Back to Journals » Nature and Science of Sleep » Volume 11
Subjective sleep measurement: comparing sleep diary to questionnaire
Authors Mallinson DC , Kamenetsky ME , Hagen EW, Peppard PE
Received 1 June 2019
Accepted for publication 6 August 2019
Published 11 September 2019 Volume 2019:11 Pages 197—206
DOI https://doi.org/10.2147/NSS.S217867
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Steven A Shea
David C Mallinson, Maria E Kamenetsky, Erika W Hagen, Paul E Peppard
Department of Population Health Sciences, School of Medicine and Public Health, University of Wisconsin-Madison, Madison, WI, USA
Correspondence: Paul E Peppard
Department of Population Health Sciences, School of Medicine and Public Health, University of Wisconsin-Madison, WARF Building #611A, 610 N. Walnut St, Madison, WI 53726-2397, USA
Tel +1 608 262 2680
Email [email protected]
Purpose: The sleep diary is the gold standard of self-reported sleep duration, but its comparability to sleep questionnaires is uncertain. The purpose of this study was to compare self-reported sleep duration between a sleep diary and a sleep questionnaire and to test whether sleep-related disorders were associated with diary-questionnaire differences in sleep duration.
Participants and methods: We compared self-reported sleep duration from 5,432 questionnaire-sleep diary pairs in a longitudinal cohort of 1,516 adults. Participants reported sleep information in seven-day sleep diaries and in questionnaires. Research staff abstracted average sleep durations for three time periods (overall; weekday; weekend) from diaries and questionnaires. For each time period, we evaluated diary-questionnaire differences in sleep duration with Welch’s two-sample t-tests. Using linear mixed effects regression, we regressed overall diary-questionnaire sleep duration difference on several participant characteristics: reporting any insomnia symptoms, having sleep apnea, sex, body mass index, smoking status, Short Form-12 Physical Health Composite Score, and Short Form-12 Mental Health Composite Score.
Results: The average diary-reported overall sleep duration (7.76 hrs) was longer than that of the questionnaire (7.07 hrs) by approximately 41 mins (0.69 hrs, 95% confidence interval: 0.62, 0.76 hrs). Results were consistent across weekday- and weekend-specific differences. Demographic-adjusted linear mixed effects models tested whether insomnia symptoms or sleep apnea were associated with diary-questionnaire differences in sleep duration. Insomnia symptoms were associated with a 17 min longer duration on the diary relative to the questionnaire (β=0.28 hrs, 95% confidence interval: 0.22, 0.33 hrs), but sleep apnea was not significantly associated with diary-questionnaire difference. Female sex was associated with greater diary-questionnaire duration differences, whereas better self-reported health was associated with lesser differences.
Conclusion: Diaries and questionnaires are somewhat disparate methods of assessing subjective sleep duration, although diaries report longer duration relative to questionnaires, and insomnia symptoms may contribute to greater perceived differences.
Keywords: comparison, self-reported sleep, sleep log, surveys
Introduction
An abundance of epidemiologic literature has established the links between sleep duration and several health outcomes. Short sleep is associated with increased risks of obesity,1 hypertension,2 and diabetes.3 Moreover, there is substantial evidence of an association between inadequate sleep duration and elevated risk of all-cause mortality.4
Given the relevance of sleep to health and well-being, there is clinical and epidemiologic justification to assess sleep duration metrics in clinical and research populations. Polysomnography is the gold standard for objective sleep duration measurement,5 although its time- and resource-consuming process can render it prohibitive for epidemiologic research. Moreover, in-lab sleep duration may not be characteristic of at-home sleep duration, as the polysomnography may interfere with typical sleep. Wrist actigraphy is an alternative approach to objective sleep duration measurement, but it is also time-intensive and its validity has not been extensively tested in populations with sleep-related disorders.6
In contrast to objective measures, subjective sleep duration measures can be efficient and practicable means of collecting sleep duration data, especially in large population-based or community-based studies. Prior studies found that subjective sleep duration measures are generally comparable to objective measures among healthy adults,6 although subjective measures may over-report sleep duration length among elderly adults or adults with poorer health.7 Nonetheless, subjective assessments offer a more efficient approach to measuring sleep duration that are commonly used in sleep research. Two common instruments for subjective sleep measurement are sleep diaries and single- or multi-item sleep questionnaires. The sleep diary is the accepted standard for self-reported sleep duration, as it elicits detailed “real time” (not recalled) information on daily sleep duration and timing over a specified time period.8 However, diaries rely on participants’ sustained adherence,9 and there is no standardized diary for research or clinical purposes.8 Simple questionnaires (for example, “On most nights, how many hours and minutes do you sleep?”) are faster, but they may be prone to inaccuracies, such as substantially rounded sleep duration estimates (e.g., to the nearest hour or half-hour), since they do not collect exact bed and wake times. While both instruments have advantages and disadvantages, their comparability and the factors that affect differences between the instruments are unknown. This information may be useful for wide-scale sleep duration surveillance that prioritizes resource- and time-efficiency.
