Back to Journals » Infection and Drug Resistance » Volume 16
The Role of HBx Protein in Diseases Beyond the Liver
Authors Ai L , Liu QQ , Li Y, Wang Y, Zhang HM
Received 18 January 2023
Accepted for publication 29 April 2023
Published 23 May 2023 Volume 2023:16 Pages 3225—3232
DOI https://doi.org/10.2147/IDR.S405316
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Liping Ai,1 Qing-Qing Liu,1 Yize Li,1 Yuanyuan Wang,2 Hong-Mei Zhang1
1Department of Clinical Oncology, Xijing Hospital, Fourth Military Medical University, Xi’an, Shaanxi, People’s Republic of China; 2Nephrology Department, Affiliated Hospital of Northwest Minzu University / Second Provincial People’s Hospital of Gansu, Lanzhou, Gansu, People’s Republic of China
Correspondence: Hong-Mei Zhang, Department of Clinical Oncology, Xijing Hospital, Fourth Military Medical University, Changle West Road No. 127, Xi’an, Shaanxi, People’s Republic of China, Email [email protected]
Abstract: HBX gene is essential for HBV replication, evading the surveillance of the immune system by integrating its sequence into the human genome. It also exists stably in human cells by inhibiting the expression and activity of mismatch repair-related pathway genes. Previous reviews have comprehensively summarized the role of HBx in liver-related diseases. Our article complements the summary of research on HBx in diseases other than liver disease. Through a comprehensive literature search and reading, we found that HBx is expressed in the kidney, placenta, lung and other organs of HBV-infected patients, and is closely related to the occurrence and development of diseases such as nephritis, diffuse large B-cell lymphoma, and gastric cancer. However, in the clinical treatment of these diseases, HBV infection and the role of HBx have not attracted sufficient attention, and there is no corresponding treatment strategy. Therefore, more research on HBx in diseases other than the liver is particularly necessary, and we hope that our article can provide some insight into the treatment of related diseases.
Keywords: HBx protein, disease, treatment, molecular mechanism, explore
Hepatitis B virus (HBV) is a hepatotropic virus and an important human pathogen. Worldwide, an estimated 296 million people are chronically infected with the virus, many of whom develop severe liver diseases, including hepatitis, cirrhosis, and hepatocellular carcinoma (HCC).1 In addition, it plays a crucial role in diseases related to organs other than the liver.
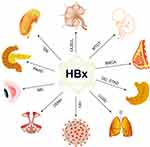
HBV is a hepatotropic DNA virus, a 3.2kb circular partial double stranded DNA molecule with four overlapping open reading frames. They encode viral envelope protein (HBsAg), core protein (HBcAg), viral polymerase (DNA-P) and HBV x protein (HBx) (Figure 1). The HBsAg, HBcAg, and DNA-P are all involved in the assembly of HBV virus. The HBV X gene is the smallest open reading frame in the HBV genome, with a length of 462 bp, encoding a protein of 154 amino acids.2 The HBX gene can be integrated into the human genome and has been shown to be closely related to a variety of human diseases3.We found through literature search that Hepatitis B x protein (HBx) plays an important role in diseases related to organs such as the kidney, blood system, reproductive system and breast (Figure 1).
HBV-GN
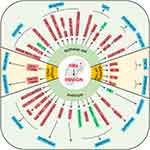
The main pathological features of glomerulonephritis are diffuse glomerular lesions, including apoptosis of epithelial cells, proliferation of endothelial cells and mesangial cells, accompanied by a large amount of immune cell infiltration in the acute phase, and hyperplasia and compression of capillaries by infiltrating cells in severe lesions. Vascular rings to narrow or block the lumen. Among these, HBV-related glomerulonephritis (HBV-GN) accounts for a large proportion of GN, and its treatment must be differentiated from GN caused by other etiologies.4 The role of HBx in kidney disease has been closely monitored by several researchers. The results showed that HBx mainly acts on the main cells (epithelial cells, podocytes and mesangial cells, etc.) that cause pathophysiological changes in HBV-GN disease through apoptosis, proliferation and Epithelial-mesenchymal transition (EMT) (Figure 2).
