Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
The Prognostic Performance of Lung Diffusing Capacity in Preserved Ratio Impaired Spirometry: An Observational Cohort Study
Authors Ogata H , Sha K, Kotetsu Y, Enokizu-Ogawa A, Katahira K, Ishimatsu A, Taguchi K, Moriwaki A, Yoshida M
Received 28 July 2022
Accepted for publication 25 October 2022
Published 28 October 2022 Volume 2022:17 Pages 2791—2799
DOI https://doi.org/10.2147/COPD.S384074
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Min Zhang
Hiroaki Ogata, Kachi Sha, Yasuaki Kotetsu, Aimi Enokizu-Ogawa, Katsuyuki Katahira, Akiko Ishimatsu, Kazuhito Taguchi, Atsushi Moriwaki, Makoto Yoshida
Department of Respiratory Medicine, National Hospital Organization Fukuoka National Hospital, Fukuoka, Japan
Correspondence: Hiroaki Ogata, Department of Respiratory Medicine, National Hospital Organization Fukuoka National Hospital, 4-39-1 Yakatabaru, Minami-ku, Fukuoka, 811-1394, Japan, Tel +81-92-565-5534, Fax +81-92-566-0702, Email [email protected]
Purpose: Similar to chronic obstructive pulmonary disease (COPD), the diffusing capacity of the lung (DLCO) might be decreased and associated with poor prognosis in preserved ratio impaired spirometry (PRISm), a clinical entity as a prodromal phase of COPD. The aims of the present study were to evaluate the distributions of DLCO and to assess the association between DLCO and mortality among subjects with PRISm.
Patients and Methods: We conducted an observational cohort study at the National Hospital Organization Fukuoka National Hospital. We classified the 899 patients ≥ 40 years of age with an assessment of DLCO into five groups based on spirometry: preserved spirometry, PRISm, mild COPD, moderate COPD, and severe/very severe COPD. The prevalence of low DLCO (< 80% per predicted) was compared among the five groups. Using PRISm patients with follow-up data, we further investigated the association of low DLCO with all-cause mortality.
Results: The prevalence of low DLCO in the PRISm group (58.8%) was significantly higher than that in the preserved-spirometry group (21.8%), the mild-COPD group (23.5%), and the moderate-COPD group (36.0%) (all P < 0.01), and it was comparable to that in the severe/very severe-COPD group (63.2%). The results remained unchanged after adjusting for potential confounders. Among the PRISm subjects, the overall survival rate was significantly lower in the low-DLCO group than in the preserved-DLCO group (P < 0.01). The multivariable-adjusted hazard ratio (HR) for all-cause mortality was significantly higher in the low-DLCO group than in the preserved-DLCO group (HR = 10.10 (95% confidence interval 2.33– 43.89)).
Conclusion: Diffusing capacity was more impaired in PRISm subjects than in those with preserved spirometry or mild to moderate COPD. Regarding PRISm, low DLCO was a significant risk factor for all-cause mortality. Clinicians should assess DLCO in the management of PRISm to predict the future risk of overall death.
Keywords: preserved ratio impaired spirometry, chronic obstructive pulmonary disease, diffusing capacity of the lungs, all-cause mortality
Introduction
Preserved ratio impaired spirometry (PRISm), also referred to as restrictive pattern or unclassified spirometry, is a clinical entity associated with an increased risk of developing chronic obstructive pulmonary disease (COPD).1,2 Epidemiological studies worldwide revealed that 3.7–22.3% of the general population were compatible with PRISm,3–11 indicating that PRISm is a common disease condition. Considering its high prevalence and clinical aspects as a prodromal phase of COPD, PRISm, whose pathophysiology is still largely unknown, is an increasing threat to public health. Since the diffusing capacity of the lung for carbon monoxide (DLCO), a useful biomarker for evaluating the gas transfer properties of the respiratory system,12 is generally deficient in COPD,13 it might be also impaired in cases of PRISm. However, this issue has not yet been investigated.
PRISm has been demonstrated to be associated with an increased risk of premature mortality,2,3,11,14 although little is known about the predictive biomarkers for death in PRISm. Since a deficit in DLCO is a strong risk factor for a poor prognosis in COPD,15 DLCO may also be inversely associated with morbidity and mortality in PRISm. However, there has been no study assessing the influence of DLCO on mortality in subjects with PRISm; therefore, the verification of this hypothesis could be of great benefit for improving the clinical management for such patients.
