Back to Journals » International Journal of Nanomedicine » Volume 16
Targeted Nanotherapeutics Using LACTB Gene Therapy Against Melanoma
Authors Liu J, Yang L, Yuan X, Xiong M, Zhu J, Wu W, Ren M, Long J, Xu X, Gou M
Received 29 July 2021
Accepted for publication 22 October 2021
Published 17 November 2021 Volume 2021:16 Pages 7697—7709
DOI https://doi.org/10.2147/IJN.S331519
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Farooq A. Shiekh
Jinlu Liu,1,* Ling Yang,1,* Xin Yuan,2,* Meimei Xiong,1 Jiao Zhu,1 Wenbi Wu,1 Min Ren,1 Jianlin Long,3 Xuewen Xu,2 Maling Gou1
1State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, Sichuan University, Chengdu, 610041, People’s Republic of China; 2Department of Plastic and Burn Surgery, West China Hospital of Sichuan University, Chengdu, 610041, People’s Republic of China; 3Department of Medical Oncology, Chongqing University Cancer Hospital, Chongqing, 400030, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Maling Gou
State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, Sichuan University, Chengdu, 610041, People’s Republic of China
Email [email protected]
Xuewen Xu
Department of Plastic and Burn Surgery, West China Hospital of Sichuan University, Chengdu, 610041, People’s Republic of China
Email [email protected]
Introduction: β-lactamase (LACTB) is a tumor suppressor gene in various tumors including melanoma. However, it remains challenging to efficiently deliver the LACTB gene into melanoma. Recently, we designed a nonviral nanocarrier iRGD/DOTAP/MPEG-PDLLA (iDPP) that could deliver gene targetedly to melanoma efficiently without obvious adverse effects.
Methods: In this study, the tumor-targeted nanoparticle iDPP was prepared to deliver LACTB gene to treat melanoma in vitro and in vivo. First, the expression level of LACTB in 6 clinical specimens of melanoma patients was evaluated. Subsequently, the characteristics of iDPP/LACTB nanocomplexes were studied. Afterwards, the in vitro and in vivo anti-tumor efficacy of the iDPP/LACTB nanocomplexes were explored utilizing the B16-F10 mouse melanoma cell line and the B16-F10 subcutaneous melanoma model.
Results: Compared with the normal epithelium, the expression level of LACTB in melanoma tissues was significantly downregulated. In vitro B16-F10 cell tests showed iDPP/LACTB nanocomplexes could increase the mRNA levels of P21, Bid, Bax, Pidd1, and Sival genes and up-regulate the p53 signaling pathway of melanoma cells, thus promoting cell apoptosis and blocking the cell cycle. Injected intravenously, iDPP nanoparticles could deliver DNA to the subcutaneous melanoma targetedly. Based on in vivo mouse xenograft model, iDPP/LACTB nanocomplexes could effectively inhibit tumor proliferation and induce tumor apoptosis, thus significantly inhibiting melanoma growth (tumor inhibition rate is about 68%) in the subcutaneous B16-F10 melanoma model.
Conclusion: The downregulated LACTB might be a potential target for melanoma therapy. The iDPP/LACTB nanocomplexes could inhibit the growth of the mouse melanoma without obvious side effects, which provide a new option for melanoma gene therapy research.
Keywords: melanoma, LACTB, nanocomplexes, gene therapy
Introduction
Cutaneous melanoma is the most lethal type of skin cancers with increasing morbidity.1,2 It was reported that 55,500 cases of diagnosed cutaneous melanoma patients died worldwide annually.3 The estimated mortality percentage of cutaneous cancer among all skin cancers reached as high as 72% in USA.4 Hence, exploring novel treatment for melanoma is under urgent need.
Mitochondrial malfunction was demonstrated to be involved in tumorigenesis and progression of cancer, which thus can be a candidate for cancer treatment.5 Recently, the downregulation of the mitochondrial β-lactamases (LACTB) has been reported to be related to a worse prognosis in a variety of cancers.6–10 LACTB, a mitochondrial serine protease resembling the bacterial penicillin-binding protein, is predominantly expressed in mitochondrial intermembrane space of mammalian liver and skeletal muscle.11–13 Besides, LACTB was significantly associated with the blood level of high-density lipoprotein cholesterol (HDL-C)14,15 and could regulate the metabolic process of mice.16 Interestingly, LACTB could enhance the upregulation of the expression of monocyte chemotactic protein-1 (MCP-1) in THP-1 macrophages as well, resulting in the occurrence of atherosclerosis.17 The function of LACTB on cancer, however, remains unclear until Keckesova found its role as a suppressor gene in breast cancer.6 LACTB can inhibit the proliferation of multiple types of breast cancer cells by disrupting mitochondrial lipid metabolism and modulating cell differentiation. Further research of LACTB in colon cancer revealed the capability of LACTB to inhibit the decomposition of p53 protein through guarding it against the interaction with MDM2.7 A recent study has also found that LACTB could suppress uveal melanoma progression,18 but how to deliver LACTB into melanoma in vivo efficiently and safely remains a challenge.