HBx is highly expressed in the epithelial cells of HBV-GN patients. High expression of HBx increases epithelial cell apoptosis by activating multiple molecular pathways, such as: MLK3-MKK7-JNKs, PI3K/AKT, NOTCH, PKC/ERK and NF-κB pathways.5–11 Apart from this, HBx can also promote EMT of epithelial cells through PI3K/AKT/mTOR, NOTCH, NF-κB and other pathways, thereby accelerating the development of GN.12–14 The remodeling of the immune microenvironment plays a crucial role in disease development. HBx can regulate the secretion of cytokines such as IL1, IL6, TNF, IL4 and IFN by up-regulating the expression of CD40, TLR4 and MHCII and other molecules in epithelial cells, thereby promoting the formation of an inflammatory microenvironment.15,16 Additionally, the proliferation and cycle changes of epithelial cells also play an important role in the development of the disease.17
In addition to its role in epithelial cells, HBx can influence disease progression by modulating the biological processes of podocytes, the major constituent cells of the glomerulus. Similarly, HBx can promote GN formation through podocyte immunity, apoptosis, pyroptosis, proliferation and cell adhesion. Besides, it influences the glomerular immune microenvironment through cellular pathways including PI3K/AKT/mTOR. Yang et al reported that podocytes can upregulate the expression of macrophage biomarkers KDMB, F4/80, MHCII, and CD40, and promote podocyte-to-macrophage transformation (PMT).18 Ying et al found that HBx can up-regulate CD59 and Crry expression in podocytes by activating the P38 pathway, resulting in decreased complement activation, which may facilitate latent HBV infection in podocytes and play a role in the development of HBV-GN.19 HBV-GN is characterized by a reduced number of podocytes due to apoptosis and shedding from the basement membrane.20–22 He et al found that HBx reduced podocyte adhesion and expression of α3β1 integrin, and increased apoptosis.23 In addition, HBx-induced changes in podocyte pyroptosis, proliferation, and cell activity also play a role in GN.24,25 Changes in the mesangial cells and mesangial interstitium caused by HBx are also the main causes of HBV-GN. Researchers have found that HBx can promote the formation and secretion of TNF-α, IL1β and IL6 by activating the ERKs and NF-κB pathways in mesangial cells, thereby promoting their proliferation of mesangial cells.26,27 On top of that, HBx can accelerate HBV-GN formation by recruiting CD4+ T cells and macrophages to the mesangial interstitium.28
In conclusion, HBx plays an important role in HBV-GN progression. When HBV-GN occurs, patients may exhibit kidney related symptoms such as proteinuria, hematuria, edema, and liver related symptoms. Therefore, antiviral drugs, including lamivudine and entecavir, are the first choice for the treatment. Additionally, immunomodulators (IFN-α) and hormonal drugs are also top priorities.4 Some researchers have also proposed that Chinese herbal medicines such as Echinacoside and Cordyceps sinensis have a certain therapeutic effect on HBV-GN-induced nephropathy.9,29
Mother-to-Child Transmission
HBV infection is a global epidemic disease. More than 50% of chronic HBV infections are caused by Mother-to-child transmission (MTCT) as HBV vaccines become widespread in the population.30 However, the mechanism underlying intrauterine HBV infection remains unclear. Existing studies have shown that HBxAg can be detected in placental trophoblast cells of HBV-infected patients. Here, we review the role and mechanism of HBx in trophoblasts.