Based on these considerations, we conducted the present study to evaluate the prevalence of reduced DLCO in subjects with PRISm and to compare it with that in cases of normal spirometry or COPD. We also assessed the clinical implication of DLCO impairment as a biomarker for all-cause mortality among subjects with PRISm.
Materials and Methods
Study Population
The current study was conducted as an observational cohort study through a review of medical records at the National Hospital Organization Fukuoka National Hospital. The entire cohort consisted of 899 patients ≥ 40 years of age who had a DLCO assessment from June 1, 2017, to May 31, 2020, regardless of department, with complete information on all relevant covariates. We classified the subjects into five groups based on the results of spirometry: preserved spirometry, PRISm, mild COPD, moderate COPD, and severe/very severe COPD. PRISm was defined as the coexistence of two major criteria: (i) post-bronchodilator forced expiratory volume in 1 second to forced vital capacity (post-BD FEV1/FVC) ≥ 70%; and (ii) reduction in FEV1, that is, post-BD FEV1 per predicted (FEV1% pred) < 80%.3 According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria,16 the severity of COPD was defined as follows: mild, FEV1% pred ≥ 80%; moderate, 50% ≤ FEV1% pred < 80%; severe and very severe, FEV1% pred < 50%.
The distribution of DLCO per predicted (DLCO % pred) and the prevalence of low DLCO by lung function category were evaluated as a cross-sectional analysis using the total cohort. With regard to PRISm, we further investigated the association of low DLCO with all-cause mortality using 111 patients (the PRISm cohort), excluding 8 patients with no follow-up data. For each case in the PRISm cohort, mortality data were collected from June 1, 2017, to December 31, 2021.
Assessment of the Diffusing Capacity and Transfer Coefficient of the Lungs
DLCO and DLCO per alveolar volume (DLCO/VA) were measured via the single-breath method using a CHESTAC-8900 spirometer (Chest MI, Inc., Tokyo, Japan) in accordance with the American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines.17 DLCO % pred and DLCO/VA % pred were calculated using the predicted values of DLCO and DLCO/VA for a person of the same age, gender, and body surface area.18 In accordance with the clinical review article,13 low DLCO was defined as DLCO % pred < 80%. In the same manner, low DLCO/VA was defined as DLCO/VA % pred < 80%. When dividing the PRISm cohort into three groups based on the tertile distribution of DLCO % pred, the cut-off values were as follows: lowest, < 64.0%; middle, 64.0–85.4%; and highest, ≥ 85.5% for DLCO % pred.
Clinical Evaluations
For each case, respiratory physicians reviewed the patient’s medical records and assessed the demographic and clinical characteristics: age, gender, height, weight, smoking exposure, and spirometry. Body mass index (BMI; kg/m2) was calculated as weight divided by height squared. Taking into consideration the guidelines for diagnosing obesity in Japanese subjects,19 obesity, normal weight, and underweight were defined as BMI ≥ 25.0 kg/m2, 18.5 to < 25.0 kg/m2, and > 18.5 kg/m2, respectively. Spirometry was performed before and 15 minutes after BD administration (ie, 200 µg of salbutamol), in line with the guidelines of the Japanese Respiratory Society,20 using CHESTAC-8900. Bronchial reversibility was defined as ≥ 12% and ≥ 200 mL reversibility in post-BD FEV1. The predicted values of FEV1 and slow vital capacity (SVC) for a person of the same age, gender, and height were estimated with the equation for the Japanese population.21
Statistical Analysis
R software version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria) was used to perform all statistical analyses. Two-sided P < 0.05 was considered to indicate statistical significance. For baseline characteristics, the heterogeneity in each variable among the lung function categories was evaluated using the analysis of variance (ANOVA), chi-square test, or Kruskal–Wallis test. Tukey’s test, logistic regression analysis, or the Mann–Whitney U-test with Bonferroni correction was used to assess the statistical difference between the PRISm group and any of the other groups. The heterogeneity in the distribution of DLCO % pred and DLCO/VA % pred across the lung-function categories was also analyzed using an ANOVA. Stratified analysis was performed according to BMI levels or smoking status. The prevalences of low DLCO and low DLCO/VA were calculated for each lung-function group and compared using unadjusted and multivariable-adjusted logistic regression models and estimated as odds ratios (ORs) with 95% confidence intervals (95% CIs). Adjustments were made for age, gender, BMI, and smoking exposure. We performed a sensitivity analysis after excluding subjects with bronchial reversibility in order to exclude asthmatic patients. Kaplan–Meier curves were constructed to show the overall survival by the levels of DLCO % pred and DLCO/VA % pred. Log rank testing was performed to study the influence of low DLCO and low DLCO/VA on all-cause mortality. The trend in overall survival according to the tertile groups of DLCO % pred was assessed with a Cox proportional hazards model. The multivariable-adjusted hazard ratios (HRs) with their 95% CIs of each level of DLCO % pred or DLCO/VA % pred for all-cause death were estimated using a Cox proportional hazards model adjusted for all aforementioned potential confounders. The same model was used to assess the linear trends in the risk of all-cause death across the tertile groups of DLCO % pred.