Gene therapy offers new therapeutic options in multiple medical fields with its great potential to achieve durable, curative clinical benefit by a single treatment.19,20 However, the most challenging issue in successfully applying gene therapy to human diseases is how to efficiently transport nucleic acids to host cells by suitable vectors.21 Compared to viral vectors, nonviral vectors possess better biodegradability, and higher safety due to the lack of oncogenic and immunogenic potential.22,23 Our previous research developed a nonviral gene delivering system (iDPP) by the self-assembly of the target peptide C18-PEG-iRGD (iRGD), N-[1-(2,3-dioleoyloxy) propyl]-N,N,N-trimethylammonium chloride (DOTAP), and monomethoxy poly(ethlene glycol)-poly(D,L-lactide) (MPEG-PDLLA), whose transfection efficiency could reach above 90% in melanoma cell line (Supplementary Figure 1). In this study, we confirmed the downregulation of LACTB expression in 6 human melanoma samples. Afterwards, we constructed the iDPP/LACTB nanocomplexes to deliver LACTB to B16-F10 melanoma cells in vitro and in vivo. Furthermore, we investigated the effect of iDPP/LACTB nanocomplexes on cell proliferation and apoptosis in vitro as well as tumor growth in vivo mouse model (Figure 1). Compared with dacarbazine (DTIC), a positive chemotherapy drug for treating melanoma in clinical practice, iDPP/LACTB nanocomplexes might be a promising choice for future melanoma therapy research.
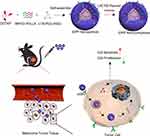 |
Figure 1 Schematic illustration of iDPP/LACTB nanocomplexes for the treatment of melanoma. |
Materials and Methods
MPEG-PDLLA was purchased from Daigang (Jinan, China). B16F10 murine melanoma cell line was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cell Counting Kit-8 (CCK8) and dacarbazine (DTIC) were purchased from Meilunbio® (Dalian, China). AnnexinV-FITC/PI apoptosis detection kit was provided by Vazyme (Nanjing, China). Cell cycle detection kit was purchased from KeyGen Biotech (Nanjing, China). Crystal violet was obtained from Beyotime Biotechnology (Shanghai, China). Animal Total RNA Isolation Kit (RE-03011), RT EasyTM II (Premix) (RT-01021) and Real Time PCR EasyTM-SYBR Green I (QP-0101T) were provided by FOREGENE (Chengdu, China).
Female C57BL/6 mice (6–8 weeks old) were obtained from Dashuo Biotechnology Company (Chengdu, China) and kept in standard specific pathogen-free (SPF) conditions. The mice were maintained for one week to adapt to the environment before experiments began. All animal experiments were conducted according to the guidelines of the Institutional Animal Care and Treatment Committee of West China Hospital of Sichuan University (ethic approval number 20211219A).
The Patient Samples
Paraffin blocks of 6 patients diagnosed with malignant melanoma were provided by West China Hospital of Sichuan University (Chengdu, China) and were conducted in accordance with the Declaration of Helsinki. Immunohistochemistry Staining (IHC) was carried out and the Image J software was used to evaluate the expression level of LACTB in melanoma patients. Patient consent and approval from the ethics committee of West China Hospital of Sichuan University were obtained (ethic approval number 2021822).
The Construction of LACTB Plasmids
The whole gene synthesis of the recombinant plasmid LACTB was carried out by using the empty plasmid of PVAX as the carrier, while the mouse LACTB sequence was obtained on the website of NCBI Nucleotide database (https://www.ncbi.nlm.nih.gov/CCDS/CcdsBrowse.cgi?REQUEST=CCDS&GO=MainBrowse&DATA=CCDS40674.1). The extraction and purification of LACTB recombinant plasmid were carried out according to the instructions of Tiangen non-endotoxin plasmid extraction kit and the concentration was measured, and plasmids were stored at −20°C for further use.