HBx inhibits apoptosis and promotes the invasion, proliferation and inflammatory response of HBV-infected trophoblasts through Smad and PI3K/p-AKT signaling.31 Cui et al used HTR-8/SVneo cells to establish a trophoblast model with HBx overexpression and confirmed that HBx and its different fragments can activate the Smad signaling pathway, accompanied by the downregulation of E-cadherin, and upregulation of vimentin and N-cadherin. After the signaling pathway was activated, reduced apoptosis, increased invasive ability and enhanced inflammatory response were observed in HTR-8/SVneo cells.32 Wang et al and other research teams confirmed that high expression of HBx in trophoblast JEG-3 can reduce apoptosis by activating the PI3K/p-AKT pathway.33,34
It is worth noting the roles of EGFR in HBx-mediated activation of the PI3K/AKT pathway; however, the conclusions of different research groups are not completely consistent. Lin et al demonstrated that HBx promotes HBV replication in trophoblasts via downregulation of Smc5/6, activates the EGFR promoter and inhibits trophoblast apoptosis via the PI3K/p-AKT downstream signalling pathway, thereby increasing the risk of HBV intrauterine infection.35 However, Wang W et al found that HBxAg suppresses apoptosis and promotes the secretion of placental hormones in human placental trophoblasts via activation of the EGFR/Akt pathway.34 In other words, The specific location of EGFR activation in HBx inhibiting trophoblast apoptosis through the PI3K/AKT pathway need to be further determined.
MTCT of HBV has become the main route of transmission of chronic hepatitis B in China; therefore, prevention of MTCT of HBV is the key to controlling chronic hepatitis B. All pregnant women need to be tested for HBsAg and other hepatitis B serological indicators before birth. Newborns of HBsAg-positive pregnant women need to be injected with hepatitis B immunoglobulin and hepatitis B vaccine within 12h after birth.36
Diffuse large B-cell lymphoma Data from researchers showed that the positive rate of serum HBV was significantly increased in Diffuse large B-cell lymphoma (DLBCL) patients (23.6%) compared to that in the general Chinese population (7.2%, P<0.001), especially in advanced stage lymphoma patients (P=0.003).37 HBx was also strongly expressed in tissues from patients with HBsAg-positive HBV surface antigen (HBsAg) positive.37,38 And it is closely related to the poor prognosis of DLBCL. Therefore, it is necessary to conduct in-depth research on the mechanism of action of HBx in DLBCL development.
Multiple research teams have confirmed that high HBx expression in DLBCL tissues leads to resistance to various chemotherapeutic drugs. Li et al found that the core component of HBV (HBX) directly upregulated the expression of lncNBAT1, which was closely associated with the chemotherapy outcomes of HBV-infected individuals with DLBCL through in vitro and in vivo experiments. lncNBAT1 interact with signal transducer and activator of transcription 1 (STAT1) to prevent its enrichment at the promoter region of the functional target gene apolipoprotein B mRNA editing enzyme catalytic subunit 3A (APOBEC3A), inhibiting the expression of APOBEC3A and inducing resistance to MTX in DLBCL cells.39 Zhao et al found that HBX specifically inhibited the phosphorylation of checkpoint kinase 2 (CHK2, a key DNA damage response protein). CHK2 depletion similarly conferred resistance to S-phase arrest-inducing chemotherapeutic, consistent with HBX overexpression in DLBCL cells. In addition, some researchers have confirmed that HBx can promote the expression of c-Myc in DLBCL tissues, and c-Myc is a marker of poor prognosis.40
In terms of treatment, for HBV antigen-positive patients, rituximab (R) and chemotherapy (chemo), the first-line treatments for DLBCL, may cause HBV reactivation, which affects the continuation of chemotherapy. Therefore, patients with hepatitis B antigen-positive DLBCL should continue to use entecavir to prevent HBV activation after R-CHOP chemotherapy.41
Gastric Ulcers and Stomach Adenocarcinoma
Guo et al indicated that HBX could induce apoptosis and G1 arrest in GES-1 (a gastric mucosal cell line) cells. They further confirmed the aggravation of Gastric ulcers (GU) by HBX using clinicopathological parameters.42
