Back to Journals » Journal of Inflammation Research » Volume 16
Systemic Immune-Inflammation Index and Long-Term Mortality in Patients with Stroke-Associated Pneumonia
Authors Xie M, Yuan K , Zhu X, Chen J, Zhang X , Xie Y , Wu M , Wang Z, Liu R, Liu X
Received 1 December 2022
Accepted for publication 2 March 2023
Published 17 April 2023 Volume 2023:16 Pages 1581—1593
DOI https://doi.org/10.2147/JIR.S399371
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Mengdi Xie,1,* Kang Yuan,2,* Xinyi Zhu,2,* Jingjing Chen,3 Xiaohao Zhang,4 Yi Xie,2 Min Wu,1 Zhaojun Wang,2 Rui Liu,2 Xinfeng Liu1,2,5
1Department of Neurology, Jinling Hospital, Nanjing Medical University, Nanjing, People’s Republic of China; 2Department of Neurology, Affiliated Jinling Hospital, Medical School of Nanjing University, Nanjing, People’s Republic of China; 3Department of Neurology, Changhai Hospital, Navy Medical University, Shanghai, People’s Republic of China; 4Department of Neurology, Nanjing First Hospital, Nanjing Medical University, Nanjing, People’s Republic of China; 5Stroke Center & Department of Neurology, Division of Life Sciences and Medicine, The First Affiliated Hospital of USTC, University of Science and Technology of China, Hefei, Anhui, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xinfeng Liu, Department of Neurology, Jinling Hospital, Nanjing Medical University, No. 305 East Zhongshan Road, Nanjing, Jiangsu Province, 210000, People’s Republic of China, Tel +86 2584801861, Fax +86 2584805169, Email [email protected] Rui Liu, Department of Neurology, Affiliated Jinling Hospital, Medical School of Nanjing University, No. 305 East Zhongshan Road, Nanjing, 210000, Jiangsu Province, People’s Republic of China, Tel +86 2584801861, Fax +86 2584805169, Email [email protected]
Background: Systemic immune inflammation has been investigated as a prognostic marker of different diseases. This study is designed to assess the association of systemic immune-inflammation index (SII) with long-term mortality of stroke-associated pneumonia (SAP) patients.
Methods: Patients aged ≥ 18 years with SAP were selected from the Nanjing Stroke Registry Program in China. We retrospectively evaluated systemic immune-inflammation response with SII and pneumonia severity with the pneumonia severity index and the confusion, uremia, elevated respiratory rate, hypotension, and aged 65 years or older score. To explore the correlation between SII and mortality in SAP patients, multivariable Cox regressions and competing risk regressions were conducted. Mediation analysis was also performed to assess the role of pneumonia severity.
Results: Among 611 patients in the SAP population, death occurred in 164 patients (26.8%) during the median follow-up of 3.0 (1.2– 4.6) years. In multivariate analysis, higher SII scores could predict increased mortality in patients with SAP (adjusted hazard ratio 2.061; 95% confidence interval, 1.256– 3.383; P = 0.004), and the association was mediated by pneumonia severity. Moreover, adding SII to traditional models improved their predictive ability for mortality.
Conclusion: Our study displayed that SII was characterized in SAP patients with different prognoses. Elevated SII scores increased the risk of mortality. Further research is required for the clinical practice of the index among SAP patients.
Keywords: systemic immune-inflammation index, stroke-associated pneumonia, mortality, China
Introduction
Stroke is the second-leading cause of death and the third-leading cause of death and disability combined globally.1 Of particular note, the incidence and prevalence of stroke have grown faster in China than in other countries due to the aging of the general population.2 It is imperative to improve the quality of stroke treatment and the quality of life in stroke patients. Stroke-associated pneumonia (SAP) is one of the most frequent medical complications in patients with stroke, whose incidence varies among different studies.3–6 Poststroke pneumonia has been proven to be a risk factor for the development of other non-pneumonia complications after acute ischemic stroke (AIS).7 In addition, SAP is significantly associated with worse prognoses,5 prolonged hospitalization durations,8 and increased medical costs.9 Previous studies have developed several risk scores or machine learning models to predict SAP.3,4,10–12 Nevertheless, risk factors that influence the outcomes of SAP remain unclear. A study from the UK demonstrates that age, pre-stroke disability, dementia, lung cancer, and previous transient ischemic attack are independently associated with the 6-month mortality of SAP patients. However, laboratory indicators are not significant in this study.13 Thus, an easy and objective predictor is needed to predict SAP outcomes.
