this issue, but the pooled results vary. With this umbrella review, we could directly analyze published systematic reviews and meta-analyses to provide robust conclusions. However, the differences among the included studies are understandable to a certain extent, as the different etiologies and heterogeneity of HCC may lead to various responses and clinical outcomes, which could partially explain why the effects of the included meta-analyses were inconsistent.54
Our umbrella review also found that the combination of TACE and sorafenib resulted in a higher incidence of adverse events such as fatigue, diarrhea, elevated bilirubin, hand and foot skin reaction, rash, and hypertension. These adverse events may lead to reduction and suspension of sorafenib therapy, which should be considered in clinical practice. However, in a meta-analysis by Chen et al,55 the authors found no significant differences in adverse events between combination therapy and sorafenib monotherapy. In addition, some clinical trials have indicated that the safety and tolerance of TACE plus sorafenib are acceptable.56–60 It remains unclear whether the combination of TACE and sorafenib increases the incidence of adverse events.
This is the first umbrella review to systematically investigate the safety and clinical effects of TACE combined with sorafenib in advanced HCC through published systematic reviews and meta-analyses. However, similar to any evidence-based study, this umbrella review had some limitations. First, some meta-analyses included only RCTs, while the rest included both RCTs and observational studies, such as cohort studies and prospective non-RCTs. Non-RCTs may be biased and lead to unreliable conclusions. Thus, we should also focus on the associations identified in observational studies. Second, the credibility of the umbrella review depends directly on the meta-analyses included, which may lead to inevitable conclusions. Third, in the GRADE assessment, we found that the methodological quality of most of the included evidence was low to very low based on AMSTAR2 analyses. Fourth, the TACE protocols in different studies were different, and the baseline of the included patients was also different. Due the low evidence certainty and the low methodological quality of most of the included evidence, the conclusions need to be verified by future clinical evidence. For example, some studies used different doses and combinations of TACE-related drugs such as lipiodol, lobaplatin, epirubicin, and mitomycin C, etc.
Conclusions
In conclusion, the association between TACE and sorafenib is supported by evidence. Our umbrella review found that TACE plus sorafenib enhanced survival and tumor response. In addition, the combination of TACE and sorafenib showed a higher incidence of adverse events such as fatigue, diarrhea, elevated bilirubin, hand and foot skin reaction, rash, and hypertension. However, clinicians should interpret our results with caution when applying them in clinical practice, and high-quality studies are required to confirm our results.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Yan J, Deng M, Kong S, et al. Transarterial chemoembolization in combination with programmed death-1/programmed cell death-ligand 1 immunotherapy for hepatocellular carcinoma: a mini review. iLIVER. 2022;1(4):225–234. doi:10.1016/j.iliver.2022.10.001
3. Yan J, Deng M, Li T, et al. Efficacy and complications of transarterial chemoembolization alone or in combination with different protocols for hepatocellular carcinoma: a Bayesian network meta-analysis of randomized controlled trials. iLIVER. 2023;2(2):130–141. doi:10.1016/j.iliver.2023.03.002
4. Paradis V, Zalinski S, Chelbi E, et al. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology. 2009;49(3):851–859. doi:10.1002/hep.22734
5. Chen F, Zhang W, Gao X, Yuan H, Liu K. The role of small interfering RNAs in hepatocellular carcinoma. J Gastrointest Cancer. 2023. doi:10.1007/s12029-023-00911-w