Cui et al found that HBV infection is associated with an increased risk of Stomach adenocarcinoma (STAD) based on a meta-analysis. Histological examination showed that gastric epithelium positive for HBx demonstrated a higher nuclear-cytoplasmic ratio than HBx-negative cells.43 In a more in-depth mechanistic study, Du et al found that HBx can promote the expression of URG11, which in turn activates the β-catenin signaling pathway to promote the growth and metastasis of GC.44
Breast invasive carcinomaKlein et al suggest that although BRCA formation is rare (<1%) in WAP-HBX animals, HBX can immortalize ME cells derived from mammary tissue segments in a p53-independent manner, a process that is cell cycle-dependent The protein D1 gene was overexpressed. Finally, both cyclin D1 induction and HBX mitotic activity are dependent on p38 and c-Jun N-terminal kinase, but not on MEK-1 kinase activity.45 Through epidemiological studies on BRCA, Adhikari et al found that HBV may also directly affect breast cells through its cis and trans effects of HBx which may act as oncoproteins.46
Other Diseases
In nasopharyngeal carcinoma (NPC), using a xenograft mouse model, it was confirmed that HBx promoted the EMT process of epithelial cells by up-regulating the expression of YAP1, and further promoted cell invasion. Anti-YAP1 can also decrease metastasis in vivo.47 In pancreatic ductal adenocarcinoma (PDAC), HBx expression significantly enhances cell proliferation and migration and induces an EMT phenotype. The expression of ErbB4 and TGF-α increased in parallel with HBx expression, and several downstream pathways including PI3K/AKT, MAPK, and ERK were upregulated. Inhibition of PI3K/AKT pathway reversed the effects of HBx in PDAC cell lines. HBx promotes pancreatic carcinogenesis by regulating the PI3K/AKT signaling pathway.48 Similarly, HBx upregulates the expression of Hepatoma upregulated protein (HURP), promotes the progression of lung squamous cell carcinoma (LUSC), and induces cisplatin resistance in H1299 lung cancer cells.49
In addition to tumors, HBx has also been shown to play a role in other diseases. HBx has also been shown to play a role in other diseases. The risk of macular degeneration (MD) was significantly higher in the HBV-infected cohort than that in the non-HBV-infected cohort (adjusted HR = 1.31; 95% CI = 1.17–1.46). In vitro, researchers demonstrated that overexpression of HBx in the human retinal pigment epithelial (RPE) cell line, ARPE19, significantly reduced cell viability and clonogenic survival upon UV and blue light irradiation. Using gene microarray analysis, we further showed that almost all genes in DNA repair pathways including base excision repair, nucleotide excision repair, mismatch repair, and homologous recombination were significantly down-regulated in UV-induced cell death of HBx-transfected ARPE19 cells.50 That is to say, the HBx may sensitize RPE cells to UV and blue light irradiation and increase the risk of HBV-infection-associated MD through the down-regulation of multiple DNA repair pathways. Multiple research teams have demonstrated that HBx may help HBV-HIV co-infected individuals developed AIDS more rapidly than patients co-infected with HBV and HIV.51–53
Conclusion
HBV is mainly transmitted through blood, mother-to-child and sexual contact. Although HBV is a hepadnavirus, infection has been reported in multiple organs in humans, including the kidneys, lymph nodes, and placenta, as described in this review. At present, it is known that persistent HBV antigenemia can form HBV antigen antibody circulating immune complex and deposit in the glomerulus. In addition, it is speculated that the mechanism of HBV intrauterine infection may be that HBV enters the fetus through the placenta or causes intrauterine infection due to placental rupture or maternal blood mixing with fetal blood, but these hypotheses lack direct evidence. Therefore, the specific mechanism by which HBV enters organs outside the liver is also a topic that deserves more attention from researchers. From our literature study of all HBx in non-liver diseases, we know that HBx in organs such as the kidney, lymph node and placenta is less studied. Except for kidney diseases, research on HBx in other diseases has only focused on its expression promoting the occurrence and development of various types of diseases. However, the specific mechanism still needs further exploration. Therefore, we call for more researchers to explore the mechanism of HBV in diseases other than the liver.