Peripheral blood cells are verified to be significantly correlated with outcomes of both stroke and pneumonia. It is suggested that the neutrophil-to-lymphocyte ratio (NLR) is associated with stroke severity, functional outcomes, and recurrent stroke in patients with AIS.14 Moreover, NLR and platelet-to-lymphocyte ratio could predict early neurological outcomes after thrombolysis in patients with AIS.15 In community-acquired pneumonia, NLR is associated with mortality, and adding NLR to the confusion, uremia, elevated respiratory rate, hypotension, and aged 65 years or older (CURB-65) score could significantly increase diagnostic accuracy.16 Moreover, higher levels of platelets, even in the normal range, are prognostic for 30-day mortality of pneumonia patients.17
Systemic immune-inflammation index (SII), calculated by peripheral platelet, neutrophil, and lymphocyte counts, was introduced to reflect the balance of host immune and inflammatory status in patients with cancers at first.18 Recently, elevated SII are reported to predict severity and adverse outcomes in stroke patients.19,20 A large-scale prospective study demonstrates that the highest group of SII is associated with the risk of stroke, myocardial infarction, and all-cause mortality in patients without cardiovascular diseases.21 Nevertheless, these studies eliminated patients with pneumonia. The value of SII for predicting mortality in patients with SAP is unclear.
Hence, our study is aimed to detect the relationship between the SII score and long-term mortality in patients with SAP. We hope to provide clues for the early detection of patients with poor prognoses.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Participants
This study retrospectively collected data on consecutive patients with AIS from January 1, 2013, to December 31, 2019 in the Nanjing Stroke Registry Program. This prospective registry was approved by the Ethics Review Board of Jinling Hospital (approval number 2010NLY-018). Detailed information about Nanjing Stroke Registry Program has been published previously.22 All patients who were clinically diagnosed with AIS were registered at admission after providing informed consent. This study was conducted according to the Declaration of Helsinki.
Patients were included in our study if they (1) had AIS diagnosed within 7 days of onset; (2) were aged ≥ 18; (3) had a brain computed tomography or magnetic resonance imaging scan right before or during hospitalization; (4) were diagnosed as stroke-associated pneumonia during the first 7 days after the index stroke;23 (5) finished at least 1 year of follow-up or deceased before then. Patients were excluded if they (1) lacked measurement of blood cells including neutrophils, lymphocytes, and platelets; (2) had an active infection within 2 weeks before admission; (3) had immunosuppression (including taking more than 10 mg of prednisone-equivalent per day for at least 2 weeks, receiving cytotoxic therapy, or having acquired immunodeficiency syndrome) or active tuberculosis; (4) had a history of hematological tumor or autoimmune disease; (5) died within a month of onset.
Clinical Assessment
Baseline characteristics of study subjects, including demographic characteristics, vascular risk factors, clinical factors, and laboratory results were all collected. Laboratory data were obtained within 24h of hospital admission. The severity of stroke was assessed by well-trained neurologists who were not involved in the study using the National Institutes of Health Stroke Scale (NIHSS) score.24 Stroke subtypes were divided according to the Trial of Org 10172 in Acute Stroke Treatment classification.25 Dysphagia was identified with a bedside screening test performed on the first day after admission. Patients were classified as nonsmokers, former smokers, and current smokers according to their smoking status. Patients were defined as former smokers if they had quit smoking for more than 30 days before the index stroke, while other smokers were classified as current smokers. A similar definition was also applied to alcohol consumers.
The diagnosis of SAP was conducted retrospectively by an infection specialist who was blind to other clinical and laboratory results according to the medical record or the antibiotic therapy. We included both probable and definite SAP, regardless of chest x-ray or CT findings.23 Well-validated scores of pneumonia burdens were assessed within 24 hours of SAP diagnoses, including the pneumonia severity index (PSI) and the CURB-65 score (Table S1).26,27 The SII score was calculated as total peripheral platelet counts (P; ×109/L) × neutrophil counts (N; ×109/L) / lymphocyte counts (L; ×109/L).18 To minimize the skewness of the distribution, the SII was transformed into a logarithmic scale.
Follow-Up and Endpoint
The primary outcome was defined as all-cause mortality, which was evaluated through structured telephone interviews conducted by a trained nurse or doctor. Patients were followed up at 3, 6, and 12 months during the first year after discharge and annually thereafter.
Statistical Analysis
Continuous variables were presented as mean ± SD or median (interquartile range) according to their distribution and compared with Student’s t-test or Mann–Whitney U-test. The χ2 test or Fisher exact test was used for categorical variables presented as n (%). Comparison of multiple values between subgroups was conducted by trend tests or Kruskal–Wallis H-tests as appropriate.
To explore the association between the SII score and long-term mortality after SAP, several common variables were selectively included in the Cox proportional hazard regression. The proportional-hazard assumptions were examined with the Schoenfeld residuals test and no violations were found. The Deviance residuals test was used to identify outliers and no extreme outliers were found. The Martingale residuals test was used to examine the log-linearity of continuous SII. Model 1 was adjusted for age and sex. Model 2 was adjusted for model 1 as well as cardiovascular risk factors, such as stroke etiology, atrial fibrillation, history of cancer, hypertension, diabetes mellitus, dyslipidemia, and smoking and drinking status. Variables with a significance level < 0.1 in univariate analysis were considered confounders and included in model 3, after a backward selection except for counts of neutrophils, platelets and lymphocytes, which were parts of SII. We used the long-rank test to compare the Kaplan-Meier curve among the 25th and 75th percentiles of the SII score.