6. Radosevic A, Quesada R, Serlavos C, et al. Microwave versus radiofrequency ablation for the treatment of liver malignancies: a randomized controlled Phase 2 trial. Sci Rep. 2022;12(1):316. doi:10.1038/s41598-021-03802-x
7. Criss CR, Makary MS. Salvage locoregional therapies for recurrent hepatocellular carcinoma. World J Gastroenterol. 2023;29(3):413–424. doi:10.3748/wjg.v29.i3.413
8. Shen J, Qi W, Dai J, et al. Tenofovir vs. entecavir on recurrence of hepatitis B virus-related hepatocellular carcinoma beyond Milan criteria after hepatectomy. Chin Med J. 2021;135(3):301–308. doi:10.1097/CM9.0000000000001864
9. Su GL, Altayar O, O’Shea R, et al. AGA clinical practice guideline on systemic therapy for hepatocellular carcinoma. Gastroenterology. 2022;162(3):920–934. doi:10.1053/j.gastro.2021.12.276
10. Shitara K, Di Bartolomeo M, Mandala M, et al. Association between gene expression signatures and clinical outcomes of pembrolizumab versus paclitaxel in advanced gastric cancer: exploratory analysis from the randomized, controlled, phase III KEYNOTE-061 trial. J Immunother Cancer. 2023;11(6):e006920. doi:10.1136/jitc-2023-006920
11. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi:10.1056/NEJMoa0708857
12. Merle P, Blanc JF, Edeline J, et al. Ipilimumab with atezolizumab-bevacizumab in patients with advanced hepatocellular carcinoma: the PRODIGE 81-FFCD 2101-TRIPLET-HCC trial. Dig Liver Dis. 2023;55(4):464–470. doi:10.1016/j.dld.2023.01.161
13. Lu J, Guo JH, Ji JS, et al. Irradiation stent with 125 I plus TACE versus sorafenib plus TACE for hepatocellular carcinoma with major portal vein tumor thrombosis: a multicenter randomized trial. Int J Surg. 2023;109(5):1188–1198. doi:10.1097/JS9.0000000000000295
14. Liu Y, Wang Y, Wei Z, et al. Exploratory study of microparticle transcatheter arterial chemoembolization combined with resection for huge hepatocellular carcinoma. iLIVER. 2022;1(1):35–42. doi:10.1016/j.iliver.2022.01.001
15. Fu J, Cao S, Song L, et al. Radiomics/Radiogenomics in hepatocellular carcinoma applications and challenges in interventional management. iLIVER. 2022;1(2):96–100 doi:10.1016/j.iliver.2022.07.001.
16. Deng Z, Wang Y. Predictors of liver failure after transarterial chemoembolization in patients with spontaneously ruptured hepatocellular carcinoma: a retrospective study. J Interv Med. 2023;6(1):35–40. doi:10.1016/j.jimed.2022.10.003
17. Yuan H, Lu H, Zeng J, Zhang Y, Shen L. Comparison of radiation doses between hepatic artery infusion chemotherapy and transarterial chemoembolization for liver cancer. J Interv Med. 2021;4(4):184–189. doi:10.1016/j.jimed.2021.08.004
18. Yang XD, Kong FE, Qi L, et al. PARP inhibitor Olaparib overcomes Sorafenib resistance through reshaping the pluripotent transcriptome in hepatocellular carcinoma. Mol Cancer. 2021;20(1):20. doi:10.1186/s12943-021-01315-9
19. Yousef EH, El-Magd NFA, El Gayar AM. Norcantharidin potentiates sorafenib antitumor activity in hepatocellular carcinoma rat model through inhibiting IL-6/STAT3 pathway. Transl Res. 2023;S1931–S5244(23):69–82.
20. Ostwal V, Ramaswamy A, Gota V, et al. Phase I study evaluating dose de-escalation of Sorafenib with Metformin and Atorvastatin in Hepatocellular Carcinoma (SMASH). Oncologist. 2022;27(3):165–e222. doi:10.1093/oncolo/oyab008
21. Zheng K, Zhu X, Fu S, et al. Sorafenib plus hepatic arterial infusion chemotherapy versus sorafenib for hepatocellular carcinoma with major portal vein tumor thrombosis: a randomized trial. Radiology. 2022;303(2):455–464. doi:10.1148/radiol.211545
22. Yau T, Park JW, Finn RS, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, Phase 3 trial. Lancet Oncol. 2022;23(1):77–90. doi:10.1016/S1470-2045(21)00604-5
23. Lyu N, Wang X, Li JB, et al. Arterial chemotherapy of oxaliplatin plus fluorouracil versus sorafenib in advanced hepatocellular carcinoma: a biomolecular exploratory, randomized, Phase III Trial (FOHAIC-1). J Clin Oncol. 2022;40(5):468–480. doi:10.1200/JCO.21.01963