Preparation and Characterization of iDPP/LACTB Nanocomplex
iDPP nanoparticles were prepared according to our previous work.24 Briefly, 9mg MPEG-PDLLA, 1mg DOTAP and 0.2mg iRGD were dissolved by 1mL dichlormethane, respectively, before mixing. Afterwards, the solvent was removed by reduced pressure distillation under 40°C. Meanwhile, MPEG-PDLLA, DOTAP and iRGD formed a thin film around the round bottom flask. 5mL 5% (w/v) glucose water was added to rehydrate the film to prepare iDPP nanoparticles under 40°C. Ultimately, the solution was stored at 4°C for further use.
Particle size and zeta potential were measured by dynamic light scattering (Nano-ZS, Malvern Instruments, Malvern, U.K.). Transmission electron microscope (TEM) was utilized to exhibit the morphology of iDPP nanoparticles. Gel retardation assay was employed to assess the binding ability of iDPP nanoparticles to the LACTB DNA. Erythrocyte agglutination and hemolytic test were carried out to evaluate the safety of iDPP/LACTB nanocomplexes.
The uptake of iDPP/LACTB nanocomplexes by mouse melanoma cell line B16-F10 was analyzed by flow cytometry. At first, B16-F10 cells were cultured in 6-well plates at a density of 1×105 cells/mL for 24 h. At the same time, iDPP nanoparticles, lipofectamine3000 and PEI25K were incubated with YOYO-1-labeled LACTB plasmids to form complexes with green fluorescence, which were then transfected into B16-F10 cells for 4 h. Finally, the cells were collected, and the transfection efficiency was detected by flow cytometry.
The mouse subcutaneous melanoma model was established to explore the targeting ability of iDPP/LACTB nanocomplexes by in vivo imaging. Firstly, the B16-F10 cells growing in logarithmic phase were collected and re-suspended with serum-free medium, and then inoculated at a density of 2×105 per mouse on the right dorsal abdomen of mice. When the tumor grew to about 1cm in diameter, iDPP/luciferase plasmid (pGL-6) nanocomplex was injected into mice via caudal vein. 72 hours later, 200 μL luciferase substrate D-luciferase sodium solution (15mg/mL) was injected into the caudal vein. After 20min, IVISLumina imager was used for observation and photography.
Cell Counting Kit-8 (CCK8) Test on B16-F10 Cells
The cell proliferation inhibition of iDPP/LACTB nanocomplexes on B16-F10 cells was evaluated by CCK8 cell count kit. Briefly, B16-F10 cells were seeded into a 96-well plate at the concentration of 1000 per well first. After 24h, NS, iDPP/PVAX (12.5 μg/0.5 μg), DTIC (150 μM) and iDPP/LACTB (12.5 μg/0.5 μg) were added into the well. 48h later, CCK8 was involved to measure the viability of B16-F10 cells. Cell viability was counted according to the following equation: cell viability = (OD-OD blank)/(OD ns-OD blank) *100%.
Cell Apoptosis Test on B16-F10 Cells
Cell apoptosis ratio was detected by flow cytometry after staining of Annexin V/Propidium iodide kit. First, cells were prepared in a 6-well plate at the concentration of 3×104 cell per well for 24h. NS, iDPP/PVAX (50 μg/2 μg), DTIC and iDPP/LACTB (50 μg/2 μg) were then added into the well for 6h. Afterwards, cells continued to grow under complete medium for 48h. The next procedures were operated according to the manufacturer’s instruction. Briefly, after digested by trypsin free of EDTA, cells were collected to be stained by Annexin V for 5min and then another 5 min by PI.
Detection of Mitochondrial Membrane Potential by JC-1 Dyes
Studies have shown that when the mitochondrial function is abnormal, there will be a series of reactions, including the decrease of mitochondrial membrane potential and the increase of ROS production.25 When the mitochondrial membrane potential (ψ mt) is high, JC-1 dyes can form polymers and emit red fluorescence, while ψ mt decreases, JC-1 dyes emit green fluorescence as monomers. JC1 mitochondrial membrane potential detection kit was used to evaluate the ability of iDPP/LACTB nanocomplex to induce ψ mt downgrades of B16-F10 cells. The cells were treated according to the method and transfection scheme, and JC-1 staining was performed 48 hours later. The experimental scheme referred to the instructions of the kit and finally the cells were observed under confocal fluorescence microscope.