Ethical Considerations
The study was approved by the National Hospital Organization Fukuoka National Hospital Institutional Review Board for Clinical Research (#F4-2). The study was conducted in accordance with the Declaration of Helsinki. Informed consent was waived due to the retrospective nature of the study. All the data was anonymized for covering the patient data confidentiality and participant privacy.
Results
Level of DLCO % Pred and Prevalence of Low DLCO for Each Lung-Function Group
The prevalence of PRISm was 13.2% among the entire cohort. Table 1 lists the demographic and clinical characteristics of the total cohort. Among the five lung function groups, the prevalence of male gender, the mean age, the mean BMI, and the amount of smoking exposure were not prominent in the PRISm group. Meanwhile, the mean values of absolute SVC and SVC % pred were lowest in the PRISm group.
 |
Table 1 Demographic and Clinical Characteristics of Study Subjects by Lung Function Category |
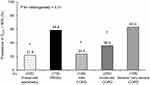
Figure 1 shows the level of DLCO % pred for each lung-function group. The mean values of DLCO % pred were 98.4%, 73.9%, 99.3%, 89.4%, and 71.0% in the preserved-spirometry group, the PRISm group, the mild-COPD group, the moderate-COPD group, and the severe/very severe-COPD group, respectively. In the PRISm group, DLCO % pred was significantly lower than in the other lung function groups except for in the severe/very severe-COPD group. Likewise, the prevalence of low DLCO was significantly higher in subjects with PRISm than in those with preserved spirometry, mild COPD, or moderate COPD (all P < 0.01); it was as high as about 60% in the PRISm group and the severe/very severe-COPD group (Figure 2). The results were substantially similar after adjustments for potential confounders; there was a significant increase in OR in the PRISm group as compared to the preserved-spirometry group, the mild-COPD group, and the moderate-COPD group (all P < 0.01) (Table 2). Broadly similar results were obtained in the analysis stratified by BMI levels (Supplementary Figures S1 and S2) or smoking status (Supplementary Figures S3 and S4). The results were not substantially changed after excluding asthmatic subjects (Supplementary Figures S5 and S6). Further, there was no significant decrease in the level of DLCO/VA % pred or increase in the prevalence of low DLCO/VA in the PRISm group compared to the group with preserved spirometry, mild COPD, or moderate COPD (all P > 0.05) (Supplementary Figures S7 and S8).
 |
Table 2 Multivariable-Adjusted Regression Analysis for Low DLCO in the Patient Groups Based on FEV1 Value |
DLCO % Pred and Risk of All-Cause Mortality in PRISm
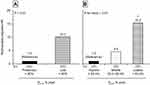
In the PRISm cohort, all-cause death occurred in 27 of 111 individuals (24.3%) with a median follow-up of 899 days (interquartile range 335–1378). As shown in Figure 3A, the overall survival rate was significantly lower in the low-DLCO group than in the preserved-DLCO group (P < 0.01). There was a significant linear trend in the association between DLCO % pred and overall survival (P < 0.01 for the trend) (Figure 3B). A more significant increase in multivariable-adjusted HR for all-cause mortality was observed in the low-DLCO group than in the others (HR = 10.10 (95% CI 2.33–43.89)) (Figure 4A). In the same analysis, the multivariable-adjusted HR of smoking failed to reach statistical significance (P = 0.27). Similarly, the multivariable-adjusted HR for all-cause death was elevated as DLCO % pred decreased (P < 0.01 for the trend); as compared with the highest tertile group, the HR was significantly higher in the lowest tertile group (HR = 15.33 (95% CI 3.39–69.37), P < 0.01) (Figure 4B). Statistical significance was also observed in the associations between low DLCO/VA and all-cause mortality (multivariable-adjusted HR = 4.23 (95% CI 1.70–10.50)) (Supplementary Figures S9 and S10).
