Back to Journals » Patient Preference and Adherence » Volume 17
SPUR-27 – Psychometric Properties of a Patient-Reported Outcome Measure of Medication Adherence in Chronic Obstructive Pulmonary Disease
Authors Wells J , Mahendran S , Dolgin K , Kayyali R
Received 23 October 2022
Accepted for publication 18 January 2023
Published 19 February 2023 Volume 2023:17 Pages 457—472
DOI https://doi.org/10.2147/PPA.S394538
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Johnny Chen
Joshua Wells,1 Siva Mahendran,2 Kevin Dolgin,3 Reem Kayyali1
1Department of Pharmacy, Kingston University, Kingston, UK; 2Respiratory Department, Kingston Hospital NHS Foundation Trust, Kingston, UK; 3Observia, Paris, France
Correspondence: Reem Kayyali, Department of Pharmacy, Kingston University, Penrhyn Road, Kingston, KT1 2EE, UK, Tel/Fax +44 208 417 2561, Email [email protected]
Purpose: People living with COPD who struggle to take their medicines often experience poorer health outcomes such as exacerbations of symptoms, more frequent and lengthy hospital admissions, and worsening mortality rates. This study aimed to evaluate the psychometric properties of the previously validated SPUR-27 model, a multi-factorial model of medication adherence.
Patients and Methods: This cross-sectional study was conducted with 100 adult patients living with COPD in a hospital setting in Southwest London. Medication adherence was assessed using a shortened SPUR model (SPUR-27) against the validated Inhaler Adherence Scale (IAS) as a comparator. In addition, objective medication adherence data, presented as the Medication Possession Ratio (MPR), were derived from patient medical and pharmacy records. The COPD Assessment Tool (CAT) score was used to examine the relationship between medication adherence and COPD symptom severity. Reliability of SPUR-27 was assessed using internal consistency estimates. Exploratory factor analysis, partial confirmatory factor analysis, and maximum likelihood analysis were conducted in conjunction with construct, concurrent, and known-group validity testing to explore the psychometric properties of the SPUR model in this population.
Results: A 7-factor model for SPUR-27 was derived with adequate factor loadings. SPUR (α=0.893) observed strong internal consistency (> 0.8). The model was significantly positively correlated with IAS score (p< 0.001) as well as MPR (p< 0.01). A significant (p< 0.01) relationship between poor medication adherence and worsening symptom severity, as defined by the CAT score, was identified for SPUR (χ2 = 8.570) using Chi-Square analysis. Furthermore, SPUR-27 demonstrated early evidence of validity with good incremental fit indices: NFI (0.96), TFI (0.97), and CFI (0.93) were all reported as > 0.9 in addition to the RMSEA, which was < 0.08 (0.059).
Conclusion: SPUR demonstrated strong psychometric properties in patients living with COPD. Further work should look to examine the test–retest reliability of the model and its application in broader sample populations.
Keywords: validity, patient-reported outcome measure, chronic obstructive pulmonary disease, factor analysis
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a respiratory condition characterised by a progressive and irreversible decline in lung function. The condition is commonly observed in those exposed to substances that can induce alveolar abnormalities as a result of inflammation, such as tobacco smoke.1 People living with COPD report episodic breathlessness and a chronic cough that is often accompanied by excessive or abnormal mucus secretions. Unfortunately, COPD is becoming an increasingly common respiratory condition, with a reported 44.2% increase in global prevalence between 1990 and 2015.2 Global mortality also significantly increased in this period with 3.2 million COPD deaths recorded in 2015 – an 11.6% rise in mortality since 1990.
A recent large-scale observational study of COPD patients in Spain (n= 59,369)3 identified an all-cause mortality rate of 5.6% when compared to 1% in the general population of adults over the age of 40 (n=1,219,749). Unsurprisingly, this increased risk of mortality is accompanied by significant impacts on an individual’s quality of life (QOL). Disease severity has been directly associated with considerably poorer QOL outcomes, even in the case of mild disease.4 Examples of psychological comorbidities that are highly prevalent among people living with COPD include depression and anxiety.5 In fact, a longitudinal population study (n=35,722) of UK primary care patients identified an incidence rate of 16.2 vs 9.4 per 1000 patients for new-onset depression in individuals with and without a COPD diagnosis, respectively. Those diagnosed with severe COPD were also twice as likely to report a diagnosis of depression (Odds Ratio (OR), 2.01; 95% Confidence Interval (CI) 1.45–2.78),6 This exposure to both increased mortality and psychological distress provides some insight into the potential social and clinical complexities of living with COPD, particularly without appropriate management such as inhaled medicines. Inhalers are an essential component in the pharmacological treatment of COPD; however, improper use of inhaled therapies can increase the risk of hospitalisation due to COPD exacerbations, which in turn is associated with worsening mortality in conjunction with other previously identified predictors such as depression, comorbidity, and a previous history of hospital admissions.7,8 Furthermore, psychological comorbidities such as depression, as well as COPD symptom severity, have been shown to have a direct negative impact on adherence to respiratory treatment regimens.9,10
There is a relationship between the poor use of inhalers and COPD exacerbations; hence, tailored interventions to address this may also serve to reduce long-term outcomes associated with poor medicines management such as higher rates of hospitalisation, increased length of stay, and greater healthcare expenditure associated with care for these patients.11,12 To design such interventions, it is important to first quantify the magnitude of the problem, which in this case specifically relates to medication adherence (MA). Only 50% of the individuals living with a chronic condition are estimated to adhere to their treatment regimens,13 with even lower estimations (<40%) reported among people living with COPD.14 However, understanding these figures alone does not help to delineate the multi-factorial elements that contribute to poor MA. Behavioural and psychosocial factors such as forgetfulness, poor inhaler technique or recognition of therapeutic benefit, and a poor patient–clinician relationship can all have a cumulative negative impact on an individual’s ability to take their medicines.15,16 Therefore, it is essential to identify poor adherence and its causative factors for each individual patient in order to deliver truly tailored MA interventions.