Measurement of Reactive Oxygen Species (ROS) Using Flow Cytometry
ROS is the single-electron reduction product of oxygen, and mitochondria is one of the main sources of endogenous ROS production.25,26 When the function of mitochondria is impaired, the level of ROS increases. Flow cytometry was used to detect the level of ROS in B16-F10 cells treated with iDPP/LACTB nanocomplexes. The cells were treated according to the method and transfection scheme before being collected for detection 48 hours later. Intracellular ROS production was detected by DCFH-DA probe method. 10 μL MDCFH-DA working solution was added to the collected cells and cells were incubated at 37°C for 20 minutes to be detected by flow cytometry.
Cell Cycle Detecting Assay on B16-F10 Cells
Cell cycle blockage of iDPP/LACTB nanocomplexes was analyzed by cell cycle detecting kit. The cell preparation and treatment were the same as above. After the treatment, cells were first fixed with precooled 70% (v/v) alcohol at 4°C overnight. Next, Propidium iodide was applied to stain the cell nucleic acids before detection by flow cytometry. Finally, the results were introduced into the Modifit software to analyze the cell cycle stages.
Colony Formation Assay
Crystal violet was utilized to visualize the cell colony formation. Cells were first seeded into the 6-well plate at the concentration of 1000 cell per well. After the same treatment, cells were grown for another week. Firstly, 4% paraformaldehyde was used to fix cells for 20 minutes, and then 0.1% crystal violet dye solution was added to the plate. Finally, cells were photographed after the plate was dried.
Transcriptome-Library Sequencing
RNA gene sequencing (RNA-seq) was used to analyze the changes of related pathways in B16-F10 cells induced by iDPP/LACTB nanocomplexes. Firstly, B16-F10 cells in logarithmic phase were collected and plated at the density of 2×105 cells per well for 24 h. After changing the culture medium to serum and antibiotics free medium, the cells were transfected with 50 μg iDPP/2 μg PVAX gene preparation and 50 μg iDPP/2 μg LACTB nanocomplexes, respectively, and then changed to complete medium 6 hours later. After 48 hours, the cells of each well were lysed with Trizol and RNA-seq test was carried out.
Evaluation of Anti-Tumor Efficacy in the Subcutaneous B16-F10 Melanoma Model
The establishment of mouse subcutaneous melanoma model was consistent with that above. On the 6th day after tumor grafting, the tumor-bearing mice were randomly divided into 4 groups. The mouse body weight, the long diameter and short diameter of the tumor were recorded. The tumor volume was calculated according to the formula (tumor volume = 0.5 × long × width × width). At the same time, the treatment of each group was started by intravenous injection of 5% normal saline (NS), iDPP/PVAX nanocomplexes (125 μg/5 μg), 6 μg/g DTIC27 and iDPP/LACTB nanocomplexes (125 μg/5 μg), respectively. The treatment was given every other day for a total of 5 times. When the mice in the NS group were weak, the peripheral blood of the mice in each group was taken for biochemical detection and then the mice in each group were killed. The tumor tissue of each group was photographed and weighed. The tumor inhibition rate of each group was calculated according to the following formula: tumor inhibition rate (%) = (average tumor weight of NS group-tumor weight of each group)/average tumor weight of NS group × 100%, some of which were stored in the refrigerator at-80 °C for subsequent RNA extraction. Some of them were fixed with 4% paraformaldehyde for pathological sections. At the same time, the sections of important organs (heart, liver, spleen, lung, kidney) were taken to evaluate the safety of iDPP/LACTB nanocomplexes in vivo.
The Histology Analysis and Its Interpretation
Various tissues like tumor, heart, spleen, etc. were fixed with 4% paraformaldehyde for 48 hours before undergoing dehydration, paraffin embedding, section and staining. Briefly, gradient dehydration was performed on the tissues before paraffin embedding and slicing (4 μm/piece). The dried slices were then dewaxed and hydrated for hematoxylin and eosin (H&E) and IHC staining. For IHC staining, 0.01M citrate buffer of PH6.0 was prepared for microwave antigen repair (medium-high heat for 8 minutes, high-fire 4min) ahead of the subsequent staining steps: 10 min peroxidase blocking, 15min sheep serum incubation, 5 μg/mL LACTB primary antibody 4°C overnight, 30min second antibody incubation at room temperature, 5min DAB coloration and hematoxylin re-staining.