To explore the predictive power of the SII score, we calculated the area under the receiver operating characteristic curve at different time points, taking variables in model 3 as confounders. Moreover, the net reclassification index was calculated to assess the added value of the SII score to conventional prognostic factors.28 To further examine the possible non-linear association of the SII index with long-term mortality, we used a restricted cubic spline with 4 knots located at the 5th, 35th, 65th, and 95th percentiles of the distribution. The final cubic spline model was adjusted for confounders included in model 3.29
Considering that pneumonia severity is also an important predictor of mortality, which is commonly evaluated using the PSI and CURB-65 score, we then investigated the relationship between SII score, pneumonia severity, and long-term mortality with mediation analysis.30 Additionally, mortality related to stroke and other causes were regarded as competing events, and then proportional-hazards analysis and Fine and Grey’s model were conducted to eliminate deaths caused by other reasons.
All statistical analyses were conducted with R version 4.1.2. (R Foundation, Vienna, Austria) and a two-sided P value <0.05 was considered to be statistically significant.
Results
Baseline Characteristics of Patients with SAP
The inclusion and exclusion process of SAP patients was displayed in Figure 1. Among 611 patients who were finally enrolled in the study, the median age was 66, most of them were men (70%), and the baseline NIHSS score was 13. The rate of thrombectomy and intravenous thrombosis was 28.2% and 12.3% respectively. During our median follow-up of 3.0 years, mortality occurred in 164 patients, who were more likely to have higher PSI (101.5 vs 76.0; P < 0.001) or CURB-65 (2 vs 1; P < 0.001) scores. As to the blood cell counts, higher levels of neutrophils (7.7 vs 6.7; P = 0.001) and lower levels of lymphocytes (1.1 vs 1.4; P < 0.001) were observed in patients who died. However, no significant difference was found in platelet counts between the 2 groups. In addition, deceased patients tended to have older age (P < 0.001), history of atrial fibrillation (P < 0.001) and cancer (P = 0.009), higher initial NIHSS scores (P < 0.001), stroke etiology of cardio-embolism (P < 0.001), dysphagia (P < 0.001), and higher levels of Hemoglobin A1c (HbA1c, P < 0.001), C-reactive protein (CRP; P < 0.001), procalcitonin (PCT; P = 0.001), and interleukin-6 (IL-6, P = 0.001). The 2 groups did not differ in terms of body mass index, drinking status, prevalence of hypertension, diabetes, and dyslipidemia (Table 1).
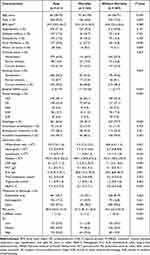 |
Table 1 Characteristics of All Patients Diagnosed with SAP |
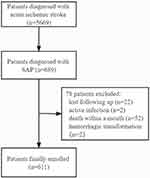 |
Figure 1 Flow chart of patients enrollment. |
The baseline characteristics stratified by the quartile of SII score are displayed in Table S2. In comparison to patients in the first quartile of SII, those with increasing SII scores had a higher probability of cardio-embolism (P < 0.001), intravenous thrombolysis (P = 0.024), endovascular treatment (P < 0.001), and a higher baseline NIHSS (P < 0.001) and PSI (P < 0.001) score. However, no significant difference was found among patients with or without successful recanalization. The distribution of continuous SII in different treatment groups is shown in Figure S1.
Association of the SII Score with Mortality
The highest quarter of SII accounted for 39.0% of patients who died during our follow-up, significantly higher than 19.9% of survivors (Table 1). In univariable analysis, higher SII scores were related to a higher probability of mortality (Table S3). The cumulative survival probability plot stratified by the score is shown in Figure 2. It was observed that different SII groups could clearly distinguish patients with different survival rates. Other predictors for mortality were age, stroke etiology, baseline NIHSS score, pneumonia severity, dysphagia, history of atrial fibrillation, cancer, smoking status, neutrophil counts, lymphocyte counts, CRP, HbA1c and use of antiplatelet and statin (Table S3). In the multivariable regression analysis, the SII score (adjusted hazard ratio [aHR] 2.061; 95% confidence interval [CI], 1.256–3.383; P = 0.004) remained significant after adjusting for potential confounders in model 3 (Table 2).
 |
Table 2 Multivariable Analysis of SII Index to Predict Mortality |
 |
Figure 2 Kaplan-Meier survival curves of mortality categorized by SII. Abbreviation: SII, systemic immune-inflammation index. |
According to the time-dependent receiver operating curve, the SII score showed good discriminative ability at 1, 3, and 6 years (Figure 3). Furthermore, adding the score to 3 models modestly improved the net reclassification index for the prediction of long-time mortality (Table 3). The restricted cubic spline curve adjusted for variables in model 3 displayed an ascending trend of SII score (P = 0.059 for nonlinearity, Figure 4) with the risk of long-term mortality within a certain range.