One such example of a tool that can identify non-adherence includes the Morisky Medication Adherence Scale (MMAS-8), an example Patient-Reported Outcome Measure (PROM), validated in a number of conditions that assesses MA using several Likert scale items which are designed to evaluate and quantify medicine-taking behaviours, for example forgetfulness.17 PROMs have seen growing interest over recent years owing to their generally simple design and ease of implementation, as well as their adaptability to measure specific factors related to an intended outcome. However, this simplicity also runs the risk of unidimensionality, or taking too narrow an approach to examining predictors of MA. Martin et al18 highlight that PROMs are subject to moderate-high result variability, even within the same sample population. Hence, a multi-faceted approach is necessary to capture a patient’s true experience of MA, which almost always presents as a complex overlap of numerous causative factors, as seen in COPD, that can rarely be addressed in isolation.
The initial model to holistically address four key drivers of MA, known as SPUR, was developed by Observia, an e-health organisation based in Paris.19,20 The four key domains of MA termed Social, Psychographic, Usage, and Rational comprise the 45-item measure and can be used to evaluate individual factors and behaviours related to non-adherence.21,22 Recently, a shorter 27-item version of the SPUR tool (SPUR-27) was validated for patients living with type 2 diabetes in a UK study by Wells et al.23 The revised 27-item model demonstrated greater reliability than the original SPUR-45 tool as well as significant correlations with objective and PROMs of MA in addition to socio-clinical factors such as body mass index (BMI) and income. The psychometric properties of the tool, as well as its relationship to patient socio-clinical factors, have yet to be evaluated in patients living with COPD.
Materials and Methods
Study Aim, Design and Ethical Approval
This study aimed to evaluate the psychometric properties of the adapted SPUR-27 as a holistic measure of MA in COPD, in addition to investigating the relationship between SPUR-27 and socio-clinical factors related to MA, such as disease severity, body mass index (BMI), the number of prescribed medicines and number of comorbidities.
This follow-up cross-sectional study to VMATT223 was conducted in a hospital inpatient setting in London, England, from January to December 2021. The study (VMATC) protocol and relevant documentation were submitted via IRAS (ID:285590) for review with approval received from the NHS Health Research Authority (HRA) research ethics committee (20/NW/0485) in January 2021. Recruitment was conducted by the lead researcher, who provided each participant with a patient information sheet and written consent form. Informed consent was obtained prior to engagement in the study. The present study was conducted in compliance with the ethical principles for research documented in the Declaration of Helsinki.
Study Population
Patients over the age of 18 with a COPD diagnosis that were prescribed ≥1 inhaled medication for their respiratory condition were considered for the study. Participants had a minimum 6-month history of prescribed medications and were able to read and write in English. Participants who reported the following were excluded from the study: too acutely unwell to participate, significant co-morbidities that may affect adherence, eg, cancer which is actively being treated, severe psychiatric illness, or registration with another study at the time of recruitment that involved an investigational medicinal product. Furthermore, patients that were unable to complete the tool independently were excluded from participation. However, specific accommodations, eg, printing larger text questionnaires, were made to include patients who had appropriate capacity for participation but may have been limited by a functional disability or impaired vision as just two examples.
Sample Size
Due to intermittent lockdowns and restrictions to research as a result of the Covid-19 pandemic, the initial recruitment phase of the study (6 months) was reduced to 4 months. Based on in-patient data derived from previous annual admissions (230–368 patients admitted per year), an optimistic expected admission ratio of 10 patients per week was estimated, providing a total sample of 160 patients over the 4-month period. A sample from a previous pilot (n=10) of COPD patients on the Trust acute admissions unit found that 30% (n=3) were too acutely unwell to participate in survey-based research and/or reported an excluding factor. Therefore, roughly 25–30% (n=40–48) were expected to be ineligible due to being acutely unwell, receiving active cancer treatment or having a significant excluding co-morbidity, eg, dementia. Raosoft24 produced a final minimum sample size (5% margin of error, 95% CI) of 92 participants, which was rounded to 100 for the final study sample.
Research Instrument
The English version of the original 45-item SPUR was used in the study, with the methods for development of the model being reported previously.19,23 The tool is a holistic PROM of MA that contains generic wording which can be adapted for use with target chronic conditions; hence, this study replaced “type 2 diabetes” with “COPD” throughout the 45-item instrument. However, previous work by Wells et al23 provided evidence for the psychometric properties of a more concise 27-item version of the main questionnaire titled, SPUR-27. Therefore, only the results for SPUR-27 tool (derived from completion of the original 45-item SPUR questionnaire) were evaluated in patients living with COPD. Validation of the SPUR-27 model for type 2 diabetes took place during recruitment for the VMATC study; hence, despite participants completing the entire 45-item tool, this study reports on the most relevant results of the more recently validated and concise SPUR-27 model.