Additionally, apart for the corresponding treatment guidance for HBV-GN, MTCT and DLBCL, there is no corresponding treatment for HBV-related GU, STAD and BRCA (Breast Cancer) diseases. Therefore, more in-depth mechanistic research and exploration of individualized treatment methods are necessary to pay attention. We hope that our article can provide some support for research on HBx in other diseases.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by Xi’an Science and Technology Plan Project (20YXJ0002(5)) and Natural Science Basic Research Program of Shaanxi (No. 2021JZ-35).
Disclosure
This manuscript has not been published previously and is not currently under consideration by any other journals. All authors declare no competing interests in this work.
References
1. Chuang YC, Tsai KN, Ou JJ. Pathogenicity and virulence of Hepatitis B virus. Virulence. 2022;13(1):258–296. doi:10.1080/21505594.2022.2028483
2. Seeger C, Mason WS. Hepatitis B virus biology. Microbiol Mol Biol Rev. 2000;64(1):51–68. doi:10.1128/MMBR.64.1.51-68.2000
3. Tang H, Oishi N, Kaneko S, Murakami S. Molecular functions and biological roles of hepatitis B virus x protein. Cancer Sci. 2006;97(10):977–983. doi:10.1111/j.1349-7006.2006.00299.x
4. Liu Y, Shi C, Fan J, Wang B, Li G. Hepatitis B-related glomerulonephritis and optimization of treatment. Expert Rev Gastroenterol Hepatol. 2020;14(2):113–125. doi:10.1080/17474124.2020.1717948
5. Wang X, Zhou Y, Yuan WJ, Zhu N, Shang MH. Role of Notch1 receptor on apoptosis of renal tubular epithelial cells transfected with HBx gene. Zhonghua Yi Xue Za Zhi. 2013;93(4):300–304.
6. Guan H, Zhu N, Tang G, Du Y, Wang L, Yuan W. DNA methyltransferase 1 knockdown reverses PTEN and VDR by mediating demethylation of promoter and protects against renal injuries in hepatitis B virus-associated glomerulonephritis. Cell Biosci. 2022;12(1):98. doi:10.1186/s13578-022-00835-1
7. He P, Zhang B, Liu D, et al. Hepatitis B virus X protein modulates apoptosis in NRK-52E cells and activates Fas/FasL Through the MLK3-MKK7-JNK3 signaling pathway. Cell Physiol Biochem. 2016;39(4):1433–1443. doi:10.1159/000447846
8. Yang Y, Wang X, Zhang Y, Yuan W. Hepatitis B virus X protein and proinflammatory cytokines synergize to enhance TRAIL-induced apoptosis of renal tubular cells by upregulation of DR4. Int J Biochem Cell Biol. 2018;97:62–72. doi:10.1016/j.biocel.2018.02.006
9. Zhang Y, Wu Q, Zhong L, Wang L, Gong D. Echinacoside promotes the proliferation of human renal tubular epithelial cells by blocking the HBX/TREM2-mediated NF-κB signalling pathway. Mol Med Rep. 2020;22(2):1137–1144. doi:10.3892/mmr.2020.11201
10. He P, Zhang D, Li H, et al. Hepatitis B virus X protein modulates apoptosis in human renal proximal tubular epithelial cells by activating the JAK2/STAT3 signaling pathway. Int J Mol Med. 2013;31(5):1017–1029. doi:10.3892/ijmm.2013.1295
11. He P, Zhou G, Qu D, Zhang B, Wang Y, Li D. HBx inhibits proliferation and induces apoptosis via Fas/FasL upregulation in rat renal tubular epithelial cells. J Nephrol. 2013;26(6):1033–1041. doi:10.5301/jn.5000304