 |
Table 3 Net Reclassification Improvement (NRI) for Mortality After the Addition of the SII Index |
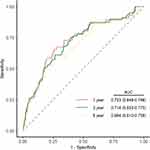 |
Figure 3 Time-dependent ROC curves at 1, 3, and 6 years for SII. Abbreviations: AUC, area under the curve; ROC, receiver operating characteristic; SII, systemic immune-inflammation index. |
 |
Figure 4 Association was fitted with restricted cubic spline with 4 knots (at 5th, 35th, 65th, 95th percentiles) adjusted for covariates included in model 3 in Table 3. The solid line represented the hazard ratio and the dashed lines represented the 95% confidence interval. |
For sensitivity analysis, we observed a significant association between the SII score and stroke-related mortality after considering mortality due to other causes as competing risks. In mediation analysis, both direct and indirect effects of SII are significant, and the proportion of mediated effects accounted for 31% and 26% when we used the PSI and CURB-65 scores as mediators (Table S4).
Discussion
In the present study, we detected the predictive ability of SII for mortality among patients with SAP. Different from previous studies, we conducted a much longer follow-up (up to 8 years at most) to observe the long-term mortality of SAP patients. The findings displayed that a higher SII score was significantly associated with the mortality of SAP patients in the 1st and 3rd years after adjusting for potential confounders. The alterations of immune and inflammatory status in the acute stage had not only short-term but also long-term influences on SAP patients.
The incidence of SAP ranges from 5% to 30% due to different diagnostic criteria.3,4,31–33 In our study, we observed 12.2% of stroke patients developing SAP, which was similar to most studies. In terms of mortality, 164 out of 611 (26.8%) patients deceased during our follow-up, which was significantly lower than Tinker’s study.13 This low mortality might be attributed to our inclusion criterion of AIS and exclusion criterion of death within a month. Previous studies have suggested many biomarkers in predicting mortality of stroke, such as cardiac troponin T,34 amino-terminal pro–B-type natriuretic peptide,35 total cholesterol,36 γ-glutamyl transferase,37 estimated glomerular filtration rate,38 and neurofilament light39 et al. Several inflammatory parameters are also meaningful in the prediction of stroke mortality. Patients with increased serum levels of procalcitonin (PCT) and C-reactive protein (CRP) have a higher mortality at 3 month or even 1 year after the index stroke.40–42 In the present study, serum levels of CRP could also predict long-term mortality in the univariable and multivariable Cox regression analysis. Nevertheless, these inflammation biomarkers are commonly regarded as acute-phase proteins and used to predict short-term outcomes in previous studies.40,41 As far as we know, effects of SAP are prolonged and comprehensive. It is suggested that both stroke and pneumonia have a long-term impact on the cardiovascular system, increasing mortality.43 Yende et al hypothesize that pathophysiologic changes after pneumonia would persist beyond recovery on account of the highly concentrated pro-inflammatory cytokines and abnormal immune responses.44 Thus, screening these high-risk patients is crucial for careful surveillance over a long time.
In the present study, SII was associated with long-term mortality in SAP patients after adjusting for potential confounders. SII was firstly introduced as a biomarker of overall immune and inflammation status in patients with cancers.18,45 As an integrated index calculated by peripheral counts of lymphocytes, neutrophils, and platelets, SII seems more comprehensive and representative than NLR or platelet-lymphocyte ratio.18,19 Studies have exhibited the significance of SII in the prediction of stroke severity and outcomes.19–21 Zhang et al find that baseline systemic inflammatory indicators including SII at admission may reflect long-term prognoses in patients with vertebrobasilar artery occlusion.46 Wang et al reveal that SII is a significant predictor for the short-term and long-term functional outcomes and all-cause mortality of AIS patients.47 These findings confirm the prediction ability of SII for the long-term prognosis. To the best of our knowledge, few studies have applied SII to predict long-term prognosis in SAP patients. However, several studies have reported significant associations between components of SII and SAP. Nam et al have indicated that NLR at admission could predict the risk of SAP in patients with AIS. Besides, there seems to be a close correlation between NLR and the severity of SAP.31 Li et al report that the platelet-to-lymphocyte ratio also acts as an independent risk factor of SAP after adjusting for potential confounders.48 Consistent with previous studies, our study found that SII at admission was an appropriate marker for predicting long-term mortality after the double strike of stroke and pneumonia. Furthermore, elevated SII at admission may imply underlying immune and inflammation alterations associated with both stroke and undetected SAP.