Discussion
The present study revealed a higher prevalence of low DLCO in patients with PRISm than in those with normal spirometry, mild COPD, or moderate COPD. The diffusing capacity in PRISm was at the same level as in severe COPD. The results were consistent across BMI levels. Our study also showed that impaired DLCO % pred was a significant risk factor for all-cause mortality in cases of PRISm. To the best of our knowledge, this is the first study to evaluate the distributions of DLCO % pred and to investigate the impact of low DLCO on overall survival in PRISm patients.
In the current study, PRISm was accompanied by deficits in diffusing capacity. It has been well known that there are inverse associations between DLCO and disease severity in COPD,12 since DLCO decreases by alveolar destruction (lung emphysema) and alveolar microvascular damage preceding emphysematous changes.22 DLCO/VA did not decrease as much as DLCO in PRISm, probably due to attenuated impairment by obesity-related deficits in VA. Recent epidemiological studies have established that PRISm is associated with adverse cardiovascular outcomes and dementia,2,3,14,23 probably due to potential hypoxia and/or systemic microvascular atherosclerosis.23 Our results were in accordance with these reports, considering that DLCO reflects not only lung function impairment but also microvascular damage. Although PRISm has been reported to be at least partly attributed to obesity,3,5 which can be linked to increased DLCO % pred,24 diffusing capacity was strongly impaired in PRISm at all BMI levels in the present study, indicating that PRISm is not merely a byproduct of obesity. Deficits in diffusing capacity among subjects with PRISm were observed regardless of subjects’ smoking status; we believe that decrease in DLCO in PRISm is unlikely to be explained solely by smoking exposure. Among PRISm subjects, impairment in DLCO might have been mainly a reflection of enhanced systemic inflammation and oxidative stress,2,4 which was considered to result in a high prevalence of cardiovascular disease and diabetes in this population.3,5,11
In the present research, low DLCO was an independent risk factor for all-cause death in PRISm. As for COPD, several cohort studies have demonstrated that impaired diffusing capacity is one of the most robust risk factors for morbidity and mortality.15,25 This can be explained by DLCO’s potential as a promising candidate for estimating exercise intolerance and physical functioning in patients with COPD.15 The current outcome extends these findings to subjects with PRISm. To expand the utility of DLCO in the management of PRISm, we are planning a prospective cohort study to investigate the associations of DLCO with the cardiovascular burden and incidence of dementia in a future work.
The strengths of our study were the relatively large sample size with DLCO assessment, the uniformity in measurements of DLCO by virtue of the single-center survey, the use of regression models adjusting for multiple confounders to evaluate the independent effects of DLCO, and the longitudinal study design to minimize the potential of reverse causation. However, some potential limitations should be noted. First, the DLCO values were based on a single measurement. This may cause misclassification of the levels of DLCO, which could have weakened the associations found in the present study, biasing the results toward a null hypothesis. Second, the present outcomes might lack external validity and generalizability due to the study design as a single-center analysis, although the lung functions of the study population were substantially comparable to those of other PRISm cohorts.3,5,6,14,26 Third, we did not have access to prescription data of the study population; it was possible that the increase in the DLCO value was due to BD use. However, long-acting bronchodilators have reportedly been unable to contribute to significant improvements in DLCO.27 In addition, there have been no established treatment options, including BD, for PRISm;12,28 we speculate that few cases of PRISm received such treatment, as another survey has demonstrated.6 Hence, this limitation may not have altered our conclusions. Fourth, we might have included some asthmatic subjects in the present study. However, the results of sensitivity analysis using only the subjects without bronchial reversibility were comparable to those from the primary analysis. We therefore speculate that this limitation did not alter the conclusion. Fifth, we did not have access to radiographic information for each subject; some of the individuals with PRISm might have had restrictive lung diseases such as interstitial pneumonia rather than pre-COPD or obesity. Considering the very low prevalence of clinically apparent interstitial lung disease,29 however, the majority of PRISm subjects were unlikely to show evidence of interstitial pneumonia.3,30,31 Lastly, we were unable to investigate the associations of impaired diffusing capacity with cause-specific mortality due to a lack of data concerning the cause of death.