Additional items were included to capture the socio-demographic information of participants including their residential status and whether they received a package of care. Relevant clinical data were also captured such as smoking status, the number of prescribed medicines including inhaled therapy and oral medications for respiratory disease, co-morbidities, COPD symptom severity, COPD exacerbations, use of rescue packs (combinations of antibiotics and steroids), and the number of GP visits. These data were either self-reported or recorded with consent from the hospital electronic patient record (EPR). Data for models/tools used to classify and characterise COPD, such as GOLD25 and the MRC Dyspnoea scale,26 as well as lung function tests including FEV1 and FVC, were collected where available from the EPR. All retrospective data were considered eligible if reported within the previous 12 months prior to study participation.
Factor Analyses
Both Exploratory Factor Analysis (EFA) and Partial Confirmatory Factor Analysis (PCFA) were conducted on SPUR-27 tool in line with COSMIN guidance for cross-validation of PROMs in a new condition.27 The Kaiser–Meyer–Olkin (KMO) measure of sampling adequacy and Bartlett’s test of sphericity were used to determine database eligibility. Visual inspection for an inflection point in scree plots were utilised to identify initial factors. EFA was conducted using Principle Components Analysis (PCA) with Direct Oblimin rotation; factor loadings >0.32 were considered valid.28 PCFA was performed using Maximum Likelihood Analysis (MLA). Fit indices including normed fit index (NFI), comparative fit index (CFI), Tucker–Lewis index (TLI), and root mean square error of approximation (RMSEA) were calculated. A value ≥0.9 was expected for NFI, CFI and TLI and <0.08 for the RMSEA value.29,30
Reliability
Cronbach’s alpha (α) was calculated as an internal consistency estimate of reliability for both the whole SPUR-27 tool, as well as for individual factors, with an alpha ≥0.8 considered as strong evidence of reliability. Item-Total Correlations (ITCs) and Intraclass Correlation Coefficients (ICC) were calculated, with a value >0.5 considered acceptable for the latter.31
Construct Validity
Construct validity was established by comparing SPUR-27 scores with the previously validated 4-item Inhaler Adherence Scale (IAS).32 The IAS provides an indication of adherence to inhalers based on a mean score across the 4 items, with a result of 5 indicating adherence.
Concurrent Validity
The Medication Possession Ratio (MPR), a crude objective measure of medication adherence, was compared with SPUR-27 scores to examine concurrent validity. MPR was derived from either the Summary Care Record (SCR), an electronic system of primary care patient data, or their EPR. An MPR ≥80% was the cut-off for determining a participant as adherent.33 The following formula was used to calculate MPR:
Known Group Validity
Known group validity properties were measured by examining expected relationships (Spearman’s Rank Correlations) between medication adherence and socio-clinical factors. It was hypothesised that a relationship would exist between adherence scores and patient age, income, comorbidity burden, the number of respiratory medicines, COPD exacerbations, use of rescue packs, and the number GP visits. A Chi-Square analysis (χ2) was conducted to evaluate the dynamic between adherence scores and the severity of COPD symptoms experienced by the patient. COPD symptom severity was assessed using the COPD Assessment Tool (CAT), with scores of 0–19 classified as low-medium and scores ≥20 classified as high–very high. SPUR-27 defined adherence as total scores ≥80%.23 It was expected that those participants with higher adherence scores would report lower CAT scores due to reduced symptom severity. It was also expected that patients with less severe COPD (lower GOLD classification, lower MRC score, and better lung function results) would report higher adherence.
Results were treated as a non-parametric given the small sample size; therefore, a Kruskal–Wallis H-test was used to examine between-group differences for SPUR-27 scores and all socio-demographic data that were collected as ordinal or categorical variables.
Cut-off Determination
Receiver operating curve (ROC) and area under the curve (AUC) procedures were used to produce an ROC-AUC analysis for determining a cut-off value with the highest sensitivity vs lowest inverse specificity.34
Adequacy Testing
Data were generated to determine the sensitivity and specificity of SPUR-27. Furthermore, predictive values (positive and negative) were included in the adequacy analysis.
Data Analyses
Data were analysed using IBM SPSS Software Version 26.0 for Windows. Spearman’s rank correlation coefficient (p) was used to examine significant correlations (p<0.05). Continuous data are expressed as a mean (x) and standard deviation (SD). Categorical data are reported as sample number (n) and percentage (%).
Results
Study Sample Characteristics
A total of 100 patients participated in this study out of 123 that were approached, providing a response rate of 81.3%. Age, education, and income were collected as ordinal data and are reported as such (Table 1). The modal age range was 70–79 years (41%, n=41). Participants were predominantly White (90%), educated to GCSE level or equivalent (51%), and classified as retired (76%). Female participants represented slightly over half the sample (52%). Over two-thirds (67%) of the study sample reported that they were ex-smokers, with an average pack-year history across both smoking and ex-smoking participants of 39.33 ± 26.03 (Table 2). Body Mass Index (BMI) data were available for the entire sample, with a mean score of 27.38 ± 6.88 kg/m2 indicating that study participants were predominantly overweight based on BMI classification.
 |
Table 1 Sample Demographic Characteristics |
 |
Table 2 Sample Socio-Clinical Characteristics |
Factor Analysis
For EFA, the initial KMO measure of sampling adequacy was 0.752 (>0.7) and Bartlett’s test of sphericity was significant (χ2 = 1489.802, p < 0.001). An 8-factor solution was obtained with eigenvalues >1 that explained 62.2% of the variance. However, the rotated model found one item with a loading <0.32 (Item 29). Once removed, an 8-factor solution was observed, with Item 33 loading as the sole item on factor 8, which was deemed inadequate. Removal of Item 33 produced a 7-factor rotated solution, with only 2 items loading onto factors 5 and 6.