The mRNA Expression Level of LACTB by Quantitative Real-Time Fluorescence Analysis
The total RNA of cells and tissues was extracted using the Total RNA extracting Kit (Fuji Biotech, Chengdu). Total RNA was reverse-transcribed to synthesize single strand DNA (cDNA) to undergoing RT-PCR. With GAPDH as the internal reference gene, the primers of each gene are as follows. Forward-GAPDH: CAACAGCAACTCCCACTCTTCCA; Reverse-GAPDH: ACCCTGTTGCTGTAGCCGTAT; Forward-LACTB: AGCTGGATCTGGACCTTCCTGTG; Reverse-LACTB: CGGCATCTGGCTTCGCTGTC.
Statistical Analysis
All the experimental data were analyzed by GraphPad Prism 6.0 and expressed as average ± standard deviation. The difference between the two groups was statistically analyzed by double-tailed Student’s t-test (ns p > 0.05, * p < 0.05, ** p < 0.01, *** p < 0.001).
Results
LACTB is Downregulated in Melanoma and Decreased LACTB Expression Predicts Poor Prognosis
In order to investigate the potential role of LACTB in melanoma, the histomorphology of 6 clinical human skin melanoma tissues was observed. As shown in Figure 2A, typical melanoma cells exhibited nest-like distribution. The immunohistochemical positive rate of LACTB in melanoma tissues was characterized by integrated optical density (IOD). From Figure 2B and C, the positive rate of LACTB in these 6 melanoma tissues was significantly lower than that in adjacent normal epithelial tissues. Furthermore, the survival of melanoma patients with different expression of LACTB based on the TCGA database (http://www.oncolnc.org/) was analyzed. The results (Figure 2D) indicated that lower level of LACTB in melanoma tissues is related to a worse survival, thus inspiring us to use the iDPP nanoparticle to deliver LACTB for melanoma treatment.
iDPP/LACTB Nanocomplexes Inhibit Proliferation of Melanoma Cells in vitro
Furthermore, to evaluate the potential anti-tumor effects of iDPP/LACTB nanocomplexes, we constructed the LACTB plasmid based on the PVAX empty vector and prepared the iDPP/LACTB nanocomplexes. These nanocomplexes were capable of protecting the LACTB plasmid from dissociating and could be effectively taken up by B16-F10 cells, along with good biocompatibility and electrical neutrality (Supplementary Figure 2). After transfection, the LACTB mRNA level of iDPP/LACTB group was significantly higher than that of the other three groups (Figure 3A). Among the other three groups, NS referred to the normal saline, iDPP/PVAX referred to the empty vector control, and DTIC referred to the positive control of chemotherapy. Meanwhile, compared with other groups, iDPP/LACTB nanocomplexes could remarkably inhibit the proliferation of B16-F10 cells, the inhibition rate was 44.54 ± 6.85% (Figure 3B). The results of clone proliferation test showed that the area of B16-F10 cell clones in iDPP/LACTB nanocomplexes was the least (Figure 3C and D), indicating that iDPP/LACTB nanocomplexes could significantly reduce the monoclonal formation of B16-F10 cells. Besides, B16-F10 cells in iDPP/LACTB nanocomplexes treated group could be blocked in the stage of G0/G1 cell cycle, with the proportion of 60.2 ± 2.85% (Figure 3E and F). The above results demonstrated that iDPP/LACTB nanocomplexes could effectively inhibit the proliferation of B16-F10 melanoma cells by blocking cells in G0/G1 cell cycle.
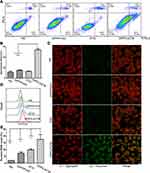
iDPP/LACTB Nanocomplexes Induce Apoptosis of Melanoma Cells in vitro
Since LACTB is located in mitochondria, which is vital in cell apoptosis, we investigated the effect of iDPP/LACTB nanocomplexes on apoptosis of melanoma cells. As shown in Figure 4A and B, the apoptotic proportion of iDPP/LACTB group was 26.89 ± 3.25%, which was significantly higher than 6.55 ± 0.62% in the positive drug group DTIC. When the mitochondrial function was impaired, the mitochondrial membrane potential decreased and ROS production increased. From Figure 4C, the mitochondrial membrane potential of the cells treated with iDPP/LACTB nanoparticles was lower so some JC-1 dyes entered the cytoplasm in the form of monomers, hence showing red and green fluorescence. Meanwhile, the other three groups had normal mitochondrial membrane potential and JC-1 dyes were retained in the mitochondrial matrix in the form of polymers with strong red fluorescence. Flow cytometry showed that the level of ROS in iDPP/LACTB group was the highest (Figure 4D and E). These results suggested that iDPP/LACTB nanocomplexes can induce apoptosis of B16-F10 melanoma cells by inducing the decrease of mitochondrial membrane potential and increasing the production of ROS.