Conclusion
The diffusing capacity of the lung was more impaired in subjects with PRISm than in those with preserved spirometry or mild to moderate COPD. More than half of the cases of PRISm presented with low DLCO. Additionally, low DLCO was an independent risk factor for all-cause mortality, and there was a linear trend in the risk of death across the levels of DLCO % pred. In managing PRISm, clinicians should assess the diffusing capacity of the lung in order to predict each patient’s future risk of death.
Abbreviations
PRISm, preserved ratio impaired spirometry; COPD, chronic obstructive pulmonary disease; DLCO, diffusing capacity of the lung for carbon monoxide; BD, bronchodilator; FEV1/FVC, forced expiratory volume in 1 second to forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; % pred, percent predicted; VA, alveolar volume; ATS/ERS, American Thoracic Society/European Respiratory Society; BMI, body mass index; SVC, slow vital capacity; ANOVA, analysis of variance; OR, odds ratio; 95% CIs, 95% confidence intervals; HR, hazard ratio.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
This study was approved by the National Hospital Organization Fukuoka National Hospital Institutional Review Board for Clinical Research (#F4-2). Given the retrospective nature of the study, written informed consent was not requested by the ethics committee.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Guerra S, Sherrill DL, Venker C, Ceccato CM, Halonen M, Martinez FD. Morbidity and mortality associated with the restrictive spirometric pattern: a longitudinal study. Thorax. 2010;65:499–504. doi:10.1136/thx.2009.126052
2. Wijnant SRA, de Roos E, Kavousi M, et al. Trajectory and mortality of preserved ratio impaired spirometry: the Rotterdam Study. Eur Respir J. 2020;55:1901217. doi:10.1183/13993003.01217-2019
3. Wan ES, Balte P, Schwartz JE, et al. Association between preserved ratio impaired spirometry and clinical outcomes in US adults. JAMA. 2021;326:2287–2298. doi:10.1001/jama.2021.20939
4. Wan ES, Castaldi PJ, Cho MH, et al. Epidemiology, genetics, and subtyping of preserved ratio impaired spirometry (PRISm) in COPDGene. Respir Res. 2014;15:89. doi:10.1186/s12931-014-0089-y
5. Kaise T, Sakihara E, Tamaki K, et al. Prevalence and characteristics of individuals with preserved ratio impaired spirometry (PRISm) and/or impaired lung function in Japan: the OCEAN Study. Int J Chron Obs Pulmon Dis. 2021;16:2665–2675. doi:10.2147/COPD.S322041
6. Washio Y, Sakata S, Fukuyama S, et al. Risks of mortality and airflow limitation in Japanese with preserved ratio impaired spirometry. Am J Respir Crit Care Med. 2022;206(5):563–572. doi:10.1164/rccm.202110-2302OC
7. Guerra S, Carsin A-E, Keidel D, et al. Health-related quality of life and risk factors associated with spirometric restriction. Eur Respir J. 2017;49:1602096. doi:10.1183/13993003.02096-2016
8. Mannino DM, McBurnie MA, Tan W, et al. Restricted spirometry in the Burden of Lung Disease Study. Int J Tuberc Lung Dis. 2012;16:1405–1411. doi:10.5588/ijtld.12.0054
9. Nonato NL, Nascimento OA, Padilla RP, et al. Occurrence of respiratory symptoms in persons with restrictive ventilatory impairment compared with persons with chronic obstructive pulmonary disease: the PLATINO study. Chron Respir Dis. 2015;12:264–273. doi:10.1177/1479972315588004
10. Schwartz A, Arnold N, Skinner B, et al. Preserved ratio impaired spirometry in a spirometry database. Respir Care. 2021;66:58–65. doi:10.4187/respcare.07712
11. Higbee DH, Granell R, Smith GD, Dodd JW. Prevalence, risk factors, and clinical implications of preserved ratio impaired spirometry: a UK Biobank cohort analysis. Lancet Respir Med. 2022;10(2):149–157. doi:10.1016/S2213-2600(21)00369-6
12. Global Initiative for Chronic Obstructive Lung Disease T. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2022 report; 2022. Available from: https://goldcopd.org/2022-gold-reports-2/.