For comparison, the EFA was conducted on the SPUR-27 model using PCA while fixing the number of factors at 7. The 7-factor solution obtained explained 68.9% of the variance, with all 27 items observing loadings >0.32.28 Only 2 items were loaded onto Factor 7; however, the average loading was 0.666. The PCA 7-factor solution was retained for further analysis (Table 3).
 |
Table 3 SPUR-27 Item Content, Factor Loadings, and Descriptive Statistics |
PCFA using MLA was performed using the same rotation. The implied model χ2 value was reported at 280.956, df = 183. These values were used to determine incremental fit indices: NFI (0.96), TFI (0.97) and CFI (0.93) were all reported as >0.9 in addition to the RMSEA, which was <0.08 (0.059).
Notably, the SPUR-27 model in this population observed items loading onto different factors from those observed in the previous type 2 diabetes cohort.23 For example, item 6 that is currently mapped to factor 1 was previously designated under the Usage domain and under Factor 5 in the former study. When comparing the type 2 diabetes and COPD models, continuity of item-to-factor mapping occurred for 18/27 items (66.7%) in this factor structure for VMATC.
Item and Mean Score Distribution
SPUR questionnaire items ranged from 1 to 5, with a higher score indicating a greater likelihood of adherence. The observed item scores demonstrated a predominantly left-skewed distribution with a range of 3.44–4.74 and mean total score 4.19 ± 1.09. The mean SPUR-27 score was 83.82 ± 11.53.
Reliability
An internal consistency estimate, reflected as a Cronbach (α) value, was strong (>0.8) for the total SPUR-27 tool (0.893). Cronbach’s values for individual factors 1–7 ranged from adequate to strong (0.504–0.850) (Table 4). There were no items that if removed would have led to an increased α for the overall SPUR-27 model. ITCs ranged from 0.263 to 0.652. ICCs for SPUR-27 were 0.893 (0.860–0.921, 95% CI) and were deemed acceptable (>0.5). Inter-factor correlations were predominantly significant (p<0.05 or p<0.01) and ranged from −0.008 to 0.535 (Table 4).
 |
Table 4 Inter-Factor Correlations, and Internal Consistency Estimates, and Descriptive Statistics for All Subscales |
Construct Validity
When compared with the IAS total score, SPUR-27 demonstrated a significant (p<0.001) strong (>0.6) positive correlations (p=0.645) providing initial evidence of construct validity.
Concurrent Validity
When compared with MPR, the SPUR-27 model demonstrated a weak significant (p<0.01) positive correlation providing evidence of concurrent validity (p=0.295).
Known Group Validity
The SPUR-27 model demonstrated a significant (p<0.01) weak positive correlation with patient income (ρ=0.269) and a weak negative correlation with the number of COPD exacerbations reported in the 12 months prior to study participation (ρ=−0.201). A positive correlation was observed with age, and a negative correlation with the number of respiratory medicines, number of comorbidities, number of GP visits, and number of rescue packs within the previous 12 months; however, these data were not significant (p>0.05).
Results for GOLD COPD classification, MRC Dyspnoea scale scores, and lung function tests (FEV1/FVC) were available for <5% (n=4/100) of the study sample and were therefore excluded from the analysis. No significant difference (p>0.05) in SPUR-27 score was observed between groups based on socio-demographic characteristics, residential status or those receiving differing levels of social care support.
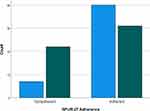
When cross-tabulating adherence scores and COPD symptom severity (CAT score), SPUR-27 observed a significant (p<0.01) Chi-Square (χ2) value (χ2 =8.570); hence, known group validity was established. Figure 1 demonstrates the distribution of adherent vs non-adherent patients against CAT score responses.
Cut-off Determination
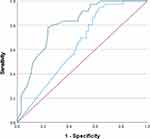
The ROC curve (Figure 2) compared SPUR-27 and MPR scores against the IAS as a reference. As per Wells et al,23 previous validation report for the SPUR-27 model, MPR was demonstrated to be a poor predictor of adherence; hence, a decision was made to use a validated PROM of MA (IAS) for the ROC-AUC analysis. Adherence assessed on IAS was poor with a positive:negative case ratio of 36:64 with positive cases defined as adherent patients. The SPUR-27 model observed an ROC-AUC >80%, whereas MPR produced an ROC-AUC of 63.8% (0.531–0.745, 95% CI) (Table 5). Based on ROC-AUC coordinates, a cut-off value of 87% was determined for SPUR-27 providing a sensitivity of 0.806 and inverse specificity of 0.281.
 |
Table 5 ROC-AUC Analysis |
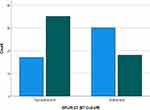
As a new cut-off score was determined for SPUR-27, the Chi-Square analysis was repeated to determine adequacy of known-group validity vs the CAT score. The χ2 value was 8.903 (p=0.003); hence, known group validity could be established using the new cut-off score (Figure 3).