The purpose of this study is to compare self-reported sleep duration measures between a sleep diary and a questionnaire in an adult population. In addition to comparing these measures, this study also investigates whether insomnia symptoms, sleep apnea, or demographic characteristics contribute to differences in self-reported sleep duration between the diary and questionnaire. Both insomnia and sleep apnea can result in atypical sleep habits. These conditions may affect responses to daily sleep reporting differently than usual sleep duration questions. Additionally, we hypothesize that observed demographic factors will not be associated with diary-questionnaire differences in reported sleep duration. Demographic characteristics may affect sleep quality,10 but there is little evidence of these factors contributing to disagreement between different methods of subjective sleep duration.
Methods
Sample
We analyzed data from participants in the Retirement and Sleep Trajectories (REST) Study. Established in 2010, REST is a prospective cohort used for investigating pre- and post-retirement sleep patterns in a community-dwelling (ie, independent living) population of middle-aged and older adults.11 REST drew its study population from the sampling frame of the Wisconsin Sleep Cohort (WSC) study, a longitudinal population-based study of the history of common sleep disorders among (then) Wisconsin state employees who were 30–60 years old upon enrollment in 1988. The WSC’s full sample collection has been described elsewhere.10,12 Individuals from the sampling frame were eligible for REST if they were alive in 2010 and met one of the following requirements: previous response to at least three mailed WSC surveys, or one overnight WSC polysomnography study and previous response to at least one mailed WSC survey.11 REST staff mailed one sleep and health questionnaire, which included sleep duration items, as well as one seven-day sleep diary per year to each eligible individual during 2010–2014. Participants were asked to record sleep information in their diaries upon completion of the sleep and health questionnaire.
There were 2,472 eligible adults at baseline, 2,341 (94.7%) of whom were locatable and alive through the fourth and final survey wave. There were 131 adults who were lost to follow up throughout the study duration. Participants returned 6,405 questionnaire-diary pairs of a total possible of 9,589 (66.8%). We excluded 1,092 observations (17.0%) that were missing information on at least one key variable for our analysis. This generated an analytic sample of 5,313 questionnaire-diary pairs from 1,516 adults. Among included adults, 1,445 (95.3%) returned at least two questionnaire-diary pairs.
Subjective sleep instruments
The two subjective sleep instruments for this study were the sleep diary and the questionnaire. The diary elicited sleep and wake times over a seven-day period. Because sleep diaries covered a seven-day period, participants could record up to six nights’ worth of sleep data in each diary. We computed daily sleep duration as the difference between the reported sleep time on a day and the reported wake time on the subsequent day. For example, if a participant reported falling asleep at 10:00 PM on a Monday and waking up at 6:00 AM on the following Tuesday, then the sleep duration was eight hours. This generated a possible total of six daily sleep durations, which we used to compute average diary-reported sleep durations. The three primary measures from the diary were average overall sleep duration, average weekday sleep duration, and average weekend sleep duration, all of which were measured in hours. The following formula calculated the weighted average overall sleep duration from the diary: 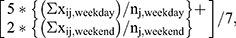 where 'x' is the recorded sleep duration, subscript 'i' is the diary-logged night, 'n' is the number of diary-logged nights slept, subscript 'j' indicates the participant, subscript 'weekday' indicates a weekday night, and subscript 'weekend' indicates a weekend night. This equation calculates the mean sleep durations for weekday nights and weekend nights, and then weights those values to calculate an average sleep duration for the entire week. Participants did not have to complete all seven days of the diary to be included in this analysis.
where 'x' is the recorded sleep duration, subscript 'i' is the diary-logged night, 'n' is the number of diary-logged nights slept, subscript 'j' indicates the participant, subscript 'weekday' indicates a weekday night, and subscript 'weekend' indicates a weekend night. This equation calculates the mean sleep durations for weekday nights and weekend nights, and then weights those values to calculate an average sleep duration for the entire week. Participants did not have to complete all seven days of the diary to be included in this analysis.