As an integrated index calculated by peripheral counts of lymphocytes, neutrophils and platelets, the association of SII and SAP could be elucidated as follows. First of all, immunodepression and the following systemic inflammatory responses play an important role in the development of SAP. The main courses include the shift of T-helper 1 responses to T-helper 2 and the following peripheral lymphocytopenia. Studies have found that these alterations in the immune system impair the immune function, such as the defense against bacteria.49 Additionally, in neutrophils and monocytes, the antimicrobial defense mechanisms are undermined, including oxidative burst and NETosis.50 These changes enhance the susceptibility to SAP in stroke patients and increase mortality.51 Of particular note, Vogelgesang et al have demonstrated that the delay of the T-lymphocyte recovery is only exhibited in patients who developed infection.52 Since the neuroinflammation in the central nervous system is influenced by peripheral infections, inflammatory responses are intense and long-lasting in SAP patients.53 Studies reveal that this proinflammatory signature could be observed in peripheral blood between 3 to 12 months after SAP, causing long-lasting cognitive decline.54 In addition, platelets are usually believed to be excessively activated and accumulated in AIS patients, hampering stroke recovery.55 However, the immune function of platelet is gradually recognized. Platelets or platelet-neutrophil interaction can recognize and kill microbial pathogens including bacteria.56,57 Moreover, endotoxin and pathogens in the circulation of SAP patients could activate platelets and generate a procoagulant status.58 Consequently, the activation of platelet seems more violent in patients with severe infection. Adding platelet to NLR is crucial for the prediction of SAP outcomes. Collectively, SII seems to be a comprehensive biomarker to reflect the integral immune and inflammation status that would maintain for a long time after stroke and pneumonia. Serum levels of SII in the acute stage are closely related to the development and outcome of SAP.
Competing risk analysis also revealed the correlation between SII and stroke-related mortality. Previous studies have confirmed that infection could act as a stroke trigger, especially in the short time window after the index infection.59 The explanations for this include inflammation, thrombophilia and other mechanisms in infected patients. These alterations also contribute to stroke to some extent. It is reported that pneumonia has a long-term influence on the cardiovascular system.59 Jennie et al discover that patients hospitalized for pneumonia are more likely to suffer from respiratory or cardiovascular diseases again and have higher mortality after a long-term follow-up.43 As in previous studies, our study indicated that SII seemed an appropriate marker of the systemic status of SAP patients and was correlated with stroke-related mortality.
We also found other factors associated with long-term mortality in SAP patients, including age, NIHSS, dysphagia, history of cancer and atrial fibrillation, HbA1c and use of statin. Most of them are regarded as risk factors for poor stroke outcomes and the development of SAP.4,5,48 Previous studies find that age is not only a risk factor for SAP but also a predictor of mortality in SAP patients,13 which is consistent with our research. Our study also indicated that HbA1c instead of glucose acts as a confounder, which might imply the importance of glycemic stability on long-term outcomes. Tsakiridou et al suggest that increased HbA1c could significantly predict repeated bloodstream infections, which might be manifested by the effect of immunodepression.60 In addition, we also discovered that cardio-embolism and large-artery atherosclerosis, usually associated with higher stroke severity, accounted for higher proportions in higher SII groups, which is consistent with Nam’s study about SAP.31
We observed that SII classifications were linearly correlated with PSI and CURB-65 scores. The association between SII and long-term mortality was partly mediated through the pneumonia severity evaluated with PSI or CURB-65 score. In a prospective study on pneumonia, higher PSI scores are consistent with higher long-term mortality.43 This is in line with our study. It is recommended that patients with more than 90 scores of PSI suffer from severe pneumonia.31 The average score of PSI was 101.5 among patients with poor prognoses in our study. Hence, it is reasonable to hypothesize that SII is a promising index in SAP patients indicating the severity of both stroke and pneumonia.
Thus, our study suggested that the calculation of SII was of vital importance for the prediction of long-term prognoses in SAP patients. The alterations of immune and inflammatory status in the acute stage had not only short-term but also long-term influences on SAP patients. To the best of our knowledge, this is the first study with a relatively large sample size to introduce SII to SAP patients and explore the potential effects of systemic changes in SAP patients over a long period. Given that the measurement of SII is based on total platelet, neutrophil, and lymphocyte counts, it is convenient to obtain during routine laboratory examination. Such a risk stratification tool can lead to more cost-efficient and personalized health care and guide clinicians on the medication. Higher SII scores represent intense inflammatory responses or worse immune responses. A recent study indicates that stroke patients with chronic inflammatory diseases who receive TNF-α inhibitors have decreased risk of mortality and SAP incidence,61 which implies the efficiency of a combination of anti-inflammation with immunomodulation therapy for SAP patients with higher SII scores. Moreover, considering non-pneumonia complications are mediated by pneumonia through similar mechanisms,7 SII is a promising biomarker of other complications after stroke. Further studies are needed to verify our hypothesis. Collectively, our study supplemented the roles of SII in poststroke pneumonia and provided new thoughts in clinical practice.
This study also has limitations. First, our data were collected from a single-center database, which might generate biases in defining and diagnosing some variables. The retrospective design might cause biases due to missing variables and follow-up information. Second, the calculation of SII was only limited to the first measure of blood sample within 24 hours of admission. The following changes in parameters were not traced and their effects on long-term mortality were uncertain. In addition, medication after discharge such as antiplatelet or anticoagulant drugs and statins was only collected once at discharge. Medication compliance is also considered an important factor in prognosis. During the follow-up, we discovered that part of patients experienced pneumonia repeatedly after discharge and whether such chronic inflammatory status could increase mortality remained to be confirmed. Furthermore, information on (antibiotic) treatment was not collected, which may interfere with outcomes.