SPUR Model Adequacy
Sensitivity and specificity data vs IAS scores were generated for SPUR-27 using the original >80% score and new ≥87% cut-off determinant score (Table 5). Sensitivity and specificity were also examined for the MPR as a comparator against the models. SPUR-27 (87 cut-off) observed sensitivities and specificities >70.0%. Predictive values (±) are also reported in Table 6. SPUR-27 (87 cut-off) provided the most consistent scores across the three examined outcomes.
 |
Table 6 Tests for SPUR-27 Model Adequacy |
Discussion
This study aimed to provide evidence for the psychometric properties of the SPUR model in patients living with COPD. SPUR-27, a shorter model that has previously demonstrated greater reliability and strong psychometric properties in patients living with in type 2 diabetes23 was used as the predominant model in the analysis. The study has provided evidence for the psychometric properties of the SPUR-27 model in this population whilst also identifying potential limitations with the cross-cultural validation of the tool.
Factor analyses for the short-form SPUR-27 model was performed in accordance with COSMIN guidelines for cross-validation.27 EFA provided an initial 8-factor solution that reduced to seven factors following item removal. However, two factors with only two items were identified in the rotated solution. This finding was not concordant with previous validation, and such factors are at risk of instability, particularly with smaller sample sizes.35 A fixed 7-factor PCA on the original SPUR-27 tool provided an improved rotated solution, with a single factor with two loadings – these items were highly correlated and hence provided justification for inclusion of the 7-factor PCA model.36 Values for fit indices including NFI, TLI, CFI (>0.9) and RMSEA (<0.08) met the expected thresholds to determine good factor-structure fit, providing additional evidence for suitability of the 7-factor solution. There were differences in item-factor loading between the 7-factor type 2 diabetes and COPD models. This result demonstrates some incongruence between the populations. This may in part be attributed to the smaller sample size used in this study or reflective of stark differences between patients’ experiences of MA when comparing living with COPD or type 2 diabetes; these findings may ultimately lead to less delineation between factors and warrant further investigation within a larger population.
Early evidence for construct validity was observed using the previously validated IAS measure. A significant moderate correlation (>0.6) was observed when comparing SPUR-27 with the IAS.37 Internal consistency estimates for SPUR-27 were strong (>0.8), without exceeding a threshold of >0.9 that may suggest redundancy of items or the need for a shorter scale.38,39 When compared to the original validation scores of the IAS, SPUR-27 demonstrated a greater internal consistency estimate (0.89 vs 0.83) and similar correlations with an objective MA measure (p=0.30 vs 0.35), such as MPR. Previous studies using MPR have highlighted the tendency for over-estimation of MA with this methodology.40 As the study recruitment was affected by Covid-19, the increased dispensing and remote delivery of medicines potentially exaggerated MPR values.23 However, in this sample, SPUR-27 observed a weak significant correlation with MPR. This finding is in contrast to previous work by Tommelein et al41 that found no significant correlation between refill adherence (MPR or equivalent) and MARS-5, a PROM that is commonly used to self-report MA among patients living with COPD.42–44 Furthermore, within this study, the correlation between SPUR-27 and MPR was in fact greater than the correlation values reported in previous studies for SPUR-27, which included additional validated PROMs; hence, early evidence of concurrent validity was observed in this sample.
The impact of socioeconomic status, comorbidity burden, and polypharmacy on medication adherence in COPD is well documented.45–48 Despite this, the specific relationship between income and MA is less established in UK population. In this study sample, a significant positive correlation between income and MA using SPUR-27 was observed. The discourse on income and MA relates predominantly to US populations who access a paid/insurance-based healthcare model, whereby low-income has been associated with poor MA in patients living with COPD.49,50 UK data are sparse; however. the link between income and MA has been previously identified in Danish populations with a similar healthcare model.48 In terms of other socio-demographic factors, it was predicted that the MA would also increase with age, and conversely decrease with an increasing number of comorbidities and prescribed medicines. Bivariate correlations for these relationships matched predicted effect directions; however. the findings were not significant. Vetrano et al45 highlight for stable convergence of correlations that a sample size of approximately 250 participants should be used. In this study, due to Covid-19, only 100 patients were recruited. Hence, the ability to detect correlated sample effects may have been limited. However, EFA conducted with samples ≤50 have been previously used and validated.51 Even with a small sample, Chi-Square analysis of MA vs symptom severity demonstrated a significant relationship between poor MA and increasing symptom severity. This finding was concordant with the literature and it constituted a key aim for developing the SPUR measure to address the relationship between MA and socio-clinical factors, such as COPD symptom severity and exacerbation risk.4
Previous validation of SPUR-27 did not provide guidance on a scoring methodology, therefore an ROC-AUC analysis was conducted to provide adequate data to determine an appropriate cut-off score.34 An ROC-AUC value of >0.8 was used as determination of a good test result.52 SPUR-27 reported an encouraging result >0.8 versus the reference IAS score. Using this method, a new cut-off score of 87 was determined for SPUR-27. Known group validity was reestablished using Chi Square analysis between the CAT score and SPUR-27, which provided a significant result. The new cut-off score was an important step of the validation process owing to the vast improvement in model adequacy for SPUR-27. The initial SPUR-27 cut-off determinant provided highly specific but poorly sensitive results, performing comparatively to the MPR methodology. However, with determination of the new cut-off score, SPUR-27 observed improved sensitivity and specificity with values >70%. In a pooled analysis of MMAS-8 validation studies, with MMAS-8 considered a gold standard PROM of MA, sensitivity and specificity were reported as 0.43 (0.33–0.53, 95% CI) and 0.73 (0.68–0.78, 95% CI), respectively.53 SPUR-27 was therefore acceptably sensitive (70.3%) and specific (80.6%) to MA, particularly when compared with similar PROMs reported in the wider literature such as MMAS-8.