The questionnaire consisted of several items regarding sleep history and general health. One question addressed sleep duration: “How many hours of sleep do you usually get during (a) a weekday night?; and (b) a weekend or non-work night?” Participants recorded their answers in hours and minutes. The three primary measures from questionnaire data were average overall sleep duration, average weekday sleep duration, and average weekend sleep duration, all of which were measured in hours. The following formula calculated weighted average overall sleep duration from the questionnaire: 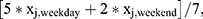 where 'x' is the recorded sleep duration, subscript 'j' indicates the participant, subscript 'weekday' indicates a weekday night, and subscript 'weekend' indicates a weekend night.
where 'x' is the recorded sleep duration, subscript 'j' indicates the participant, subscript 'weekday' indicates a weekday night, and subscript 'weekend' indicates a weekend night.
Variables
There were three primary sleep-related disorder predictor variables in this analysis: insomnia symptoms (yes or no), insomnia symptom-days per month, and self-reported sleep apnea (yes or no). The insomnia symptoms variable was a composite of four questionnaire items: difficulty falling asleep, difficulty getting back to sleep, repeated nocturnal awakenings, and early morning awakening. Participants could respond to each item with “never,” “rarely,” “sometimes,” “often,” or “almost always.” If a participant responded with “often” or “almost always” to any item, then the participant was indicated as having reported insomnia symptoms. Insomnia symptom-days per month was defined as the sum of a participant’s estimated symptomatic days for those items (for example, an individual could report 15 days of difficulty falling asleep and 25 days of repeated nocturnal awakenings, yielding 40 insomnia symptom-days per month). There was a range of 0–100 insomnia days per month in our sample. Self-reported sleep apnea was indicated by a survey question that asked respondents if they have ever been diagnosed with sleep apnea by a physician, to which respondents could answer “yes” or “no.” If a respondent selected “yes” to this question, every future observation reported “yes” to self-reported sleep apnea.
Other variables in our analysis included age at the time of the sleep diary (years), sex (female or male), body mass index (kilograms/meters2), smoking status (current smoker or not current smoker), the Short Form-12 (SF-12) Physical Health Composite Score, and the SF-12 Mental Health Composite Score. We rescaled both SF-12 scores from a 0–1 point range to a 0–100 point range for ease of interpretation, with higher scores indicating better health. The development and validation process for these scores has been fully described previously.13
Analysis
We used a five-step approach to describe the sample and its sleep duration trends. Using only the first observed questionnaire-diary pair for each participant, we calculated the baseline distributions of all variables and we measured the mean self-reported sleep durations for the diary and questionnaire for all time periods (overall, weekday, and weekend). Welch's two-sample t-tests evaluated the difference between mean diary- and questionnaire-reported sleep durations for each time period, and Pearson correlation coefficients and Spearman’s rank correlation coefficients measured the relationship between diary- and questionnaire-reported sleep durations for each time period. Using all observed questionnaire-diary pairs (ie including multiple questionnaire-diary pairs per participant, if available) we generated overlapping histograms of overall diary- and questionnaire-reported sleep durations, and we generated overlapping density plots of diary-to-questionnaire sleep duration ratios by time period (see supplemental appendix for overlapping density plots). We calculated diary-to-questionnaire ratios by dividing an observation’s diary-reported sleep duration by its questionnaire-reported sleep duration for a given time period.