24. Pereira H, Bouattour M, Dioguardi Burgio M, et al. Health-related quality of life in locally advanced hepatocellular carcinoma treated by either radioembolisation or sorafenib (SARAH trial). Eur J Cancer. 2021;154:46–56. doi:10.1016/j.ejca.2021.05.032
25. Ioannidis J. Next-generation systematic reviews: prospective meta-analysis, individual-level data, networks and umbrella reviews. Br J Sports Med. 2017;51(20):1456–1458. doi:10.1136/bjsports-2017-097621
26. Shi X, Wallach JD. Umbrella reviews: a useful study design in need of standardization. BMJ. 2022;378. doi:10.1136/bmj.o1740
27. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi:10.1186/2046-4053-4-1
28. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi:10.1136/bmj.j4008
29. Bortolato B, Köhler CA, Evangelou E, et al. Systematic assessment of environmental risk factors for bipolar disorder: an umbrella review of systematic reviews and meta-analyses. Bipolar Disord. 2017;19(2):84–96. doi:10.1111/bdi.12490
30. Dragioti E, Evangelou E, Larsson B, Gerdle B. Effectiveness of multidisciplinary programmes for clinical pain conditions: an umbrella review. J Rehabil Med. 2018;50(9):779–791. doi:10.2340/16501977-2377
31. He Y, Li X, Gasevic D, et al. Statins and multiple noncardiovascular outcomes: umbrella review of meta-analyses of observational studies and randomized controlled trials. Ann Intern Med. 2018;169(8):543–553. doi:10.7326/M18-0808
32. Kalliala I, Markozannes G, Gunter MJ, et al. Obesity and gynaecological and obstetric conditions: umbrella review of the literature. BMJ. 2017;359:j4511. doi:10.1136/bmj.j4511
33. Veronese N, Solmi M, Caruso MG, et al. Dietary fiber and health outcomes: an umbrella review of systematic reviews and meta-analyses. Am J Clin Nutr. 2018;107(3):436–444. doi:10.1093/ajcn/nqx082
34. Li D, Pang Y, Xu L, Xu X. Efficacy and safety of sorafenib combined with TACE in the treatment of advanced hepatocellular carcinoma: a meta-analysis. J BUON. 2021;26(4):1355–1364.
35. Hu MD, Jia LH, Liu HB, Zhang KH, Guo GH. Sorafenib in combination with transarterial chemoembolization for hepatocellular carcinoma: a meta-analysis. Eur Rev Med Pharmacol Sci. 2016;20(1):64–74.
36. Xie Y, Tian H, Xiang H. Is transcatheter arterial chemoembolization plus sorafenib better than chemoembolization plus placebo in the treatment of hepatocellular carcinoma? Tumori. 2021;107(4):292–303. doi:10.1177/0300891620945029
37. Cheng Z, He L, Guo Y, Song Y, Song S, Zhang L. The combination therapy of transarterial chemoembolisation and sorafenib is the preferred palliative treatment for advanced hepatocellular carcinoma patients: a meta-analysis. World J Surg Oncol. 2020;18(1):243. doi:10.1186/s12957-020-02017-0
38. Zeng J, Lv L, Mei ZC. Efficacy and safety of transarterial chemoembolization plus sorafenib for early or intermediate stage hepatocellular carcinoma: a systematic review and meta-analysis of randomized controlled trials. Clin Res Hepatol Gastroenterol. 2016;40(6):688–697. doi:10.1016/j.clinre.2016.04.006
39. Zhang L, Hu P, Chen X, Bie P, Yang L-Y. Transarterial chemoembolization (TACE) plus sorafenib versus TACE for intermediate or advanced stage hepatocellular carcinoma: a meta-analysis. PLoS One. 2014;9(6):e100305. doi:10.1371/journal.pone.0100305
40. Li L, Zhao W, Wang M, et al. Transarterial chemoembolization plus sorafenib for the management of unresectable hepatocellular carcinoma: a systematic review and meta-analysis. BMC Gastroenterol. 2018;18(1):138. doi:10.1186/s12876-018-0849-0
41. Zhang X, Wang K, Wang M, et al. Transarterial chemoembolization (TACE) combined with sorafenib versus TACE for hepatocellular carcinoma with portal vein tumor thrombus: a systematic review and meta-analysis. Oncotarget. 2017;8(17):29416–29427. doi:10.18632/oncotarget.15075
42. Jin PP, Shao SY, Wu WT, et al. Combination of transarterial chemoembolization and sorafenib improves outcomes of unresectable hepatocellular carcinoma: an updated systematic review and meta-analysis. Jpn J Clin Oncol. 2018;48(12):1058–1069. doi:10.1093/jjco/hyy138