The results of the study indicate strong psychometric properties of SPUR-27 to reliably assess MA in patients living with COPD. With this early evidence of psychometric properties for SPUR-27 in this population, future work should seek to support the development of tailored MA interventions as discussed by Dolgin.19 The applicability of SPUR-27 model may also extend to settings such as community pharmacies, GP surgeries, and social care facilities (eg, nursing/residential homes) as a self-report measure than can facilitate the early identification and characterisation of poor MA and the risks associated with worsening COPD symptom severity as identified in this study sample. Proactive monitoring of MA and health status in these patients may also have positive health economic and quality of life outcomes that future validation studies may look to address such as a reduction in hospitalisation or COPD exacerbations.
There were several limitations in this study. Due to the Covid-19 pandemic, there was a significant impact on the recruitment period that resulted in a small sample size; hence, the study results should be treated cautiously. This is particularly true given the impact on the availability of routinely collected clinical data such as GOLD, MRC and lung function tests, which had not been reported as frequently during the Covid-19 pandemic that limited some of the analysis for this sample. Furthermore, due to the pandemic, patients were unable to be seen for follow-up testing given that the risk of Covid-19 outweighed the benefit of reattendance for a non-interventional study. This limited the capacity to conduct a test–retest reliability evaluation for this sample. Most participants in this study were >70 years of age. Although participants were only included if they could complete the study independently and were recruited by an experienced healthcare professional, the inclusion of a validated model to assess cognitive deficiency, such as the mini mental-state examination (MMSE) would have benefitted the current study. Although this model observed strong psychometric properties and the ability to discriminate between adherent and non-adherent patients in this sample, the factorial structure of the SPUR-27 tool in this study was also subject to incongruence when compared to the previously validated model. Future research should look to expand the sample size to provider further evidence for confirmation of a consistent factor structure, as well as providing an opportunity to apply a test–retest approach to determine suitability of the measure for this validity criterion.
Conclusion
This study has demonstrated the strong psychometric properties of SPUR-27 version in patients living with COPD. This is the first study to demonstrate psychometric properties of the SPUR model in patients who report a chronic respiratory condition that predominantly relies on inhaled pharmacotherapy. Furthermore, this study employed both a comparative PROM and objective measure of adherence to improve the reliability of the results in this population. Future studies should look to explore cross-cultural validation in additional comorbidities for wider application of the tool, as well as future translation of SPUR measures for implementation in different sample populations beyond those with English as their first language. Furthermore, future work should look to explore the relationship between the SPUR model and psychological comorbidity given the extensive evidence of the latter’s impact on both MA and quality of life in patients living with COPD.
Acknowledgments
The authors thank the Kingston Hospital NHS Foundation Trust research department, as well as the respiratory team for their support with this study during the COVID-19 pandemic. We would also like to give huge thank you to all the participants who supported this study, particularly when faced with the challenges of the COVID-19 pandemic.
Disclosure
KD reports being the General Manager of Observia. JW receives funding for his PhD via Observia; however, he did not receive financial remuneration for this work. The authors report no other conflicts of interest in this work.
References
1. Burney P, Jarvis D, Perez-Padilla R. The global burden of chronic respiratory disease in adults. Int J Tuberc Lung Dis. 2015;19(1):10–20. doi:10.5588/ijtld.14.0446
2. Soriano JB, Abajobir AA, Abate KH, et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet Respir Med. 2017;5(9):9. doi:10.1016/S2213-2600(17)30293-X
3. Izquierdo JL, Morena D, González Y, et al. Clinical management of COPD in a real-world setting. A big data analysis. Arch Bronconeumol. 2021;57(2):94–100. doi:10.1016/j.arbres.2019.12.025
4. Zamzam A, Azab NY, El Wahsh RA, Ragab AZ, Allam EM. Quality of life in COPD patients. Egypt J Chest Dis Tuberc. 2012;61(4):281–289. doi:10.1016/J.EJCDT.2012.08.012
5. Mikkelsen RL, Middelboe T, Pisinger C, Stage KB. Anxiety and depression in patients with chronic obstructive pulmonary disease (COPD). A review. Nord J Psychiatry. 2004;58(1):65–70. doi:10.1080/08039480310000824
6. Schneider C, Jick SS, Bothner U, Meier CR. COPD and the risk of depression. Chest. 2010;137(2):341–347. doi:10.1378/CHEST.09-0614