We employed a three-step approach to determine whether insomnia symptoms or sleep apnea were associated with diary-questionnaire differences in sleep duration. First, Bland-Altman graphs plotted the average of the diary- and questionnaire-reported durations against the difference between the two durations, and plot points were symbolically coded by insomnia symptoms and sleep apnea status, respectively. Second, we built a series of linear mixed effects regression models to identify factors associated with diary-questionnaire differences in sleep durations. Model 1A adjusted for any insomnia symptoms, sleep apnea, sex, body mass index, smoking status, SF-12 Physical Health Composite Score, and SF-12 Mental Health Composite score. The model included squared-terms for age and body mass index to account for nonlinear relationships with sleep duration differences. Models 1B and 1C replicated 1A, except 1B excluded sleep apnea and 1C excluded any insomnia symptoms. This accounted for potential over-control by including multiple sleep-related disorder variables. Models 2A and 2B replicated 1A and 1B, respectively, except they included insomnia days per month instead of any insomnia symptoms. All models included random intercepts at the participant level.
We set statistical significance for all analyses at the 5% level and calculated 95% two-sided profile confidence intervals. We conducted the statistical analyses in R 3.4.4,14 and we used two R packages for linear mixed-effects modeling in this analysis: lme4 and lmeTest.15,16 The University of Wisconsin Health Sciences Institutional Review Board approved study protocols and informed consent documents.
Results
Baseline results
At baseline observation for each participant, approximately 58% of the sample reported at least one insomnia symptom with a mean (standard deviation, SD) 21.3 (18.7) insomnia days per month, and about 17% of the sample reported sleep apnea (Table 1). Respondents at baseline observation were generally middle-aged or older, and about half of the sample was female. A little less than half of the sample were current smokers at baseline observation, and the average body mass index was near the threshold of obesity (30 kg/m2). On a range of 0–100 points, the average SF-12 physical health score and the average SF-12 mental health score were 48 (10) points and 52 (9) points, respectively. Both SF-12 scores for this sample are comparable to those of similarly aged populations.17
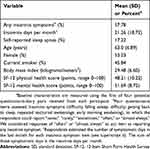 |
Table 1 Baseline characteristics of analyzed participants in the retirement and sleep trajectories study (n=1,516) |
The mean (SD) overall reported sleep durations for the diary and for the questionnaire at baseline were 7.76 (1.01) hours and 7.07 (1.04) hours, respectively, with a 0.69 hr (41 min) mean difference (95% confidence interval (CI): 0.62, 0.76 hrs) (Table 2). Overlapping histograms of diary- and questionnaire-reported overall sleep durations for baseline questionnaire-diary observations revealed somewhat similar distributions (Figure 1). Of note, the histogram for questionnaire-reported sleep duration exhibited binning at the half hour-level, suggesting that participants may round their sleep estimates to the nearest half hour. Mean sleep durations at baseline were shorter for weekdays and longer for weekends regardless of reporting method, and mean questionnaire-reported durations were consistently shorter than mean diary-reported durations. Relative to the overall mean difference in diary- and questionnaire-reported baseline sleep duration, weekday-specific mean difference was similar (0.73 hrs, 95% CI: 0.65, 0.81), whereas the weekend-specific mean difference was somewhat smaller (0.60 hrs, 95% CI: 0.51, 0.68 hrs).
 |
Table 2 Self-reported sleep duration measures on the sleep diary and questionnaire among participants in the retirement and sleep trajectories study at baseline (n=1,516)a |
The Pearson correlation coefficients exhibit a moderate positive relationship between diary- and questionnaire-reported sleep durations – 0.48 (95% CI: 0.44, 0.52) overall, 0.48 (95% CI: 0.44, 0.52) for weekday-specific durations, and 0.39 (95% CI: 0.34, 0.43) for weekend-specific durations. Spearman’s rank correlation coefficients were nearly identical. The diary-to-questionnaire ratio of sleep duration was constant and demonstrated nearly identical patterns across time periods (Figure S1). For those reasons, we focus on diary-questionnaire differences in overall reported sleep duration for the remainder of this section.
Sleep-related predictors of diary-questionnaire differences
Bland-Altman plots suggest minimal differences in diary- and questionnaire-reported sleep duration by insomnia symptom status or self-reported sleep apnea (Figure 2). However, mixed-effects regression models demonstrated that both the presence of any insomnia symptom and the number of insomnia symptom-days per month were significantly and positively associated with differences between diary- and questionnaire-reported sleep duration. Having any insomnia symptoms was associated with a 0.28 hr (17 min) average increase in diary-reported sleep duration relative to the questionnaire-reported duration, regardless of whether we adjusted for self-reported sleep apnea (Table 3). Likewise, each insomnia day per month is associated with a 0.01 hr (0.6 min) average increase in diary-reported sleep duration relative to the questionnaire-reported duration (Table 4). A 10-day increase in insomnia days, for example, is related to an expected 6 min increase in the diary-to-questionnaire difference of reported sleep duration. Again, adjusting for self-reported sleep apnea did not alter the association between insomnia days per month and reported sleep duration difference. Self-reported sleep apnea was not significantly associated with diary-questionnaire differences in reported sleep duration in any model. Therefore, there is no evidence of over-adjustment by including multiple sleep disorder-related variables.