12. Du R, Huang C, Bi Q, et al. URG11 mediates hypoxia-induced epithelial-to-mesenchymal transition by modulation of E-cadherin and beta-catenin. Biochem Biophys Res Commun. 2010;391(1):135–141. doi:10.1016/j.bbrc.2009.11.019
13. Hong L, Zhang J, Min J, et al. A role for MHBst167/HBx in hepatitis B virus-induced renal tubular cell apoptosis. Nephrol Dial Transplant. 2010;25(7):2125–2133. doi:10.1093/ndt/gfp737
14. Li M, Hu L, Zhu F, Zhou Z, Tian J, Ai J. Hepatitis B virus X protein promotes renal epithelial-mesenchymal transition in human renal proximal tubule epithelial cells through the activation of NF-κB. Int J Mol Med. 2016;38(2):513–520. doi:10.3892/ijmm.2016.2637
15. Wang X, Zhou Y, Zhu N, Wang L, Gu LJ, Yuan WJ. The deposition of Notch1 in hepatitis B virus-associated nephropathy and its role in hepatitis B virus X protein-induced epithelial-mesenchymal transdifferentiation and immunity disorder in renal tubular epithelial cells. J Viral Hepat. 2014;21(10):734–743. doi:10.1111/jvh.12244
16. Wang X, Zhou Y, Yuan WJ, Shang MH, Bao JF, Zhu N. Effect of Notch1 signal on hepatitis B virus X-mediated abnormal immune activity in renal tubular epithelial cells. Zhonghua Yi Xue Za Zhi. 2013;93(24):1906–1910.
17. Han W, Luo M, He M, et al. HBx gene transfection affects the cycle of primary renal tubular epithelial cells through regulating cyclin expression. Mol Med Rep. 2018;18(2):1947–1954. doi:10.3892/mmr.2018.9197
18. Yang YT, Du Y, Yuan WJ, Wang L. Role of histone demethylase KDM6B in HBx-mediated podocyte-macrophage transdifferentiation. Zhonghua Yi Xue Za Zhi. 2021;101(12):866–871. doi:10.3760/cma.j.cn112137-20210119-00170
19. Yin XL, Zhou JH. 乙型肝炎病毒X蛋白对小鼠足细胞补体调节蛋白CD59和Crry表达的影响 [Hepatitis B virus X protein upregulates the expression of CD59 and Crry in mouse podocytes]. Zhonghua Er Ke Za Zhi. 2010;48(12):934–938. Chinese.
20. Lei XY, Chen XX, Sun YH, Gao MD, Hu XX, Suo YH. Hepatitis B virus X protein decreases nephrin expression and induces podocyte apoptosis via activating STAT3. Exp Ther Med. 2019;17(5):4223–4229. doi:10.3892/etm.2019.7453
21. Zhang Y, Chen Y, Yang F, Zhou J. HBx transfection limits proliferative capacity of podocytes through cell cycle regulation. Acta Biochim Biophys Sin. 2014;46(12):1016–1023. doi:10.1093/abbs/gmu102
22. Sun YH, Lei XY, Chen XX, Cui WJ, Liu J. Effect and molecular mechanism of interferon-α on podocyte apoptosis induced by hepatitis B virus X protein. Zhongguo Dang Dai Er Ke Za Zhi. 2019;21(9):930–935. doi:10.7499/j.issn.1008-8830.2019.09.017
23. He P, Liu D, Zhang B, et al. Hepatitis B virus X protein reduces podocyte adhesion via downregulation of α3β1 integrin. Cell Physiol Biochem. 2017;41(2):689–700. doi:10.1159/000458428
24. Yu Y, Dong H, Sun J, et al. Hepatitis B virus X mediates podocyte pyroptosis by regulating the ROS/NLRP3 signaling pathway in hepatitis B virus-associated glomerulonephritis. Iran J Basic Med Sci. 2022;25(1):103–109. doi:10.22038/IJBMS.2022.61105.13520
25. Yu Y, Dong H, Zhang Y, et al. MicroRNA-223 downregulation promotes HBx-induced podocyte pyroptosis by targeting the NLRP3 inflammasome. Arch Virol. 2022;167(9):1841–1854. doi:10.1007/s00705-022-05499-3