7. Humenberger M, Horner A, Labek A, et al. Adherence to inhaled therapy and its impact on chronic obstructive pulmonary disease (COPD) 11 medical and health sciences 1102 cardiorespiratory medicine and haematology. BMC Pulm Med. 2018;18(1):1–6. doi:10.1186/S12890-018-0724-3/TABLES/3
8. Almagro P, Calbo E, Ochoa de Echaguïen A, et al. Mortality after hospitalization for COPD. Chest. 2002;121(5):1441–1448. doi:10.1378/CHEST.121.5.1441
9. Albrecht JS, Park Y, Hur P, et al. Adherence to maintenance medications among older adults with chronic obstructive pulmonary disease the role of depression. Ann Am Thorac Soc. 2016;13(9):1497–1504. doi:10.1513/ANNALSATS.201602-136OC/SUPPL_FILE/DISCLOSURES.PDF
10. Turan O, Yemez B, Itil O. The effects of anxiety and depression symptoms on treatment adherence in COPD patients. Prim Health Care Res Dev. 2014;15(3):244–251. doi:10.1017/S1463423613000169
11. Dal negro RW, Tognella S, Tosatto R, Dionisi M, Turco P, Donner CF. Costs of chronic obstructive pulmonary disease (COPD) in Italy: the SIRIO study (social impact of respiratory integrated outcomes). Respir Med. 2008;102(1):92–101. doi:10.1016/J.RMED.2007.08.001
12. Ngo CQ, Phan DM, Vu van G, et al. Inhaler technique and adherence to inhaled medications among patients with acute exacerbation of chronic obstructive pulmonary disease in Vietnam. Int J Environ Res Public Health. 2019;16(2):185. doi:10.3390/IJERPH16020185
13. Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011;86(4):304–314. doi:10.4065/mcp.2010.0575
14. Sanduzzi A, Balbo P, Candoli P, et al. COPD: adherence to therapy. Multidiscip Respir Med. 2014;9(1):1–9. doi:10.1186/2049-6958-9-60/TABLES/5
15. Giraud V, Roche N. Misuse of corticosteroid metered-dose inhaler is associated with decreased asthma stability. Eur Respir J. 2002;19(2):246–251. doi:10.1183/09031936.02.00218402
16. Unni EJ, Farris KB. Unintentional non-adherence and belief in medicines in older adults. Patient Educ Couns. 2011;83(2):265–268. doi:10.1016/J.PEC.2010.05.006
17. Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens. 2008;10(5):348–354. doi:10.1111/j.1751-7176.2008.07572.x
18. Martin LR, Williams SL, Haskard KB, Dimatteo MR. The challenge of patient adherence. Ther Clin Risk Manag. 2005;1(3):189–199.
19. Dolgin K. The SPUR model: a framework for considering patient behavior. Patient Prefer Adherence. 2020;14:97–105. doi:10.2147/PPA.S237778
20. Tugaut B, Shah S, Dolgin K, et al. Development of the SPUR tool: a profiling instrument for patient treatment behavior. J Patient Rep Outcomes. 2022;6(1):1–10. doi:10.1186/S41687-022-00470-X
21. de Bock E, Dolgin K, Kombargi L, et al. Finalization and validation of questionnaire and algorithm of SPUR, a new adherence profiling tool. Patient Prefer Adherence. 2022;16:1213–1231. doi:10.2147/PPA.S354705
22. de Bock E, Dolgin K, Arnould B, Hubert G, Lee A, Piette JD. The SPUR adherence profiling tool: preliminary results of algorithm development. Curr Med Res Opin. 2021;1–9. doi:10.1080/03007995.2021.2010437
23. Wells JS, Husseini A, Okoh S, et al. SPUR: psychometric properties of a patient-reported outcome measure of medication adherence in type 2 diabetes. BMJ Open. 2022;12(9):e058467. doi:10.1136/BMJOPEN-2021-058467
24. Raosoft I. Raosoft sample size calculator; 2004. Available from: http://www.raosoft.com/samplesize.html.
25. Venkatesan P. GOLD COPD report: 2023 update. Lancet Respir Med. 2023;11(1):18. doi:10.1016/S2213-2600(22)00494-5
26. UK Research and Innovation. MRC dyspnoea scale – UKRI; 2022. Available from: https://www.ukri.org/councils/mrc/facilities-and-resources/find-an-mrc-facility-or-resource/mrc-dyspnoea-scale/.
27. Mokkink CAC, Prinsen DL, Patrick Jordi Alonso Lex M, Bouter LB, Mokkink CL. COSMIN study design checklist for patient-reported outcome measurement instruments. Available from: www.cosmin.nl.
28. Field A. Discovering Statistics Using IBM SPSS Statistics. Vol. 58. sage; 2013.
29. Naqvi AA, Hassali MA, Rizvi M, et al. Validation of the general medication adherence scale in Pakistani patients with rheumatoid arthritis. Front Pharmacol. 2020;11:1039. doi:10.3389/FPHAR.2020.01039/BIBTEX
30. Pett MA, Lackey NR, Sullivan JJ. Making sense of factor analisys; 2003. Available from: https://books.google.com/books/about/Making_Sense_of_Factor_Analysis.html?id=9M3CQgAACAAJ.
31. Lahey MA, Downey RG, Saal FE. Intraclass correlations: there’s more there than meets the eye. Psychol Bull. 1983;93(3):586–595. doi:10.1037/0033-2909.93.3.586
32. Erickson SR, Coombs JH, Kirking DM, Azimi AR. Compliance from self-reported versus pharmacy claims data with metered-dose inhalers. Ann Pharmacother. 2001;35(9):997–1003. doi:10.1345/aph.10379
33. Ahmadipour H, Farajzadegan Z, Kachoei A, Pirdehghan A. Secondary prevention by enhancing adherence in diabetic patients. Int J Prev Med. 2010;1(1). doi:10.4103/ijpvm.IJPVM_302_16
34. Fawcett T. An introduction to ROC analysis. Pattern Recognit Lett. 2006;27(8):861–874. doi:10.1016/j.patrec.2005.10.010
35. Costello AB, Osborne J. Best practices in exploratory factor analysis: four recommendations for getting the most from your analysis. Pract Assess Res Eval. 2019;10(1):7. doi:10.7275/jyj1-4868
36. Yong A. For SPT in quantitative methods, 2013 undefined. A beginner’s guide to factor analysis: focusing on exploratory factor analysis. coris.uniroma1.it. Available from: https://www.coris.uniroma1.it/sites/default/files/Factor2_0.pdf.