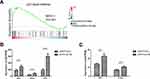
The in vitro tests above showed that iDPP/LACTB nanocomplexes could inhibit cell proliferation and induce apoptosis of melanoma cells. In order to further explore the anti-melanoma mechanism of iDPP/LACTB nanocomplexes, samples were subjected to BGI (Huada Genomics Institute Co. Ltd, China) and RNA-seq was used to analyze the changes of related signal pathway after treatment with iDPP/LACTB nanocomplexes. Huada bgi system was used to analyze the related pathway enrichment of gene sequencing results. As shown in Figure 5A, GASE enrichment analysis showed that p53 pathway was up-regulated in B16-F10 cells treated with iDPP/LACTB nanocomplexes (NES > 1, p < 0.001). p53 signal pathway is an important pathway to regulate cell growth and death, which can affect cell cycle and apoptosis.28 As shown in Figure 5B and C, the mRNA levels of cell cycle-related genes (P21) and apoptosis-related genes (Bid, Pidd1, Bax, Sival) on p53 pathway in B16-F10 cells treated with iDPP/LACTB nanocomplexes were significantly higher than those in iDPP/PVAX group. The above results suggested that the anti-tumor effect of iDPP/LACTB nanocomplexes in melanoma cells may be related to the upregulation of p53 signal pathway.
iDPP/LACTB Nanocomplexes Inhibit Melanoma Growth in vivo
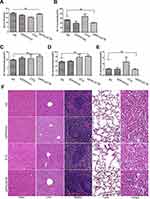
After tail vein injection, the nanocomplexes could concentrate in the melanoma tissue, demonstrating the targeting ability of this nanocomplexes in vivo (Figure 6A). The RT-PCR analysis of tumor tissues showed that iDPP/LACTB nanocomplexes significantly increased the expression level of LACTB in melanoma (Figure 6B).
In the B16-F10 subcutaneous melanoma model, compared with NS, iDPP/PVAX and DTIC (6 μg/g) group, the growth rate of tumor volume decreased significantly in iDPP/LACTB group (Figure 6C and D). After weighing the tumor weight of each group, the tumor inhibition rate of each group was calculated. As shown in Figure 6E, the tumor inhibition rate of iDPP/LACTB gene preparation was about 68%, which was significantly higher than that of DTIC treatment (about 39%). Besides, H&E staining showed that the density of tumor cells in melanoma tissue in iDPP/LACTB group was significantly lower than that in other groups (Figure 6F). Furthermore, the positive rate of Ki67 in tumor tissues of each group was NS 65.77 ± 2.42%, iDPP/PVAX 54.11 ± 0.99%, DTIC 30.86 ± 1.13%, iDPP/LACTB 11.63 ± 0.60% (Figure 6F and G). The positive rate of Ki67 in iDPP/LACTB treatment group was significantly lower than that in other groups. At the same time, the apoptosis rate of tumor tissue in each group was 0.74 ± 0.60% in NS group, 1.08 ± 1% in iDPP/PVAX group, 5.55 ± 4.75% in DTIC group and 21.62 ± 16.49% in iDPP/LACTB group (Figure 6F and H). The apoptosis rate of tumor tissue in iDPP/LACTB group was significantly higher than that in other groups. The results above demonstrated that iDPP/LACTB nanocomplexes could inhibit proliferation and induce cell apoptosis of melanoma cells in vivo.
Safety Assessment of the iDPP/LACTB Nanocomplexes in vivo
As shown in Figure 7A, the body weight of mice treated with iDPP/LACTB nanocomplexes was not significantly different from that of NS. After treatment, the peripheral blood of mice in each group was collected for the determination of biochemical indexes such as serum creatinine (Scr), glutamic pyruvic transaminase (ALT), blood glucose (Glu) and blood triglyceride (TG). As shown in Figure 7B–E, the levels of Scr, ALT, Glu and TG in iDPP/LACTB group were not significantly different from those in NS group. No pathological changes such as edema, inflammation, degeneration and necrosis were found in the five important organs of each treatment group (Figure 7F). Above all, intravenous injection of iDPP/LACTB nanocomplexes is safe and exhibits no obvious toxicity in vivo.