26. Lu H, Zhou J. HBV X gene transfection upregulates IL-1beta and IL-6 gene expression and induces rat glomerular mesangial cell proliferation. J Huazhong Univ Sci Technolog Med Sci. 2008;28(3):247–250. doi:10.1007/s11596-008-0304-5
27. Lu HZ, Zhou JH. Hepatitis B virus X protein up-regulates tumor necrosis factor-α expression in cultured mesangial cells via ERKs and NF-κB pathways. Asian Pac J Trop Biomed. 2013;3(3):217–222. doi:10.1016/S2221-1691(13)60053-2
28. Wang X, Wang L, Zhu N, Zhou Y, Gu LJ, Yuan WJ. Hepatitis B virus X protein modulates renal tubular epithelial cell-induced T-cell and macrophage responses. Immunol Cell Biol. 2016;94(3):266–273. doi:10.1038/icb.2015.85
29. He P, Lei J, Miao JN, Wu D, Wang C. Cordyceps sinensis attenuates HBx-induced cell apoptosis in HK-2 cells through suppressing the PI3K/Akt pathway. Int J Mol Med. 2020;45(4):1261–1269. doi:10.3892/ijmm.2020.4503
30. Zhao X, Bai X, Xi Y. Intrauterine infection and mother-to-child transmission of hepatitis B virus: route and molecular mechanism. Infect Drug Resist. 2022;15:1743–1751. doi:10.2147/IDR.S359113
31. Bai G, Wang Y, Zhang L, Tang Y, Fu F. The study on the role of hepatitis B virus X protein and apoptosis in HBV intrauterine infection. Arch Gynecol Obstet. 2012;285(4):943–949. doi:10.1007/s00404-011-2096-2
32. Cui H, Li QL, Chen J, Na Q, Liu CX. Hepatitis B virus X protein modifies invasion, proliferation and the inflammatory response in an HTR-8/SVneo cell model. Oncol Rep. 2015;34(4):2090–2098. doi:10.3892/or.2015.4172
33. Bai G, Fu F, Tang Y, Wang Y. Effect of hepatitis B virus infection on apoptosis of a human choriocarcinoma cell line in vitro. J Obstet Gynaecol Res. 2013;39(6):1200–1211. doi:10.1111/jog.12046
34. Wang W, Bai G, Zhang Y, et al. HBxAg suppresses cell apoptosis and promotes the secretion of placental hormones in human placental trophoblasts via activation of the EGFR/Akt pathway. Cell Biol Int. 2018;42(2):237–247. doi:10.1002/cbin.10891
35. Lin Y, Liu Y, Xu D, et al. HBxAg promotes HBV replication and EGFR activation in human placental trophoblasts. Exp Ther Med. 2021;22(5):1211. doi:10.3892/etm.2021.10645
36. Yonghao G, Yanping C, Qiaohua D, et al. The effectiveness of 20 μg hepatitis B vaccine used for the prevention of HBV vertical transmission. Sci Rep. 2022;12(1):11759. doi:10.1038/s41598-022-15744-z
37. Wang Y, Wang H, Pan S, et al. Capable infection of hepatitis B virus in diffuse large B-cell lymphoma. J Cancer. 2018;9(9):1575–1581. doi:10.7150/jca.24384
38. Huang X, Young KH, Guo W, et al. Identification of hepatitis B virus aetiologic antigens, HBx and Pre-S2, in diffuse large B-cell lymphoma. J Viral Hepat. 2020;27(9):948–950. doi:10.1111/jvh.13301
39. Li J, Chen Y, Guo X, et al. lncNBAT1/APOBEC3A is a mediator of HBX-induced chemoresistance in diffuse large B cell lymphoma cells. Mol Ther Nucleic Acids. 2022;27:1064–1077. doi:10.1016/j.omtn.2022.01.015
40. Zhao X, Guo X, Xing L, et al. HBV infection potentiates resistance to S-phase arrest-inducing chemotherapeutics by inhibiting CHK2 pathway in diffuse large B-cell lymphoma. Cell Death Dis. 2018;9(2):61. doi:10.1038/s41419-017-0097-1