37. Chan Y. Biostatistics 104: correlational analysis. Singapore Med J. 2003;1(44):614–619.
38. Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16(3):297–334. doi:10.1007/BF02310555
39. Streiner DL. Starting at the beginning: an introduction to coefficient alpha and internal consistency. J Pers Assess. 2003;80(1):99–103. doi:10.1207/S15327752JPA8001_18
40. Hamdidouche I, Jullien V, Boutouyrie P, Billaud E, Azizi M, Laurent S. Drug adherence in hypertension: from methodological issues to cardiovascular outcomes. J Hypertens. 2017;35(6):1133–1144. doi:10.1097/HJH.0000000000001299
41. Tommelein E, Mehuys E, van Tongelen I, Brusselle G, Boussery K. Accuracy of the medication adherence report scale (mars-5) as a quantitative measure of adherence to inhalation medication in patients with COPD. Ann Pharmacother. 2014;48(5):589–595. doi:10.1177/1060028014522982/ASSET/IMAGES/LARGE/10.1177_1060028014522982-FIG2.JPEG
42. Elander A, Gustafsson M. Inhaler technique and self-reported adherence to medications among hospitalised people with asthma and COPD. Drugs Real World Outcomes. 2020;7(4):317–323. doi:10.1007/S40801-020-00210-X/TABLES/3
43. Hanania NA, Braman S, Adams SG, et al. The role of inhalation delivery devices in COPD: perspectives of patients and health care providers. Chronic Obstr Pulm Dis. 2018;5(2):111. doi:10.15326/JCOPDF.5.2.2017.0168
44. VanderSchaaf K, Olson KL, Billups S, Hartsfield CL, Rice M. Self-reported inhaler use in patients with chronic obstructive pulmonary disease. Respir Med. 2010;104(1):99–106. doi:10.1016/J.RMED.2009.07.003
45. Vetrano DL, Bianchini E, Onder G, et al. Poor adherence to chronic obstructive pulmonary disease medications in primary care: role of age, disease burden and polypharmacy. Geriatr Gerontol Int. 2017;17(12):2500–2506. doi:10.1111/GGI.13115
46. Rolnick SJ, Pawloski PA, Hedblom BD, Asche SE, Bruzek RJ. Patient characteristics associated with medication adherence. Clin Med Res. 2013;11(2):54–65. doi:10.3121/CMR.2013.1113
47. Gershon AS, Dolmage TE, Stephenson A, Jackson B. Chronic obstructive pulmonary disease and socioeconomic status: a systematic review. J Chron Obstruct Pulmon Dis. 2012;9(3):216–226. doi:10.3109/15412555.2011.648030
48. Tøttenborg SS, Lange P, Johnsen SP, Nielsen H, Ingebrigtsen TS, Thomsen RW. Socioeconomic inequalities in adherence to inhaled maintenance medications and clinical prognosis of COPD. Respir Med. 2016;119:160–167. doi:10.1016/J.RMED.2016.09.007
49. Krauskopf K, Federman AD, Kale MS, et al. Chronic obstructive pulmonary disease illness and medication beliefs are associated with medication adherence. J Chron Obstruct Pulmon Dis. 2015;12(2):151–164. doi:10.3109/15412555.2014.922067
50. Simoni-Wastila L, Wei YJ, Qian J, et al. Association of chronic obstructive pulmonary disease maintenance medication adherence with all-cause hospitalization and spending in a medicare population. Am J Geriatr Pharmacother. 2012;10(3):201–210. doi:10.1016/J.AMJOPHARM.2012.04.002
51. de Winter JCF, Dodou D, Wieringa PA. Exploratory factor analysis with small sample sizes. Multivariate Behav Res. 2009;44(2):147–181. doi:10.1080/00273170902794206
52. Applied Logistic Regression - David W. Hosmer, Jr., Stanley Lemeshow, Rodney X. Sturdivant - google books. Available from: https://books.google.co.uk/books?hl=en&id=64JYAwAAQBAJ&oi=fnd&pg=PR13&ots=DtfM1V9rgJ&sig=PUyKms_XGHAXpNoZi6DTlnA8Dqk&redir_esc=y#v=onepage&q&f=false.
53. Moon SJ, Lee W-Y, Hwang JS, Hong YP, Morisky DE, Reboldi G. Accuracy of a screening tool for medication adherence: a systematic review and meta-analysis of the Morisky medication adherence scale-8. PLoS One. 2017;12(11):e0187139. doi:10.1371/JOURNAL.PONE.0187139
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Validation of the Spanish Activity Questionnaire in COPD (SAQ-COPD) in Patients with Chronic Obstructive Pulmonary Disease
Soler-Cataluña JJ, Puente Maestu L, Román Rodríguez M, Esteban C, Gea J, Bernabeu Mora R, Pleguezuelos Cobo E, Ancochea J, García-Río F
International Journal of Chronic Obstructive Pulmonary Disease 2022, 17:2835-2846
Published Date: 5 November 2022
Psychometric Evaluation of the Grit Psychological Resources Scale (GPRS)
Schimschal SE, Cleary M, Kornhaber RA, Barnett T, Visentin DC
Journal of Multidisciplinary Healthcare 2023, 16:913-925
Published Date: 5 April 2023