Demographic predictors of diary-questionnaire differences
Relative to male participants, female participants had a consistent and statistically significant 7 to 8 min larger difference between diary- and questionnaire-reported sleep duration. In contrast to the other predictors, there is evidence of an association between SF-12 physical health score and the SF-12 mental health score with shorter diary-reported sleep. Specifically, a one-point increase in either score was associated with a 0.01 hr (0.6 min) shorter sleep duration on the diary compared to the questionnaire. There was no evidence that any other observed demographic variables were associated with diary-questionnaire differences in reported sleep duration.
Discussion
To our knowledge, this is the first study to compare subjective sleep duration between sleep diaries and questionnaires. The findings from our study suggest that sleep diaries and questionnaires are comparable instruments for collecting self-reported sleep duration, although questionnaires may elicit shorter self-reported sleep duration than diaries. Indeed, questionnaire-reported sleep durations were on average 40 mins shorter than diary-reported sleep durations in our sample. Furthermore, questionnaire-reported durations exhibited a pattern of rounding to the nearest half-hour, whereas diary-reported sleep durations did not. This indicates that the diary elicits more precise data, supporting the conventional wisdom that the sleep diary is the preferred mode for self-reported sleep duration.8 Despite these differences, there was overlap in the distributions of diary-reported and questionnaire-reported sleep durations.
Our results partially corroborate our hypothesis that sleep-related disorders contribute to diary-questionnaire differences in reported sleep duration. Insomnia symptoms were associated with recording longer sleep duration on the diary compared to the questionnaire; the adjusted mean (standard error) diary-questionnaire difference for persons with no insomnia symptoms was 34.44 (2.11) minutes and the mean difference for those with any insomnia symptoms was 51.48 (2.04) minutes. Additionally, for every 10 insomnia symptom-days per month, an additional 6 mins of sleep were reported on the diary compared to the questionnaire. However, there was no evidence that self-reported sleep apnea was related to differences in reported sleep duration between the sleep diary and the questionnaire.
In contrast to our hypothesis, we found evidence that some observed demographic variables were associated with diary-questionnaire differences in reported sleep duration. Being female was associated with a 7 to 8 min greater difference between diary- and questionnaire-reported sleep duration. It is unclear why sex would predict differences in subjective sleep duration between diaries and questionnaires. A wealth of clinical, epidemiological, and sociological literature establishes that, on average, women report longer sleep duration than men,18 but this would not explain discordance between different subjective sleep duration measures. Perhaps sleep onset latency – the duration between “lights out” (the time of first attempted initiation of sleep) and actual sleep onset19 – provides insight into this difference. Recent literature suggests that women report longer sleep onset latency relative to men.20 Rather than recording the time at which they fell asleep in their sleep diaries, participants may have recorded the time at which they initiated the ritual of sleep (ie, getting into bed, turning off the lights, etc.). If participants responded to questionnaires in such a way that it did not capture sleep onset latency – that is, participants reported only the time spent asleep, excluding the time spent trying to fall asleep – then diary-questionnaire differences in reported sleep duration will be greater for women relative to men.
Additionally, higher SF-12 physical and mental health scores were associated with marginally lower diary-reported sleep duration relative to the questionnaire-reported duration. This pattern is analogous to insomnia symptoms’ relation with diary-questionnaire differences in sleep duration measurement. Compared to individuals with better self-reported health, those with worse self-reported health may report longer sleep duration on the diary relative to the questionnaire. This could translate to closer diary-questionnaire correspondence among healthier individuals. However, to the best of our knowledge, there is no prior empirical evidence that self-reported health predicts differences between self-reported sleep duration measures.