Discussion
LACTB, a mitochondrial protein, has been demonstrated as a tumor suppressor in various cancers.6–10 Our research confirmed the downregulation of LACTB in 6 melanoma patients and prepared the iDPP/LACTB nanocomplexes to deliver LACTB for melanoma treatment. In this study, the iDPP/LACTB nanocomplexes could promote cell apoptosis, accompanied by the decrease of mitochondrial membrane potential and increase of ROS. Besides, the iDPP/LACTB nanocomplexes could also inhibit the proliferation of melanoma cells obviously. Currently, it has been widely studied that p53 protein can regulate various biological processes like cell apoptosis, cell cycle, DNA damage repair and senescence.29 The RNA-seq test found a significant enrichment of the p53 pathway after treatment of iDPP/LACTB nanocomplexes, and upregulation of target genes regulating cell apoptosis and cell cycle as well (p21, Bax, Bid, Pidd, Sival).
In this study, we showed for the first time that systematic LACTB gene therapy with a non-viral vector is feasible for melanoma. Viruses are naturally evolved vectors which efficiently integrate their genes into host cells.30 However, the related adverse-effects of carcinogenesis and immunogenicity, difficulty in vector production and limited DNA packaging capacity restrict their application.23,31 Therefore, non-viral gene delivery systems attracted more and more attentions in cancer gene therapy.32 Nevertheless, commonly used nonviral vectors like PEI25K form cationic complexes after binding with DNA, which cause their interaction and aggregation with negatively charged serum protein, thus resulting in obvious toxicity and poor targeting ability.33 Here, the constructed iDPP/LACTB nanocomplex was a neutral targeted system which was readily prepared by complexing iDPP nanoparticle with LACTB plasmid through electrostatic interactions. The obtained iDPP/LACTB nanocomplex had a high transfection efficiency over PEI25K and Lipo3000, which benefited from the specific binding of iRGD to the integrin receptors overexpressed on the surface of melanoma cells.34,35 After intravenous administration, the iDPP/LACTB nanocomplex could efficiently target and transfect cancer cells, resulting in the effective cancer treatment.
Therefore, this work designed an effective and safe system to deliver LACTB to melanoma cells, which may provide potential options for melanoma treatment.
Conclusion
In this study, we found that the expression of LACTB in melanoma tissues was downregulated compared with normal tissues. Here, an electrically neutral nanocomplex iDPP/LACTB was prepared and targeted to treat melanoma, which provides a potential new choice for melanoma gene therapy.
Acknowledgments
This work was supported by the National Natural Science Foundation (82073363), Sichuan Science and Technology Program (2020YFQ0059), 1·3·5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYYC08007), Chongqing Natural Science Foundation (cstc2019jcyj-msxmX0614).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34.
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
3. Zhang Y, Hou J, Shi S, et al. CSN6 promotes melanoma proliferation and metastasis by controlling the UBR5-mediated ubiquitination and degradation of CDK9. Cell Death Dis. 2021;12(1):118.
4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30.
5. Weinberg SE, Chandel NS. Targeting mitochondria metabolism for cancer therapy. Nat Chem Biol. 2015;11(1):9–15.
6. Keckesova Z, Donaher JL, De Cock J, et al. LACTB is a tumour suppressor that modulates lipid metabolism and cell state. Nature. 2017;543(7647):681.
7. Zeng K, Chen X, Hu X, et al. LACTB, a novel epigenetic silenced tumor suppressor, inhibits colorectal cancer progression by attenuating MDM2-mediated p53 ubiquitination and degradation. Oncogene. 2018;37(41):5534–5551.
8. Zhang J, He Y, Yu Y, et al. Upregulation of miR-374a promotes tumor metastasis and progression by downregulating LACTB and predicts unfavorable prognosis in breast cancer. Cancer Med. 2018;7(7):3351–3362.
9. Xue C, He Y, Zhu W, et al. Low expression of LACTB promotes tumor progression and predicts poor prognosis in hepatocellular carcinoma. Am J Transl Res. 2018;10(12):4152.
10. Li H-T, Dong D-Y, Liu Q, Xu Y-Q, Chen L. Overexpression of LACTB, a mitochondrial protein, that inhibits proliferation and invasion in glioma cells. Oncol Res. 2017;27(4):423.