41. Marignani M, Marzano A. Surveillance and treatment protocols to detect and treat hepatitis B virus reactivation in hepatitis B surface antigen-negative/antibody to hepatitis B core antigen-positive patients receiving chemotherapy for onco-hematologic malignancies. J Clin Oncol. 2011;29(8):e210–e211. doi:10.1200/JCO.2010.33.6016
42. Guo PT, Yang D, Sun Z, Xu HM. Hepatitis B virus X protein plays an important role in gastric ulcers. Oncol Rep. 2012;28(5):1653–1658. doi:10.3892/or.2012.2011
43. Cui H, Jin Y, Chen F, et al. Clinicopathological evidence of hepatitis B virus infection in the development of gastric adenocarcinoma. J Med Virol. 2020;92(1):71–77. doi:10.1002/jmv.25584
44. Du R, Xia L, Sun S, et al. URG11 promotes gastric cancer growth and invasion by activation of beta-catenin signalling pathway. J Cell Mol Med. 2010;14(3):621–635. doi:10.1111/j.1582-4934.2008.00622.x
45. Klein A, Guhl E, Tzeng YJ, et al. HBX causes cyclin D1 overexpression and development of breast cancer in transgenic animals that are heterozygous for p53. Oncogene. 2003;22(19):2910–2919. doi:10.1038/sj.onc.1206539
46. Adhikari VP, Lu LJ, Kong LQ. Does hepatitis B virus infection cause breast cancer? Chin Clin Oncol. 2016;5(6):81. doi:10.21037/cco.2016.08.04
47. Huang Z, Su B, Liu F, et al. YAP1 promotes tumor invasion and metastasis in nasopharyngeal carcinoma with hepatitis B virus infection. Onco Targets Ther. 2020;13:5629–5642. doi:10.2147/OTT.S247699
48. Chen Y, Bai X, Zhang Q, et al. The hepatitis B virus X protein promotes pancreatic cancer through modulation of the PI3K/AKT signaling pathway. Cancer Lett. 2016;380(1):98–105. doi:10.1016/j.canlet.2016.06.011
49. Chao CC. Inhibition of apoptosis by oncogenic hepatitis B virus X protein: implications for the treatment of hepatocellular carcinoma. World J Hepatol. 2016;8(25):1061–1066. doi:10.4254/wjh.v8.i25.1061
50. Chou RH, Lee CY, Chong LW, et al. HBV infection increases the risk of macular degeneration: the roles of HBx-mediated sensitization of retinal pigment epithelial cells to UV and blue light irradiation. J Transl Med. 2018;16(1):221. doi:10.1186/s12967-018-1594-4
51. Li YJ, Wang HL, Li TS. Hepatitis B virus/human immunodeficiency virus coinfection: interaction among human immunodeficiency virus infection, chronic hepatitis B virus infection, and host immunity. Chin Med J. 2012;125(13):2371–2377.
52. Gómez-Gonzalo M, Carretero M, Rullas J, et al. The hepatitis B virus X protein induces HIV-1 replication and transcription in synergy with T-cell activation signals: functional roles of NF-kappaB/NF-AT and SP1-binding sites in the HIV-1 long terminal repeat promoter. J Biol Chem. 2001;276(38):35435–35443. doi:10.1074/jbc.M103020200
53. Barak O, Aronheim A, Shaul Y. HBV X protein targets HIV Tat-binding protein 1. Virology. 2001;283(1):110–120. doi:10.1006/viro.2001.0883
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Could the Systemic Immune Inflammation Index Predict Diagnosis, Recovery Time, Hypothyroidism, and Recurrence Rates in Subacute Thyroiditis?
Çiftel S, Tüzün Z
International Journal of General Medicine 2023, 16:1375-1382
Published Date: 18 April 2023