In conclusion, SII acts as a predictor of long-term all-cause and stroke-related mortality after SAP. The association between SII and mortality appears to be mediated by the severity of stroke and pneumonia. Further research with a prospective design and a large sample is required to confirm the clinical relevance between SII and pneumonia after stroke.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
This study was approved by the Ethics Review Board of Jinling Hospital (approval number 2010NLY-018). All patients were registered at admission after providing informed consent.
Acknowledgments
We are sincerely grateful to all the researchers and patients who participated in this study.
Funding
The project is supported by National Natural Science Foundation of China (NO. U20A20357, 81901248, 81870946).
Disclosure
The authors have declared no conflicts of interest with respect to the authorship or publication of this article.
References
1. Feigin VL, Stark BA, Johnson CO.; Collaborators GBDS. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. 2021;20:795–820. doi:10.1016/S1474-4422(21)00252-0
2. Tu WJ, Hua Y, Yan F, et al. Prevalence of stroke in China, 2013–2019: a population-based study. Lancet Reg Health West Pac. 2022;28:100550. doi:10.1016/j.lanwpc.2022.100550
3. Smith CJ, Bray BD, Hoffman A, et al. Can a novel clinical risk score improve pneumonia prediction in acute stroke care? A UK multicenter cohort study. J Am Heart Assoc. 2015;4:e001307. doi:10.1161/JAHA.114.001307
4. Ji R, Shen H, Pan Y, et al. Novel risk score to predict pneumonia after acute ischemic stroke. Stroke. 2013;44:1303–1309. doi:10.1161/STROKEAHA.111.000598
5. Finlayson O, Kapral M, Hall R, et al. Risk factors, inpatient care, and outcomes of pneumonia after ischemic stroke. Neurology. 2011;77:1338–1345. doi:10.1212/WNL.0b013e31823152b1
6. Kishore AK, Vail A, Chamorro A, et al. How is pneumonia diagnosed in clinical stroke research? A systematic review and meta-analysis. Stroke. 2015;46:1202–1209. doi:10.1161/STROKEAHA.114.007843
7. Ji R, Wang D, Shen H, et al. Interrelationship among common medical complications after acute stroke: pneumonia plays an important role. Stroke. 2013;44:3436–3444. doi:10.1161/STROKEAHA.113.001931
8. Annette IM, Andersen G, Heidi H, Marie L, Svendsen MSPJ. In-hospital medical complications, length of stay, and mortality among stroke unit patients. Stroke. 2011;2011:1.
9. Katzan IL, Dawson NV, Thomas CL, Votruba ME, Cebul RD. The cost of pneumonia after ischemic stroke. Neurology. 2007;68:1938–1943. doi:10.1212/01.wnl.0000263187.08969.45
10. Hoffmann S, Malzahn U, Harms H, et al. Development of a clinical score (a²ds²) to predict pneumonia in acute ischemic stroke. Stroke. 2012;43:2617–2623. doi:10.1161/STROKEAHA.112.653055
11. Huang J, Liu M, He W, Liu F, Cheng J, Wang H. Use of the a2ds2 scale to predict morbidity in stroke-associated pneumonia: a systematic review and meta-analysis. BMC Neurol. 2021;21:33. doi:10.1186/s12883-021-02060-8
12. Li X, Wu M, Sun C, et al. Using machine learning to predict stroke-associated pneumonia in Chinese acute ischaemic stroke patients. Eur J Neurol. 2020;27:1656–1663. doi:10.1111/ene.14295
13. Tinker RJ, Smith CJ, Heal C, et al. Predictors of mortality and disability in stroke-associated pneumonia. Acta Neurol Belg. 2021;121:379–385. doi:10.1007/s13760-019-01148-w
14. Xue J, Huang W, Chen X, et al. Neutrophil-to-lymphocyte ratio is a prognostic marker in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2017;26:650–657. doi:10.1016/j.jstrokecerebrovasdis.2016.11.010
15. Gong P, Liu Y, Gong Y, et al. The association of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and lymphocyte to monocyte ratio with post-thrombolysis early neurological outcomes in patients with acute ischemic stroke. J Neuroinflammation. 2021;18:51. doi:10.1186/s12974-021-02090-6
16. Cury VF, Antoniazzi LQ, Oliveira PHK, et al. Developing the pneumonia-optimized ratio for community-acquired pneumonia: an easy, inexpensive and accurate prognostic biomarker. PLoS One. 2021;16:e0248897. doi:10.1371/journal.pone.0248897
17. Moulis G, Christiansen CF, Darvalics B, Andersen IT, Norgaard M. Platelet count within the normal range at hospital admission is associated with mortality in patients with community-acquired pneumonia. Clin Epidemiol. 2020;12:711–716. doi:10.2147/CLEP.S245067
18. Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20:6212–6222. doi:10.1158/1078-0432.CCR-14-0442
19. Zhou YX, Li WC, Xia SH, et al. Predictive value of the systemic immune inflammation index for adverse outcomes in patients with acute ischemic stroke. Front Neurol. 2022;13:836595. doi:10.3389/fneur.2022.836595
20. Hou D, Wang C, Luo Y, et al. Systemic immune-inflammation index (sii) but not platelet-albumin-bilirubin (palbi) grade is associated with severity of acute ischemic stroke (ais). Int J Neurosci. 2021;131:1203–1208. doi:10.1080/00207454.2020.1784166
21. Jin Z, Wu Q, Chen S, et al. The associations of two novel inflammation indexes, sii and siri with the risks for cardiovascular diseases and all-cause mortality: a ten-year follow-up study in 85,154 individuals. J Inflamm Res. 2021;14:131–140. doi:10.2147/JIR.S283835
22. Liu X, Xu G, Wu W, Zhang R, Yin Q, Zhu W. Subtypes and one-year survival of first-ever stroke in Chinese patients: the Nanjing stroke registry. Cerebrovasc Dis. 2006;22:130–136. doi:10.1159/000093241
23. Smith CJ, Kishore AK, Vail A, et al. Diagnosis of stroke-associated pneumonia: recommendations from the pneumonia in stroke consensus group. Stroke. 2015;46:2335–2340. doi:10.1161/STROKEAHA.115.009617
24. Brott T, Adams HP
25. Adams HP
26. Shah BA, Ahmed W, Dhobi GN, Shah NN, Khursheed SQ, Haq I. Validity of pneumonia severity index and curb-65 severity scoring systems in community acquired pneumonia in an Indian setting. Indian J Chest Dis Allied Sci. 2010;52:9–17. doi:10.5005/ijcdas-52-1-9
27. Aujesky D, Fine MJ. The pneumonia severity index: a decade after the initial derivation and validation. Clin Infect Dis. 2008;47(Suppl 3):S133–139. doi:10.1086/591394
28. Thomas LE, O’Brien EC, Piccini JP, D’Agostino RB, Pencina MJ. Application of net reclassification index to non-nested and point-based risk prediction models: a review. Eur Heart J. 2019;40:1880–1887. doi:10.1093/eurheartj/ehy345
29. Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi:10.1002/sim.4780080504
30. Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi:10.1037/0022-3514.51.6.1173
31. Nam KW, Kim TJ, Lee JS, et al. High neutrophil-to-lymphocyte ratio predicts stroke-associated pneumonia. Stroke. 2018;49:1886–1892. doi:10.1161/STROKEAHA.118.021228
32. Ding R, Logemann JA. Pneumonia in stroke patients: a retrospective study. Dysphagia. 2000;15:51–57. doi:10.1007/s004550010001
33. Katzan IL, Cebul RD, Husak SH, Dawson NV, Baker DW. The effect of pneumonia on mortality among patients hospitalized for acute stroke. Neurology. 2003;60:620–625. doi:10.1212/01.WNL.0000046586.38284.60
34. Roever L, Resende ES, Roerver-Borges AS. Hypertroponinemia, structural cardiac disease, and stroke mortality. Stroke. 2017;48:1134–1135. doi:10.1161/STROKEAHA.117.017061
35. Dieplinger B, Bocksrucker C, Egger M, Eggers C, Haltmayer M, Mueller T. Prognostic value of inflammatory and cardiovascular biomarkers for prediction of 90-day all-cause mortality after acute ischemic stroke-results from the linz stroke unit study. Clin Chem. 2017;63:1101–1109. doi:10.1373/clinchem.2016.269969
36. Yi SW, Shin DH, Kim H, Yi JJ, Ohrr H. Total cholesterol and stroke mortality in middle-aged and elderly adults: a prospective cohort study. Atherosclerosis. 2018;270:211–217. doi:10.1016/j.atherosclerosis.2017.12.003
37. Tu WJ, Liu Q, Cao JL, Zhao SJ, Zeng XW, Deng AJ. Gamma-glutamyl transferase as a risk factor for all-cause or cardiovascular disease mortality among 5912 ischemic stroke. Stroke. 2017;48:2888–2891. doi:10.1161/STROKEAHA.117.017776
38. Hojs Fabjan T, Penko M, Hojs R. Newer glomerular filtration rate estimating equations for the full age spectrum based on serum creatinine and cystatin c in predicting mortality in patients with ischemic stroke. Eur J Intern Med. 2018;52:67–72. doi:10.1016/j.ejim.2018.02.005
39. Gendron TF, Badi MK, Heckman MG, et al. Plasma neurofilament light predicts mortality in patients with stroke. Sci Transl Med. 2020;12:eaay1913. doi:10.1126/scitranslmed.aay1913
40. Marta-Enguita J, Navarro-Oviedo M, Rubio-Baines I, et al. Association of calprotectin with other inflammatory parameters in the prediction of mortality for ischemic stroke. J Neuroinflammation. 2021;18:3. doi:10.1186/s12974-020-02047-1