11. Pagliarini DJ, Calvo SE, Chang B, et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134(1):112–123.
12. Peitsaro N, Polianskyte Z, Tuimala J, et al. Evolution of a family of metazoan active-site-serine enzymes from penicillin-binding proteins: a novel facet of the bacterial legacy. BMC Evol Biol. 2008;8(1):1.
13. Polianskyte Z, Peitsaro N, Dapkunas A, et al. LACTB is a filament-forming protein localized in mitochondria. Proc Natl Acad Sci USA. 2009;106(45):18960–18965.
14. Teslovich TM, Musunuru K, Smith AV, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466(7307):707–713.
15. Willer CJ, Schmidt EM, Sengupta S, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45(11):1274–1283.
16. Yang X, Deignan JL, Qi H, et al. Validation of candidate causal genes for obesity that affect shared metabolic pathways and networks. Nat Genet. 2009;41(4):415–423.
17. Lu J-B, Yao -X-X, Xiu J-C, Hu Y-W. MicroRNA-125b-5p attenuates lipopolysaccharide-induced monocyte chemoattractant protein-1 production by targeting inhibiting LACTB in THP-1 macrophages. Arch Biochem Biophys. 2016;590:64–71.
18. Ma Y, Wang L, He F, et al. LACTB suppresses melanoma progression by attenuating PP1A and YAP interaction. Cancer Lett. 2021;506:67–82.
19. Dunbar CE, High KA, Joung JK, Kohn DB, Ozawa K, Sadelain M. Gene therapy comes of age. Science. 2018;359(6372):175.
20. Piperno A, Sciortino MT, Giusto E, Montesi M, Panseri S, Scala A. Recent advances and challenges in gene delivery mediated by polyester-based nanoparticles. Int J Nanomedicine. 2021;16:5981–6002.
21. Hecker JG. Non-Viral, lipid-mediated DNA and mRNA gene therapy of the Central Nervous System (CNS): chemical-based transfection. Methods Mol Biol. 2016;1382:307–324.
22. Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014;15(8):541–555.
23. Wang Y, Li C, Du L, Liu Y. A reactive oxygen species-responsive dendrimer with low cytotoxicity for efficient and targeted gene delivery. Chin Chem Lett. 2020;31(1):275–280.
24. Luo L, Yang Y, Du T, et al. Targeted nanoparticle-mediated gene therapy mimics oncolytic virus for effective melanoma treatment. Adv Funct Mater. 2018;28:29.
25. Lleonart ME, Grodzicki R, Graifer DM, Lyakhovich A. Mitochondrial dysfunction and potential anticancer therapy. Med Res Rev. 2017;37(6):1275–1298.
26. Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol. 2020;21(7):363.
27. Liu Q, Xu N, Liu L, et al. Dacarbazine-loaded hollow mesoporous silica nanoparticles grafted with folic acid for enhancing antimetastatic melanoma response. ACS Appl Mater Interfaces. 2017;9(26):21673–21687.
28. Hafner A, Bulyk ML, Jambhekar A, Lahav G. The multiple mechanisms that regulate p53 activity and cell fate. Nat Rev Mol Cell Biol. 2019;20(4):199–210.
29. Kandoth C, McLellan MD, Vandin F, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502(7471):333–339.
30. Chen YH, Keiser MS, Davidson BL. Viral vectors for gene transfer. Curr Protoc Mouse Biol. 2018;8(4):e58–e58.
31. Nguyen GN, Everett JK, Kafle S, et al. A long-term study of AAV gene therapy in dogs with hemophilia A identifies clonal expansions of transduced liver cells. Nat Biotechnol. 2021;39(1):47–55.
32. Li J, Roise JJ, He M, Das R, Murthy N. Non-viral strategies for delivering genome editing enzymes. Adv Drug Deliv Rev. 2021;168:99–117.
33. Ibba ML, Ciccone G, Esposito CL, Catuogno S, Giangrande PH. Advances in mRNA non-viral delivery approaches. Adv Drug Deliv Rev. 2021;177:113930.
34. Cheng Y, Ji Y. RGD-modified polymer and liposome nanovehicles: recent research progress for drug delivery in cancer therapeutics. Eur J Pharm Sci. 2019;128:8–17.
35. Mohammed-Saeid W, Soudy R, Tikoo R, Kaur K, Verrall R, Badea I. Design and evaluation of gemini surfactant-based lipoplexes modified with cell-binding peptide for targeted gene therapy in melanoma model. J Pharm Pharm Sci. 2018;21:363–375.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.