If insomnia truly contributed to differences between diary-reported and questionnaire-reported sleep duration, the exact mechanisms by which they affect differences is uncertain. Insomnia symptomatic individuals may have unusual and less predictable sleep habits relative to non-symptomatic individuals due to the nature of their disorder. In addition to altering sleep cycles, insomnia symptoms could act as stressors that worsen an individual’s ability to accurate perceive and, later, recall sleep state. A sleep diary could elicit more accurate information by requesting specific sleep and wake times for each reported cycle, which encourages affected participants to be more aware of their atypical or varying sleep behaviors. Conversely, our sleep diary may over-report sleep duration relative to the questionnaire, as it only collected sleep and wake times but not nocturnal awakenings. Insomnia-symptomatic adults, who may be prone to these awakenings, may consider this when reporting sleep duration on the questionnaire but not on the diary. This hypothesis parallels findings from studies that compared self-reported sleep durations from diaries or questionnaires to objective actigraphy measures, which found that poor sleep quality explained some over-reported duration on subjective measures relative to actigraphy.7,21 We cannot explore these hypotheses, as our study did not compare subjective sleep duration measures to objective measures.
Differential processes for recalling sleep duration may also contribute to variation between the diary and questionnaire. Respondents only need to know sleep time and wake time for a given cycle to complete the diary. Assuming daily adherence, the cognitive burden for using the sleep diary is low. In contrast, questionnaire respondents need to consider what qualifies as a typical sleep cycle, compute the difference between a sleep onset and wake time, and average those sleep durations in their final reported average duration estimates. Differences in these processes for reporting sleep duration may give rise to divergent self-reported sleep durations.
These findings highlight some implications for using diaries or questionnaires for eliciting sleep duration. Diary- and questionnaire-reported sleep durations were reasonably comparable among non-insomnia symptomatic respondents, so questionnaires may be less expensive and less burdensome in measuring self-reported sleep durations in unaffected populations. However, questionnaires were disproportionately prone to rounding at the half-hour. It is unknown whether this measurement bias is random or systematic. Random rounding would reduce the precision of sleep duration estimates but not bias them, whereas systematic rounding would induce bias, which is problematic if using questionnaire-reported sleep duration to identify factors associated with sleep duration.22 Careful questionnaire design, such as providing instructions in questionnaires to estimate sleep time to the nearest 5 or 10 mins, may prevent excessive rounding. Otherwise, sleep diaries may be preferred to minimize measurement error via rounding.
It is important to consider this study’s limitations. First, the generalizability of these findings may be limited due to the sample’s homogeneity. REST participants are current or former Wisconsin state employees, most of whom have stable incomes and adequate access to medical care.10,11 Sleep diary-questionnaire comparability may vary in other populations, particularly in populations with more limited access to medical care for sleep-related disorders. Second, there may be a spillover effect between sleep diaries and questionnaires. Participants completed diaries and questionnaires close in time, so diary responses may have informed questionnaire responses or vice-versa. We cannot confirm that respondents would have reported the same sleep durations on the questionnaire or the sleep diary if they only used one of the modes. Lastly, this study cannot speak to the objective or clinical accuracy of either measure. We did not compare the diary or questionnaire to a gold standard objective sleep measure (e.g., polysomnography), so it is possible that the diary and questionnaire are comparable but not valid. Likewise, these findings cannot directly inform best practices for subjective sleep measurement in a clinical setting (for example, whether practitioners can use a sleep diary or questionnaire in diagnosing sleep-related disorders such as insomnia).
Conclusion
Sleep diaries and questionnaires obtain somewhat disparate self-reported sleep duration estimates, with diaries eliciting longer durations, on average. Insomnia symptoms may, in part, drive these differences, so the questionnaire and diary may be similarly valid instruments for self-reported sleep duration measurement in persons unaffected by insomnia. To lesser extents, sex and general self-reported health were associated with diary-questionnaire differences in reported sleep duration. Future research should investigate the mechanisms by which insomnia symptoms lead to diary-questionnaire differences, as well as other sources of potential disagreement.
Ethics statement
All protocols and informed consent documents were approved by the University of Wisconsin-Madison Health Sciences Institutional Review Board (UW HS-IRB). All Wisconsin Sleep Cohort Study participants provide written informed consent for laboratory-based protocols. All Retirement and Sleep Trajectories (REST) Study participants – REST is an ancillary study of the Wisconsin Sleep Cohort Study – additionally provided implied consent as approved by UW HS-IRB according to this language that accompanied the mailed REST surveys: “Your permission is voluntary. If you wish to participate, a returned survey will be taken to indicate that you consent to participate. If you do not wish to participate, you can simply discard the surveys. You also may choose to participate but skip any questions that you do not feel comfortable answering.”