41. Shi G, Li M, Zhou R, et al. Procalcitonin related to stroke-associated pneumonia and clinical outcomes of acute ischemic stroke after iv rt-pa treatment. Cell Mol Neurobiol. 2022;42:1419–1427. doi:10.1007/s10571-020-01031-w
42. Li YM, Liu XY. Serum levels of procalcitonin and high sensitivity c-reactive protein are associated with long-term mortality in acute ischemic stroke. J Neurol Sci. 2015;352:68–73. doi:10.1016/j.jns.2015.03.032
43. Johnstone J, Eurich DT, Majumdar SR, Jin Y, Marrie TJ. Long-term morbidity and mortality after hospitalization with community-acquired pneumonia: a population-based cohort study. Medicine. 2008;87:329–334. doi:10.1097/MD.0b013e318190f444
44. Yende S, Angus DC, Ali IS, et al. Influence of comorbid conditions on long-term mortality after pneumonia in older people. J Am Geriatr Soc. 2007;55:518–525. doi:10.1111/j.1532-5415.2007.01100.x
45. Huang H, Liu Q, Zhu L, et al. Prognostic value of preoperative systemic immune-inflammation index in patients with cervical cancer. Sci Rep. 2019;9:3284. doi:10.1038/s41598-019-39150-0
46. Zhang P, Xu P, Duan Z, et al. Effects of admission systemic inflammatory indicators on clinical outcomes in patients with vertebrobasilar artery occlusion: insight from the persist registry. J Neurointerv Surg;2022:jnis-2022–019437. doi:10.1136/jnis-2022-019437
47. Wang N, Yang Y, Qiu B, et al. Correlation of the systemic immune-inflammation index with short- and long-term prognosis after acute ischemic stroke. Aging. 2022;14:6567–6578. doi:10.18632/aging.204228
48. Li W, He C. Association of platelet-to-lymphocyte ratio with stroke-associated pneumonia in acute ischemic stroke. J Healthc Eng. 2022;2022:1033332. doi:10.1155/2022/1033332
49. Faura J, Bustamante A, Miro-Mur F, Montaner J. Stroke-induced immunosuppression: implications for the prevention and prediction of post-stroke infections. J Neuroinflammation. 2021;18:127. doi:10.1186/s12974-021-02177-0
50. Ruhnau J, Schulze K, Gaida B, et al. Stroke alters respiratory burst in neutrophils and monocytes. Stroke. 2014;45:794–800. doi:10.1161/STROKEAHA.113.003342
51. Dirnagl U, Klehmet J, Braun JS, et al. Stroke-induced immunodepression: experimental evidence and clinical relevance. Stroke. 2007;38:770–773. doi:10.1161/01.STR.0000251441.89665.bc
52. Vogelgesang A, Grunwald U, Langner S, et al. Analysis of lymphocyte subsets in patients with stroke and their influence on infection after stroke. Stroke. 2008;39:237–241. doi:10.1161/STROKEAHA.107.493635
53. Elkind MSV, Boehme AK, Smith CJ, Meisel A, Buckwalter MS. Infection as a stroke risk factor and determinant of outcome after stroke. Stroke. 2020;51:3156–3168. doi:10.1161/STROKEAHA.120.030429
54. Tsai AS, Berry K, Beneyto MM, et al. A year-long immune profile of the systemic response in acute stroke survivors. Brain. 2019;142:978–991. doi:10.1093/brain/awz022
55. Xu XR, Zhang D, Oswald BE, et al. Platelets are versatile cells: new discoveries in hemostasis, thrombosis, immune responses, tumor metastasis and beyond. Crit Rev Clin Lab Sci. 2016;53:409–430. doi:10.1080/10408363.2016.1200008
56. Clark SR, Ma AC, Tavener SA, et al. Platelet tlr4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi:10.1038/nm1565
57. Semple JW, Italiano JE
58. Cangemi R, Pignatelli P, Carnevale R, et al. Low-grade endotoxemia, gut permeability and platelet activation in community-acquired pneumonia. J Infect. 2016;73:107–114. doi:10.1016/j.jinf.2016.05.013
59. Corrales-Medina VF, Musher DM, Shachkina S, Chirinos JA. Acute pneumonia and the cardiovascular system. Lancet. 2013;381:496–505. doi:10.1016/S0140-6736(12)61266-5
60. Tsakiridou E, Makris D, Chatzipantazi V, et al. Diabetes and hemoglobin a1c as risk factors for nosocomial infections in critically ill patients. Crit Care Res Pract. 2013;2013:279479. doi:10.1155/2013/279479
61. Dylla L, Herson PS, Poisson SN, Rice JD, Ginde AA. Association between chronic inflammatory diseases and stroke-associated pneumonia - an epidemiological study. J Stroke Cerebrovasc Dis. 2021;30:105605. doi:10.1016/j.jstrokecerebrovasdis.2021.105605
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