43. Fu QH, Zhang Q, Bai XL, et al. Sorafenib enhances effects of transarterial chemoembolization for hepatocellular carcinoma: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2014;140(8):1429–1440. doi:10.1007/s00432-014-1684-5
44. Yang M, Yuan JQ, Bai M, Han GH. Transarterial chemoembolization combined with sorafenib for unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Mol Biol Rep. 2014;41(10):6575–6582. doi:10.1007/s11033-014-3541-7
45. Cai R, Song R, Pang P, et al. Transcatheter arterial chemoembolization plus sorafenib versus transcatheter arterial chemoembolization alone to treat advanced hepatocellular carcinoma: a meta-analysis. BMC Cancer. 2017;17(1):714. doi:10.1186/s12885-017-3707-5
46. Abou-Alfa GK. TACE and sorafenib: a good marriage? J Clin Oncol. 2011;29(30):3949–3952. doi:10.1200/JCO.2011.37.9651
47. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi:10.1016/S1470-2045(08)70285-7
48. Bergamini C, Leoni I, Rizzardi N, et al. MiR-494 induces metabolic changes through G6pc targeting and modulates sorafenib response in hepatocellular carcinoma. J Exp Clin Cancer Res. 2023;42(1):145. doi:10.1186/s13046-023-02718-w
49. Lien RY, Tung HH, Wu SL, Hu SH, Lu LC, Lu SF. Validation of the prophylactic efficacy of urea-based creams on sorafenib-induced hand-foot skin reaction in patients with advanced hepatocellular carcinoma: a randomised experiment study. Cancer Rep. 2022;5(7):e1532. doi:10.1002/cnr2.1532
50. Chen LT, Martinelli E, Cheng AL, et al. Pan-Asian adapted ESMO clinical practice guidelines for the management of patients with intermediate and advanced/relapsed hepatocellular carcinoma: a TOS-ESMO initiative endorsed by CSCO, ISMPO, JSMO, KSMO, MOS and SSO. Ann Oncol. 2020;31(3):334–351. doi:10.1016/j.annonc.2019.12.001
51. Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(5):871–873. doi:10.1093/annonc/mdy510
52. Gordan JD, Kennedy EB, Abou-Alfa GK, et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J Clin Oncol. 2020;38(36):4317–4345. doi:10.1200/JCO.20.02672
53. Park JW, Kim YJ, Kim DY, et al. Sorafenib with or without concurrent transarterial chemoembolization in patients with advanced hepatocellular carcinoma: the phase III STAH trial. J Hepatol. 2019;70(4):684–691. doi:10.1016/j.jhep.2018.11.029
54. Shuen TWH, Alunni-Fabbroni M, Öcal E, et al. Extracellular vesicles may predict response to radioembolization and sorafenib treatment in advanced hepatocellular carcinoma: an exploratory analysis from the SORAMIC Trial. Clin Cancer Res. 2022;28(17):3890–3901. doi:10.1158/1078-0432.CCR-22-0569
55. Chen A, Li S, Yao Z, et al. Adjuvant transarterial chemoembolization to sorafenib in unresectable hepatocellular carcinoma: a meta-analysis. J Gastroenterol Hepatol. 2021;36(2):302–310. doi:10.1111/jgh.15180
56. Zhao Y, Wang WJ, Guan S, et al. Sorafenib combined with transarterial chemoembolization for the treatment of advanced hepatocellular carcinoma: a large-scale multicenter study of 222 patients. Ann Oncol. 2013;24(7):1786–1792. doi:10.1093/annonc/mdt072
57. Chao Y, Chung YH, Han G, et al. The combination of transcatheter arterial chemoembolization and sorafenib is well tolerated and effective in Asian patients with hepatocellular carcinoma: final results of the START trial. Int J Cancer. 2015;136(6):1458–1467. doi:10.1002/ijc.29126
58. Cosgrove DP, Reyes DK, Pawlik TM, Feng AL, Kamel IR, Geschwind JF. Open-label single-arm Phase II trial of sorafenib therapy with drug-eluting bead transarterial chemoembolization in patients with unresectable hepatocellular carcinoma. Clin Results Radiol. 2015;277(2):594–603.
59. Geschwind JF, Kudo M, Marrero JA, et al. TACE treatment in patients with sorafenib-treated unresectable hepatocellular carcinoma in clinical practice final analysis of GIDEON. Radiology. 2016;279(2):630–640.
60. Lencioni R, Kudo M, Ye SL, et al. GIDEON (Global Investigation of therapeutic DEcisions in hepatocellular carcinoma and of its treatment with sorafeNib): second interim analysis. Int J Clin Pract. 2014;68(5):609–617. doi:10.1111/ijcp.12352
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