Acknowledgments
The authors thank Jodi Barnet for preparing the data for analysis. This work was supported by US National Institutes of Health (NIH) grants 1R01AG036838, R01HL62252, and 1UL1RR02501.
Author contributions
DCM conducted the literature review, co-developed the analysis plan, and wrote the original manuscript. MEK co-developed the analysis plan, conducted statistical analyses, created figures, and revised the manuscript. EWH co-managed the data acquisition, reviewed the analysis plan, and revised the manuscript. PEP developed the original research question, co-managed the data acquisition, reviewed the analysis plan, and revised the manuscript. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity. 2008;16(3):643–653. doi:10.1038/oby.2007.118
2. Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: analyses of the first national health and nutrition examination survey. Hypertension. 2006;47(5):833–839. doi:10.1161/01.HYP.0000217362.34748.e0
3. Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165(8):863–867. doi:10.1001/archinte.165.8.863
4. Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):585–592. doi:10.1093/sleep/33.5.585
5. Penzel T, Conradt R. Computer based sleep recording and analysis. Sleep Med Rev. 2000;4(2):131–148. doi:10.1053/smrv.1999.0087
6. Sadeh A. The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev. 2011;15(4):259–267. doi:10.1016/j.smrv.2010.10.001
7. Van Den Berg JF, Van Rooij FJ, Vos H, et al. Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. J Sleep Res. 2008;17(3):295–302. doi:10.1111/j.1365-2869.2008.00638.x
8. Carney CE, Buysse DJ, Ancoli-Israel S, et al. The consensus sleep diary: standardizing prospective sleep monitoring. Sleep. 2012;35(2):287–302. doi:10.5665/sleep.1642
9. Thurman SM, Wasylyshyn N, Roy H, et al. Individual differences in compliance and agreement for sleep logs and wrist actigraphy: a longitudinal study of naturalistic sleep in healthy adults. PLoS One. 2018;13(1):e0191883. doi:10.1371/journal.pone.0191883
10. Young T. Rationale, design, and findings from the wisconsin sleep cohort study: toward understanding the total societal burden of sleep-disordered breathing. Sleep Med Clin. 2009;4(1):37–46. doi:10.1016/j.jsmc.2008.11.003
11. Harden C Changes in Self-Reported Napping Behaviors Across the Retirement Transition [dissertation]. Madison, WI: University of Wisconsin-Madison; 2017.
12. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi:10.1056/NEJM199304293281704
13. Ware J, Kosinski M, Keller SM. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi:10.1097/00005650-199603000-00003
14. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2018.
15. Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1–48. doi:10.18637/jss.v067.i01
16. Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest package: tests in linear mixed effects models. J Stat Softw. 2017;82(13):1–26. doi:10.18637/jss.v082.i13
17. Office of Public Health Assessment. Health Status in Utah: the Medical Outcomes Study SF-12. Salt Lake City, UT: Utah Department of Health; 2004.
18. Burgard SA, Ailshire JA. Gender and time for sleep among U.S. adults. Am Sociol Rev. 2013;78(1):51–69. doi:10.1177/0003122412472048
19. Cajochen C, Kräuchi K, Danilenko KV, Wirz-Justice A. Evening administration of melatonin and bright light: interactions on the EEG during sleep and wakefulness. J Sleep Res. 1998;7(3):145–157.
20. Ohayon MM, Reynolds CF, Dauvilliers Y. The link between excessive quantity of sleep and deteriorated quality wakefulness – implications for the DSM-5. Ann Neurol. 2013;73(6):785–794. doi:10.1002/ana.23818
21. Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Sleep duration: how well do self-reports reflect objective measures? The CARDIA sleep study. Epidemiol. 2008;19(6):838–845. doi:10.1097/EDE.0b013e318187a7b0
22. Kan YK, Pudney S. Measurement error in stylized and diary data on time use. Sociol Methodol. 2008;38(1):101–132. doi:10.1111/j.1467-9531.2008.00197.x
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