13. Hughes JMB, Pride NB. Examination of the carbon monoxide diffusing capacity (DLCO) in relation to its KCO and VA components. Am J Respir Crit Care Med. 2012;186:132–139. doi:10.1164/rccm.201112-2160CI
14. Marott JL, Ingebrigtsen TS, Çolak Y, Vestbo J, Lange P. Trajectory of preserved ratio impaired spirometry: natural history and long-term prognosis. Am J Respir Crit Care Med. 2021;204:910–920. doi:10.1164/rccm.202102-0517OC
15. Balasubramanian A, Macintyre NR, Henderson RJ, et al. Diffusing capacity of carbon monoxide in assessment of COPD. Chest. 2019;156:1111–1119. doi:10.1016/j.chest.2019.06.035
16. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557–582. doi:10.1164/rccm.201701-0218PP
17. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi:10.1183/09031936.05.00034805
18. Burrows B, Kasik JE, Niden AH, Barclay WR. Clinical usefulness of the single-breath pulmonary diffusing capacity test. Am Rev Respir Dis. 1961;84:789–806. doi:10.1164/arrd.1961.84.6.789
19. The examination committee of criteria for “obesity disease” in Japan, Japan Society for the Study of Obesity T. New criteria for “obesity disease” in Japan. Circ J. 2002;66:987–992. doi:10.1253/circj.66.987
20. Clinical pulmonary functions committee of the Japanese Respiratory Society. Guidelines of Respiratory Function Tests: Spirometry, Flow-Volume Curve, Diffusion Capacity of the Lung [in Japanese]. Tokyo: The Japanese Respiratory Society; 2004.
21. Kubota M, Kobayashi H, Quanjer PH, Omori H, Tatsumi K, Kanazawa M. Reference values for spirometry, including vital capacity, in Japanese adults calculated with the LMS method and compared with previous values. Respir Investig. 2014;52:242–250. doi:10.1016/j.resinv.2014.03.003
22. Santos S, Peinado VI, Ramırez J, et al. Characterization of pulmonary vascular remodelling in smokers and patients with mild COPD. Eur Respir J. 2002;19:632–638. doi:10.1183/09031936.02.00245902
23. Xiao T, Wijnant SRA, Licher S, et al. Lung function impairment and the risk of incident dementia: the Rotterdam Study. J Alzheimer’s Dis. 2021;82:621–630. doi:10.3233/JAD-210162
24. Ray CS, Sue DY, Bray G, Hansen JE, Wasserman K. Effects of obesity on respiratory function. Am Rev Resipir Dis. 1983;128:501–506. doi:10.1164/arrd.1983.128.3.501
25. Lee HY, Kim JW, Lee SH, et al. Lower diffusing capacity with chronic bronchitis predicts higher risk of acute exacerbation in chronic obstructive lung disease. J Thorac Dis. 2016;8:1274–1282. doi:10.21037/jtd.2016.04.66
26. Anami K, Murata S, Nakano H, et al. Physical performance in relation to preserved ratio impaired spirometry: a cross‑sectional study of community‑dwelling older Japanese adults. Sci Rep. 2021;11:17411. doi:10.1038/s41598-021-96830-6
27. Santus P, Radovanovic D, Mascetti S, et al. Effects of bronchodilation on biomarkers of peripheral airway inflammation in COPD. Pharmacol Res. 2018;133:160–169. doi:10.1016/j.phrs.2018.05.010
28. Wan ES. The clinical spectrum of PRISm (preserved ratio impaired spirometry). Am J Respir Crit Care Med. 2022;206(5):524–525. doi:10.1164/rccm.202205-0965ED
29. Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810–816. doi:10.1164/rccm.200602-163OC
30. Washko GR, Hunninghake GM, Fernandez IE, et al.; COPDGene Investigators. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. 2011;364:897–906. doi:10.1056/NEJMoa1007285
31. Wan ES, Fortis S, Regan EA, et al.; COPDGene Investigators. Longitudinal phenotypes and mortality in preserved ratio impaired spirometry in the COPDGene Study. Am J Respir Crit Care Med. 2018;198:1397–1405. doi:10.1164/rccm.201804-0663OC
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.